Abstract
Context:
Patients with type V osteogenesis imperfecta (OI) are heterozygous for a dominant IFITM5 c.-14C>T mutation, which adds five residues to the N terminus of bone-restricted interferon-induced transmembrane-like protein (BRIL), a transmembrane protein expressed in osteoblasts. Type V OI skeletal findings include hyperplastic callus formation, ossification of the forearm interosseous membrane, and dense metaphyseal bands.
Objective:
The objective of this study was to examine the role of osteoblasts in the active mineralization traits of type V OI and the effect of the IFITM5 mutation on type I collagen.
Methods:
We identified eight patients with the IFITM5 c.-14C>T mutation. Cultured osteoblasts from type V OI patients were used to study osteoblast differentiation and mineralization.
Results:
We verified the expression and stability of mutant IFITM5 transcripts. In differentiated type V OI primary osteoblasts in culture, the IFITM5 expression and BRIL level is comparable with control. Both early and late markers of osteoblast differentiation are increased in type V OI osteoblasts. Mineralization, assayed by alizarin red staining, was increased in type V OI osteoblasts compared with control. However, type V OI osteoblasts have significantly decreased COL1A1 transcripts in mid- to late differentiation. Type I collagen protein is concomitantly decreased, with decreased cross-linked collagen in matrix and altered appearance of fibrils deposited in culture.
Conclusions:
This study demonstrates that type V OI mineralization has a gain-of-function mechanism at the osteoblast level, which likely underlies the overactive tissue mineralization seen in patients. Decreased type I collagen expression, secretion, and matrix incorporation establish type V OI as a collagen-related defect.
Osteogenesis imperfecta (OI) is a genetically heterogeneous heritable connective tissue disorder characterized by intrinsic bone fragility, resulting in frequent fractures and deformities of the long bones and spine (1). The phenotypic severity of OI ranges from perinatal lethal to subtle fracture susceptibility and generalized osteopenia (2). Most cases of osteogenesis imperfecta are caused by autosomal dominant mutations in the genes encoding type I collagen, COL1A1 or COL1A2 (3). Autosomal recessive osteogenesis imperfecta, with lethal to moderate phenotypes, is caused by defects in genes whose products interact with type I collagen for folding, post-translation modification or processing (4). Most recessive cases have null mutations in genes whose proteins are involved in collagen prolyl 3-hydroxylation (CRTAP, LEPRE1, and PPIB) (5–7) or proper helical folding (FKBP10 and SERPINH1) (8–11). Additional genes causing recessive OI include SERPINF1, which encodes pigment epithelium-derived factor (PEDF) (12, 13), collagen C-propeptide cleavage enzyme (BMP1) (14), and WNT1 (15, 16).
Type V OI is the only dominantly inherited form of OI not caused by mutations in COL1A1 or COL1A2 (4). It has distinctive clinical and radiographic features, including variable occurrence of hyperplastic callus, calcification of the forearm interosseous membrane, radial-head dislocation, and a subphyseal metaphyseal radiodense band (17, 18). The histomorphometry of type V OI is also distinct, with a mesh-like lamellation pattern (17). Recent reports have shown that type V OI is caused by a single recurrent heterozygous mutation in interferon-induced transmembrane protein 5 (IFITM5), which encodes bone-restricted (IFITM)-like protein (BRIL), a protein involved in mineralization and expressed in the skeleton (19, 20). The type V OI mutation is a C>T transition at position −14 of the 5′ untranslated region of IFITM5 (c.-14C>T) (21, 22). This mutation generates a new start codon, adding five amino acids to the N terminus of the protein. However, the mutant protein maintains the same intramembrane topology and palmitoylation as normal BRIL (23) and has been speculated to have a gain-of-function mechanism. The understanding that the same mutation is responsible for all cases of type V OI then led to the recognition of phenotypic variability (24). We describe here eight patients with type V OI from five different families, who display phenotypic variability.
The mechanism of type V OI is not understood at the cellular level, in this case, the bone-forming osteoblasts. Using primary cultured osteoblasts from type V OI patients, we verified the expression of mutant IFITM5 transcript and protein. We used these primary mutant osteoblasts to demonstrate that the presence of the IFITM5 mutation increases multiple markers of osteoblast differentiation. Type V OI osteoblasts also display increased mineral deposition in culture, demonstrating that a gain-of-function mechanism at the cellular level underlies the active mineralization traits in type V OI patients. Despite increased osteoblast developmental markers, COL1A1 expression, secretion, and deposition in matrix by type V OI osteoblasts is significantly decreased, which establishes type V OI as a collagen-related dysplasia.
Materials and Methods
Patients and cells
Patients 2, 3, 5, and 8 are patients at the National Institutes of Health Clinical Center (Bethesda, Maryland), whose samples were collected under an institutional review board-approved protocol. Osteoblasts were outgrowths from bone chips collected as surgical discard during medically indicated orthopedic procedures. Patient 7 is followed up at the Genetic Clinic at Mt Sinai Hospital (Toronto, Ontario, Canada); samples were collected with parental consent. Detailed case reports on the patients are presented in Supplemental Materials. Control fibroblasts were American Type Culture Collection line 2127, which is well validated for collagen biochemistry, whereas the control osteoblasts were obtained with parental consent from surgical discard bone chips of an unaffected child during an elective orthopedic procedure. These control cells have been used extensively in our laboratory and validated for experiments involving osteoblast development and collagen biochemistry.
Mutation identification and verification
We screened genomic DNA (gDNA) of dermal fibroblasts, leukocytes, or both from the control, patients, parents, or unaffected siblings. Sequencing of cDNA and gDNA from the patients revealed no mutations in COL1A1, COL1A2, CRTAP, LEPRE1, PPIB, SERPINH1, FKBP10, or SERPINF1. All patient skin and bone biopsies were obtained with informed consent under a protocol approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board.
The two exons and flanking intronic sequences of IFITM5 gDNA from leukocytes of control, patients, sibling, and parents were amplified by PCR, as previously described (25), and sequenced. Patient and control IFITM5 cDNA from fibroblasts was also sequenced and the mutation was confirmed by BsmAI restriction enzyme digestion.
Cell culture
Dermal fibroblast cultures were established from skin punch biopsies. Fibroblasts were grown in DMEM (Life Technologies) containing 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin. Osteoblast primary cultures were established from surgical bone chips (26) of normal control and OI patients who had not received bisphosphonates. Osteoblasts were cultured in MEMα (Life Technologies) supplemented with 10% fetal bovine serum, penicillin, and streptomycin at 37°C and 8% CO2. Osteoblast cultures were grown to confluence and then treated with osteoblast differentiation medium [containing 25 μg/mL L-ascorbic acid, 10−8 M dexamethasone, and 2.5 mM 2-glycerophosphate, with or without 100 ng/mL human recombinant bone morphogenetic protein (BMP)-2 (the generous gift of Wyeth Inc)].
RT-PCR
Total RNA was extracted from control and patient primary osteoblast and fibroblast cultures using TriReagent (Molecular Research Center, Cincinnati, Ohio). cDNA was reverse transcribed from 5 μg RNA using a high-capacity cDNA archive kit or murine leukemia virus reverse transcriptase and oligodeoxythymidine16 (Life Technologies). Transcript levels of IFITM5 (Hs00942485_g1), RUNX2 (Hs00231692_m1), COL1A1 (Hs00164004_m1), ALPL (Hs01029144_m1), BGLAP (Hs01587814_g1), SPP1 (Hs00959010_m1), SERPINF1 (Hs01106934_m1), and IBSP (Hs00173720_m1) (Life Technologies) were determined with TaqMan gene expression assays on a 7500 Fast real-time PCR system (Applied Biosystems). Relative expression of each gene of interest was performed in triplicate and normalized to the expression of GAPDH (Hs99999905_m1). Expression levels were compared with age-matched control osteoblasts (day 1 or 5). Expression of IFITM5 in osteoblasts and fibroblasts by PCR (as shown in Supplemental Figure 1C) was demonstrated using primers in exon 1 (forward, 5′-TTGATCTGGTCGGTGTTCAG-3′) and exon 2 (5′-GTCAGTCATAGTCCGCGTCA-3′) as previously described (21) and generated the expected 292-bp product.
Western blot
Cell lysates were collected in radioimmunoprecipitation assay buffer supplemented with a protease inhibitor cocktail (Sigma-Aldrich). Osteoblast-conditioned media were collected after incubation for 24 hours in serum-free media and supplemented with protease inhibitors. Media were concentrated using centrifugal filters (EMD Millipore) and normalized to cell counts for equal loading.
Proteins were separated on precast 4%–15% Ready Gels (Bio-Rad Laboratories), transferred to 0.2-μm nitrocellulose membranes, and blocked with 5% BSA plus 1× casein in PBS before probing with antibody overnight in 2.5% BSA and 0.5× casein. Antibodies used were as follows: IFITM5 (Abcam), COL1A1 LF-68 (the generous gift of Dr Larry W. Fisher, National Institutes of Health), and actin (Sigma-Aldrich). Blots were washed, incubated with secondary infrared-conjugated antibodies for 1 hour, washed, and visualized on a LI-COR Odyssey infrared imager (LI-COR).
Mineralization assay
Osteoblast cultures were grown to confluence in 12-well culture dishes and stimulated with mineralization medium containing 25 μg/mL L-ascorbic acid, 10−8 M dexamethasone and 2.5 mM 2-glycerophosphate. Mineralization of the nodules was determined by Alizarin Red-S staining. Cells were washed with PBS and fixed in 4% paraformaldehyde in PBS for 1 hour, washed with water, and incubated in 1% Alizarin Red-S in 2% ethanol, followed by a final water wash. Alizarin Red was solubilized with 0.5 mL of 0.5 N HCl/5% sodium dodecyl sulfate (SDS), and absorbance was measured with a spectrophotometer at 405 nm (27).
Steady-state collagen
Control and patient fibroblasts or osteoblasts were grown to confluence in six-well culture dishes. Steady-state type I collagen analysis was conducted as previously described (28). Cells were incubated with 437.5 μCi/mL L-[2,3,4,5-3H] proline for 16–18 hours, prior to collection and ammonium sulfate precipitation, and were then pepsin digested and analyzed by 6% SDS-urea-PAGE.
Matrix deposition
Postconfluent osteoblasts were stimulated with 100 μg/mL ascorbic acid three times a week for 14 days and then labeled for 24 hours with 400 μCi/mL L-[2,3,4,5-3H] proline. The media and extracellular matrix were harvested as described previously (29). The 3H-proline-labeled collagens were sequentially extracted from the matrix fraction with neutral salt, acetic acid, and pepsin and then electrophoresed on 6% SDS-urea-PAGE. Collagen content of each fraction was measured by densitometry and normalized to total sample volume.
Immunofluorescence microscopy
Fibroblasts and osteoblasts were grown to confluence on chamber slides and then treated with 200 μM ascorbic acid every day for 5 days and fixed in 4% paraformaldehyde. Staining of extracellular matrix was performed essentially as described (30). The matrix was blocked in 1% BSA in PBS plus 0.02% Tween 20, washed with PBS, and incubated with primary antibody (LF-68, α1(I) C-telopeptide) in 1% BSA/PBS. After washing, the matrix was incubated with secondary antibody, and then washed slides were mounted with 4′,6′-diamino-2-phenylindole (Vector Laboratories) and imaged using a Zeiss LSM 510 Inverted Meta microscope and LSM510 software.
Statistical analysis
The data were analyzed by Excel using a repeated-measures t test. Values are presented as mean ± SD unless noted. Significance was achieved at P ≤ .05.
Results
Identification of type V OI patients and phenotypic variability
We identified eight affected individuals from five families who are heterozygous for the IFITM5 c.-14C>T mutation found in all individuals with type V OI. Seven patients were classified clinically as type V OI prior to sequencing, while patient 8, an adult with progressive deforming OI not previously classified as type V OI, was found to have the same mutation. For each patient, the mutation was confirmed by BsmAI restriction digest; the mutation eliminates a BsmAI restriction site (Supplemental Figure 1C). Sequencing of patients and family members, as well as patients' case reports are detailed in the Supplemental Material (Supplemental Figure 1, A and B, Table 1, and Supplemental Table 1).
Table 1.
Patients Characteristics
| Patient | Age, y | Sex | Hypertrophic Callus | Radial Head Dislocation | Forearm Interosseous Membrane Calcification | Sclera | Scoliosis | Age at First Fracture | Height (50th Centile for Age) | Age of Diagnosis of OI Type V |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Deceased at 44 | M | + | Unknown | Unknown | Normal | Yes | Unknown | 7 y | Postmortem |
| 2 | 27 | F | + | + | + | Blue | Mild | In utero, 7 mo | 6.5 y | 11 y |
| 3 | 26 | M | + | + | + | Normal | Yes | Birth | 12.5 y | 10 y |
| 4 | 50 | F | + | Unknown | Unknown | Blue | Yes | Unknown | 3.5 y | 33 y |
| 5 | 22 | M | + | + | + | Blue | Mild | In utero, birth | 2.5 y | 6 y |
| 6 | 15 | M | + | Unknown | Unknown | Blue | No | 1 mo | 4.5 mo at age 30 mo | <3 mo |
| 7 | 5 | M | + | + | + | Blue | Yes | In utero | At CA 3.8 y HA 19 mo | 5 y |
| 8 | Deceased at 73 | F | − | + | Unknown | Normal | Yes | 18 mo | 3 y | Postmortem |
Abbreviations: F, female; M, male; CA, chronological age; HA, height age.
Type V OI is understood to have variability of clinical manifestations and the timing of their appearance (17, 18). Patients 2, 3, and 5 (Supplemental Figures 2–4) have characteristic bone histology and radiographic findings. Patients 1, 4, 6, and 7 (Supplemental Figure 5) have typical radiographic findings, but bone histology was not examined. The patients in all five families developed at least one of the classic characteristics of type V OI. Patients 1, 2, 3, 5, 6, and 7 developed hypertrophic callus. Radial head dislocation was noted in patients 2, 3, 5, 7, and 8, and ossification of the forearm interosseous membrane was documented in patients 2, 5, and 7. Although the initial clinical description of type V OI reported white sclerae (17, 18), patients 2, 5, 6, and 7 have blue sclerae (Table 1 and Supplemental Table 1).
IFITM5 expression in osteoblasts and fibroblasts
IFITM5 transcripts were detected by RT-PCR in both fibroblasts and osteoblasts from normal controls, with the expected higher expression in osteoblasts than fibroblasts (Supplemental Figure 1D). IFITM5 transcripts were also detected in cultured cells from patients 3 and 7 (Supplemental Figure 1D). Sanger sequencing was used to confirm the expression of the mutant IFITM5 transcripts containing the 5′-terminal addition. These transcripts could be detected in total RNA from the untreated cells, indicating they have normal stability (Supplemental Figure 1E).
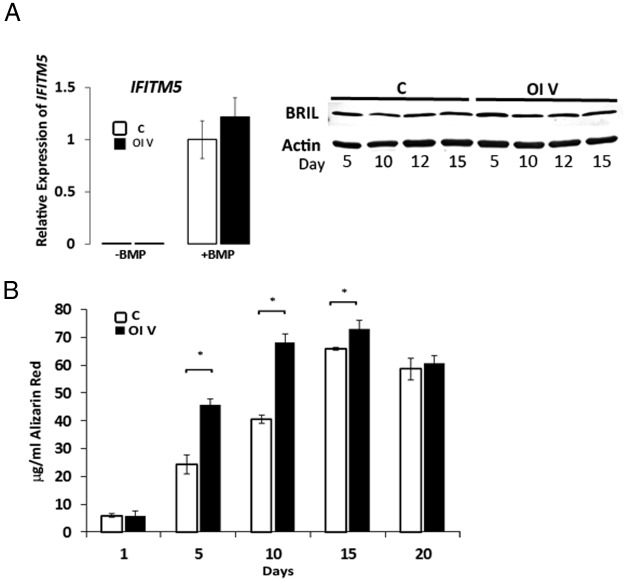
In osteoblasts from type V OI patients and controls, IFITM5 expression was barely detectable by real-time RT-PCR throughout differentiation with osteogenic media, but the addition of BMP2 significantly and comparably increased IFITM5 expression in both control and type V osteoblasts (Figure 1A, left panel). In differentiated osteoblasts, the BRIL protein level was generally comparable in type V OI and control cells (Figure 1A, right panel).
Figure 1.
IFITM5 expression and osteoblast mineralization. A, left panel, Relative IFITM5 expression in control (C) and patient 3 (OI V) osteoblasts (OBs) stimulated with osteogenic media for 10 days with or without the addition of BMP2. Patient 3 shows normal IFITM5 expression levels by quantitative PCR (n = 3). Right panel, Representative BRIL Western blot from OBs stimulated with osteogenic media showing protein stability in patient (n = 3). B, In vitro mineralization assays of control (C) and patient 3 (OI V) OB assessed by alizarin red staining shows increased mineralization of OI V OB at earlier time points than control (n = 3). *, P < .05.
IFITM5 mutation affects osteoblast markers and mineralization
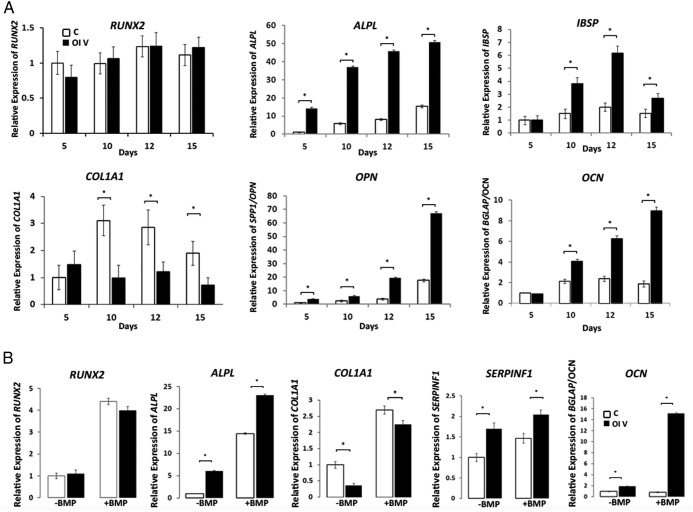
Multiple markers of osteoblast differentiation have significantly increased expression in type V OI osteoblasts from patient 3 during a differentiation time course (Figure 2A). Although the preosteoblast marker RUNX2 had equivalent expression in mutant and control osteoblasts, early osteoblast marker alkaline phosphatase (ALPL) expression was 3- to 6-fold greater than control throughout differentiation, and the mid-differentiation marker bone sialoprotein (IBSP) (31) peaked on day 12 at approximately 3-fold greater than control. Osteocalcin (BGLAP/OCN) and osteopontin (SPP1/OPN) are markers of late osteoblast maturation (31). Their peak expression in type V osteoblasts is approximately 4-fold greater than control and came late in the time course (day 15), with BGLAP/OCN expression rising over time, whereas SPP1/OPN had a late sharp peak. The addition of BMP2 to maintain IFITM5 expression during osteoblast differentiation increased the transcript levels of differentiation markers but maintained their relative level in type V and control osteoblasts (Figure 2B). Type V OI osteoblast ALPL, BGLAP, and SERPINF1 levels remained greater than control on day 10 in BMP2-treated cells.
Figure 2.
Expression of osteoblast (OB) differentiation markers. A, Relative expression of Runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALPL), bone sialoprotein (IBSP), type I collagen α1 (COL1A1), osteopontin (SPP1/OPN), and osteocalcin (BGLAP/OCN) in control (C) and patient 3 (OI V) OBs stimulated with osteogenic media for up to 15 days. Patient 3 shows increased expression of ALPL, IBSP, OPN, and OCN genes throughout the time course (n = 3). *, P < .05. B, Relative expression of RUNX2, ALPL, COL1A1, SERPINF1, and OCN in control (C) and patient 3 (OI V) OBs on day 10 of stimulation with osteogenic media with or without the addition of BMP2. Patient 3 shows significantly increased expression of ALPL, SERPINF1, and OCN ± BMP2; however, COL1A1 expression ± BMP2 is significantly decreased.
This acceleration of osteoblast markers was coordinated with increased osteoblast mineralization. Mineralization of differentiating osteoblasts was measured by alizarin red staining. Type V OI osteoblasts displayed a more brisk mineralization response than did control, increasing earlier in differentiation and remaining elevated through day 15 (Figure 1B).
IFITM5 mutation affects type I collagen expression, secretion, and matrix incorporation
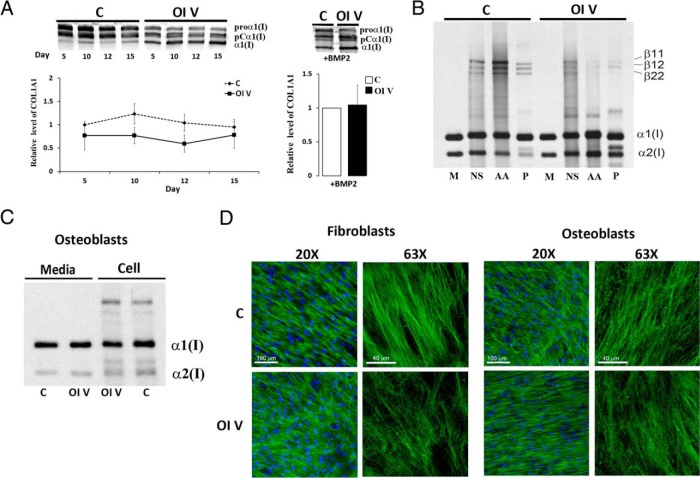
Expression of COL1A1 in type V OI osteoblasts from two typical patients (patients 3 and 5), with hyperplastic callus, calcification of the forearm interosseous membrane, and radial-head dislocation, peaked at day 5 and was significantly (2- to 3-fold) lower than control during mid- to late differentiation (Figure 2A and Supplemental Figure 6A). The relative decrease in COL1A1 expression on day 10 was partially rescued by addition of BMP2 but was still significantly lower than control (Figure 2B). Electrophoretic migration of steady-state collagen synthesized by osteoblasts of a type V OI patient (patient 3) with a typical phenotype (Figure 3C) is normal. Fibroblast collagen from several, but not all, type V patients displayed reproducible migration anomalies, which did not correlate with phenotype (Supplemental Figure 6B).
Figure 3.
Type I collagen secretion and deposition. A, Left panel, Representative Western blot of type I procollagen secretion from control (C) or patient 3 (OI V) osteoblasts (OBs) stimulated with osteogenic media for up to 15 days, or, right panel, for 10 days with BMP2 treatment. Patient 3 has decreased collagen secretion and was equal with BMP2 treatment. B, Extracellular collagen matrix deposited by control (C) or patient 3 (OI V) OBs. Normalized densitometry of control and OI V α1(I) chains after balanced loading revealed a 3.5-fold decrease in mature cross-linked collagen as well as a decrease in cross-linked β-forms in acetic acid (AA) and pepsin (P) fractions. C, Steady-state type I collagen protein in osteoblasts from control (C) and patient 3 (OI V). Migration of the α1(I) and α2(I) chains is normal in the patient. D, Immunofluorescence microscopy of collagen matrix deposited in culture by control (C) and patient 3 (OI V) fibroblasts and osteoblasts. Type V OI matrix has patchy, parallel fibers in comparison with the networked, well-formed fibers in control. M, media; NS, neutral salt.
Patient collagen protein secreted during osteoblast differentiation reflected transcript levels and was reduced compared with control (Figure 3A). Secreted collagen protein was quantified as total secreted α1(I) chain by combining measurements of all detected α1(I) forms at each time point. Interestingly, collagen from type V osteoblasts appears to be processed more rapidly in the pericellular space than does collagen from control osteoblasts, with relatively more proband mature α1(I), or partially processed pCα1(I), than proα1(I). We do not currently understand the mechanism of this difference.
Type V OI cultured osteoblasts deposit substantially less (∼4-fold) collagen into matrix fractions containing cross-links, with notable reduction in cross-linked β-forms (Figure 3B). Matrix deposited by type V OI fibroblasts (Figure 3D, left panel) and osteoblasts (Figure 3D, right panel) in long-term culture was stained for collagen. Type V OI matrix had fewer well-delineated fibrils and more amorphous ground material. Collagen fibril packing in the patient matrix was patchy compared with control, with parallel fibril bundles rather than the network appearance of control.
Discussion
Type V OI is an autosomal dominant form of OI (1, 17). It is the only rare type of OI that shares the inheritance pattern of classical OI types I-IV, which are caused by mutations in the genes that code for type I collagen, COL1A1 and COL1A2 (1, 2, 4). Type V OI was first described with clinical criteria more than a decade ago, based on distinctive radiographic and bone histology features (17). In 2012, two teams of investigators reported that a unique heterozygous mutation in IFITM5 (21, 22), which encodes the protein BRIL, was responsible for all cases of type V OI (24, 32, 33). BRIL is a transmembrane protein expressed predominantly in osteoblasts (19, 20), where it has a type II orientation in the plasma membrane with the N-terminal end in the cytoplasm (23). BRIL is also tethered to the membrane by S-palmitoylation modifications at Cys52 and Cys53, which insert into the cytoplasmic side of the membrane (23, 34). In the osteoblast membrane, the association of BRIL with FK506-binding protein 11 is promoted by S-palmitoylation of the cysteine residues and leads to a larger complex containing CD81 and CD9 (34).
Two distinct heterozygous IFITM5 mutations have been reported in OI patients: 1) the c.-14C>T mutation at the 5′-end of IFITM5, which generates a new start codon, adding five residues to the N-terminal end of BRIL but leaving the membrane insertion intact (21, 22); and 2) a missense mutation c.119C>T, resulting in a p.S40L substitution (25, 35, 36), which interferes with palmitoylation and membrane insertion (23). All cases of type V OI are caused by the 5′-end mutation, while the p.S40L substitution causes an atypical progressive deforming OI with the typical bone histology of type VI OI (25).
The IFITM5 mutation causing type V OI was quickly speculated to have a gain-of-function mechanism because of its N-terminal location and the hyperactive mineralization traits of the type V OI phenotype (22, 23). Additional indirect support for this interpretation was provided by functional studies of normal BRIL, in which adenovirus-mediated BRIL overexpression in UMR106 cells stimulated mineralization and lentivirus-mediated Ifitm5 short hairpin RNA knockdown in MC3T3 cells inhibited mineralization (19). Furthermore, comparison of the two IFITM5 mutations in primary patient osteoblasts in studies focusing not on mineralization but on PEDF also implicated a gain-of-function mechanism for the IFITM5 mutation causing type V OI (25). We demonstrated that the type V OI mutation had complementarity with the BRIL p.S40L substitution function, in that the BRIL p.S40L substitution decreased expression of PEDF by an unknown mechanism, while primary osteoblasts from type V OI patients have increased PEDF expression and secretion (25), again supporting a putative gain-of-function mechanism in type V OI. The increased mineralization of type V OI has not been demonstrated directly at the cellular level of primary patient osteoblasts, nor has a relationship of the type V OI phenotype to type I collagen been demonstrated.
We report here eight additional patients with type V OI and the first studies of type V OI primary osteoblasts focused on mineralization and differentiation. We confirmed the expression of the mutant allele in patient osteoblasts and fibroblasts and the stability of the mutant transcript. Patients in all five families developed some of the clinical features of type V OI, but there was considerable variation in the features manifested and in their timing of appearance. Even the elderly adult with severe OI, who died prior to delineation of her OI type and mutation, had a history of radial head dislocation. This clinical variability among individuals heterozygous for the IFITM5 c.-14C>T mutation has also been noted by others (24, 32).
IFITM5 transcripts were below the level of detectability by real-time RT-PCR in primary human osteoblasts, whereas BRIL protein was detectable by Western blot at a comparable level in type V and in control. Treatment with BMP2 to stimulate the osteogenic differentiation increased IFITM5 transcripts in type V OI and control osteoblasts to a comparable level and did not alter the relative levels of transcripts for differentiation markers, providing reassurance that BMP2 treatment had not fundamentally altered type V OI osteoblast differentiation. In primary type V OI osteoblasts, multiple markers of bone differentiation were increased, including early (alkaline phosphatase), middle (bone sialoprotein), and late (osteocalcin and osteopontin) markers, consistent with a generalized acceleration in type V bone cell differentiation.
Furthermore, the type V primary osteoblast studies support a mechanism for type V OI, which is specifically collagen related. The type V OI IFITM5 mutation is shown here to cause decreased type I collagen expression and secretion. Given the general increase in other osteoblast maturation markers, the decrease in type I collagen expression stands out as reflecting a specific pathway that is altered in the opposite direction to the generalized increase in cell maturation. Consequent to the decreased collagen secretion, type V OI osteoblasts have decreased collagen deposition into matrix and an altered deposition pattern when fibrils are compared with control by fluorescent microscopy. These data support the collagen-related character of BRIL defects. The full pathway for BRIL functioning in bone cells is an active area of investigation.
Finally, we demonstrated increased mineral deposition by primary type V osteoblasts in culture. These data indicate that increased osteoblast mineralization activity is the underlying cause of the hyperactive mineralization seen in patient traits such as hyperplastic callus and dense metaphyseal bands. The studies with type V OI primary osteoblasts presented here provide direct confirmation for a cell-based gain-of-function mechanism and support the collagen-related character of BRIL defects.
In summary, these data demonstrate a gain-of-function mechanism for the BRIL mutation causing type V OI at the cellular level. In combination with the dominant inheritance of type V OI, these data indicate that a BRIL protein replacement strategy for treating type V OI is unlikely to be successful. Instead, targeted therapy will need to focus on the intracellular signaling pathways and partners of BRIL to interfere with the mechanisms activating cell differentiation and mineralization. Delineation of these pathways and their connection to PEDF (25) is under investigation.
Acknowledgments
We thank the patients and their families for their dedicated long-term support of OI research. We also thank the Eunice Kennedy Shriver National Institute of Child Health and Human Development Microscopy and Imaging Core in which the confocal microscopy was conducted.
Authors' roles included the following: study design: A.R., A.S.B., A.M.B., and J.C.M.; data collection: A.R., A.S.B., A.M.B., W.A.C., A.H., and D.C.; data analysis and interpretation: A.R., A.S.B., A.M.B., W.A.C., A.H., D.C., J.S., S.C.H., and J.C.M.; drafting the manuscript: J.C.M., A.R., and A.S.B.; revising the manuscript content: A.R., A.S.B., A.M.B., W.A.C., A.H., D.C., and J.C.M.; approving the final version of manuscript: A.R., A.S.B., A.M.B., W.A.C., A.H., D.C., J.S., S.C.H., and J.C.M. J.C.M. takes responsibility for the integrity of the data analysis.
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding (to J.C.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALPL
- alkaline phosphatase
- BMP
- bone morphogenetic protein
- BRIL
- bone-restricted (IFITM)-like protein
- gDNA
- genomic DNA
- IFITM5
- interferon-induced transmembrane protein 5
- OI
- osteogenesis imperfecta
- PEDF
- pigment epithelium-derived factor
- SDS
- sodium dodecyl sulfate.
References
- 1. Marini JC, Blissett AR. New genes in bone development: what's new in osteogenesis imperfecta. J Clin Endocrinol Metab. 2013;98(8):3095–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7(9):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marini JC, Forlino A, Cabral WA, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28(3):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marini JC, Reich A, Smith SM. Osteogenesis imperfecta due to mutations in non-collagenous genes: lessons in the biology of bone formation. Curr Opin Pediatr. 2014;26(4):500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes AM, Carter EM, Cabral WA, et al. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N Engl J Med. 2010;362(6):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnes AM, Chang W, Morello R, et al. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med. 2006;355(26):2757–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cabral WA, Chang W, Barnes AM, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007;39(3):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes AM, Cabral WA, Weis M, et al. Absence of FKBP10 in recessive type XI osteogenesis imperfecta leads to diminished collagen cross-linking and reduced collagen deposition in extracellular matrix. Hum Mutat. 2012;33(11):1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christiansen HE, Schwarze U, Pyott SM, et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86(3):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alanay Y, Avaygan H, Camacho N, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86(4):551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drogemuller C, Becker D, Brunner A, et al. A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta. PLoS Genet. 2009;5(7):e1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Becker J, Semler O, Gilissen C, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2011;88(3):362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Homan EP, Rauch F, Grafe I, et al. Mutations in SERPINF1 cause osteogenesis imperfecta type VI. J Bone Miner Res. 2011;26(12):2798–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez-Glez V, Valencia M, Caparros-Martin JA, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat. 2012;33(2):343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keupp K, Beleggia F, Kayserili H, et al. Mutations in WNT1 cause different forms of bone fragility. Am J Hum Genet. 2013;92(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laine CM, Joeng KS, Campeau PM, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013;368(19):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glorieux FH, Rauch F, Plotkin H, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res. 2000;15(9):1650–1658. [DOI] [PubMed] [Google Scholar]
- 18. Cheung MS, Glorieux FH, Rauch F. Natural history of hyperplastic callus formation in osteogenesis imperfecta type V. J Bone Miner Res. 2007;22(8):1181–1186. [DOI] [PubMed] [Google Scholar]
- 19. Moffatt P, Gaumond MH, Salois P, et al. Bril: a novel bone-specific modulator of mineralization. J Bone Miner Res. 2008;23(9):1497–1508. [DOI] [PubMed] [Google Scholar]
- 20. Hanagata N, Li X, Morita H, Takemura T, Li J, Minowa T. Characterization of the osteoblast-specific transmembrane protein IFITM5 and analysis of IFITM5-deficient mice. J Bone Miner Metab. 2011;29(3):279–290. [DOI] [PubMed] [Google Scholar]
- 21. Cho TJ, Lee KE, Lee SK, et al. A single recurrent mutation in the 5′-UTR of IFITM5 causes osteogenesis imperfecta type V. Am J Hum Genet. 2012;91(2):343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Semler O, Garbes L, Keupp K, et al. A mutation in the 5′-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type V with hyperplastic callus. Am J Hum Genet. 2012;91(2):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patoine A, Gaumond MH, Jaiswal PK, Fassier F, Rauch F, Moffatt P. Topological mapping of BRIL reveals a type II orientation and effects of osteogenesis imperfecta mutations on its cellular destination. J Bone Miner Res. 2014;29(9):2004–2016. [DOI] [PubMed] [Google Scholar]
- 24. Rauch F, Moffatt P, Cheung M, et al. Osteogenesis imperfecta type V: marked phenotypic variability despite the presence of the IFITM5 c.-14C>T mutation in all patients. J Med Genet. 2013;50(1):21–24. [DOI] [PubMed] [Google Scholar]
- 25. Farber CR, Reich A, Barnes AM, et al. A novel IFITM5 mutation in severe atypical osteogenesis imperfecta type VI impairs osteoblast production of pigment epithelium-derived factor. J Bone Miner Res. 2014;29(6):1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robey PG, Termine JD. Human bone cells in vitro. Calcif Tissue Int. 1985;37(5):453–460. [PubMed] [Google Scholar]
- 27. Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329(1):77–84. [DOI] [PubMed] [Google Scholar]
- 28. Cabral WA, Makareeva E, Colige A, et al. Mutations near amino end of α1(I) collagen cause combined osteogenesis imperfecta/Ehlers-Danlos syndrome by interference with N-propeptide processing. J Biol Chem. 2005;280(19):19259–19269. [DOI] [PubMed] [Google Scholar]
- 29. Bateman JF, Golub SB. Deposition and selective degradation of structurally-abnormal type I collagen in a collagen matrix produced by osteogenesis imperfecta fibroblasts in vitro. Matrix Biol. 1994;14(3):251–262. [DOI] [PubMed] [Google Scholar]
- 30. Venturi G, Gandini A, Dalle Carbonare L, et al. Lack of expression of SERPINF1, the gene coding for pigment epithelium-derived factor, causes progressively deforming osteogenesis imperfecta with normal type I collagen. J Bone Miner Res. 2012;27(3):723–728. [DOI] [PubMed] [Google Scholar]
- 31. Miron RJ, Zhang YF. Osteoinduction: a review of old concepts with new standards. J Dent Res. 2012;91(8):736–744. [DOI] [PubMed] [Google Scholar]
- 32. Balasubramanian M, Parker MJ, Dalton A, et al. Genotype-phenotype study in type V osteogenesis imperfecta. Clin Dysmorphol. 2013;22(3):93–101. [DOI] [PubMed] [Google Scholar]
- 33. Shapiro JR, Lietman C, Grover M, et al. Phenotypic variability of osteogenesis imperfecta type V caused by an IFITM5 mutation. J Bone Miner Res. 2013;28(7):1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsukamoto T, Li X, Morita H, et al. Role of S-palmitoylation on IFITM5 for the interaction with FKBP11 in osteoblast cells. PLoS One. 2013;8(9):e75831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guillen-Navarro E, Ballesta-Martinez MJ, Valencia M, et al. Two mutations in IFITM5 causing distinct forms of osteogenesis imperfecta. Am J Med Genet A. 2014;164(5):1136–1142. [DOI] [PubMed] [Google Scholar]
- 36. Hoyer-Kuhn H, Semler O, Garbes L, et al. A non-classical IFITM5 mutation located in the coding region causes severe osteogenesis imperfecta with prenatal onset. J Bone Miner Res. 2014;29(6):1387–1391. [DOI] [PubMed] [Google Scholar]