Abstract
Context:
GH and IGF-1 are believed to be physiological regulators of skeletal muscle mitochondria.
Objective:
The objective of this study was to examine the relationship between GH/IGF-1 and skeletal muscle mitochondria in obese subjects with reduced GH secretion in more detail.
Design:
Fifteen abdominally obese men with reduced GH secretion were treated for 12 weeks with recombinant human GH. Subjects underwent 31P-magnetic resonance spectroscopy to assess phosphocreatine (PCr) recovery as an in vivo measure of skeletal muscle mitochondrial function and percutaneous muscle biopsies to assess mRNA expression of IGF-1 and mitochondrial-related genes at baseline and 12 weeks.
Results:
At baseline, skeletal muscle IGF-1 mRNA expression was significantly associated with PCr recovery (r = 0.79; P = .01) and nuclear respiratory factor-1 (r = 0.87; P = .001), mitochondrial transcription factor A (r = 0.86; P = .001), peroxisome proliferator-activated receptor (PPAR)γ (r = 0.72; P = .02), and PPARα (r = 0.75; P = .01) mRNA expression, and trended to an association with PPARγ coactivator 1-α (r = 0.59; P = .07) mRNA expression. However, serum IGF-1 concentration was not associated with PCr recovery or any mitochondrial gene expression (all P > .10). Administration of recombinant human GH increased both serum IGF-1 (change, 218 ± 29 μg/L; P < .0001) and IGF-1 mRNA in muscle (fold change, 2.1 ± 0.3; P = .002). Increases in serum IGF-1 were associated with improvements in total body fat (r = −0.53; P = .04), trunk fat (r = −0.55; P = .03), and lean mass (r = 0.58; P = .02), but not with PCr recovery (P > .10). Conversely, increase in muscle IGF-1 mRNA was associated with improvements in PCr recovery (r = 0.74; P = .02), but not with body composition parameters (P > .10).
Conclusion:
These data demonstrate a novel association of skeletal muscle mitochondria with muscle IGF-1 mRNA expression, but independent of serum IGF-1 concentrations.
Mitochondrial dysfunction is associated with insulin resistance. For example, in subjects with obesity and non-insulin-dependent diabetes mellitus, the mitochondria are smaller and morphologically different in skeletal muscle, and mitochondrial function as determined by NADH:O2 oxidoreductase activity is reduced (1). Furthermore, treatment with the mitochondrial toxin, stavudine, reduces both mitochondrial function and insulin sensitivity in a prospective, randomized, placebo-controlled trial (2). Interestingly, adult subjects with diabetes mellitus also have decreased expression of nuclear encoded mitochondrial genes, including peroxisome proliferator-activated receptorγ coactivator 1-α (PGC-1α) (3, 4). PGC-1α is a transcriptional regulator of mitochondrial biogenesis and function, which regulates peroxisome proliferator-activated receptor (PPAR)γ and PPARα signaling, as well as nuclear respiratory factor-1 (NRF-1) and mitochondrial transcription factor A (TFAM) (5, 6).
Recent evidence suggests that GH and IGF-1 are physiological regulators of mitochondria. For instance, acromegaly, a condition of pathological GH excess, is associated with alterations in skeletal muscle mitochondrial morphology, which resolve after surgical treatment (7). Conversely, we have previously shown that reduced GH and IGF-1 secretion in obesity is associated with reduced mitochondrial function, as represented by delayed phosphocreatine (PCr) recovery after submaximal exercise on 31P-magnetic resonance spectroscopy (31P-MRS) (8). 31P-MRS is a noninvasive technique to assess mitochondrial function in vivo that has been validated in both animal models and humans (9–11). 31P-MRS allows reproducible measurements of high-energy phosphate molecules such as PCr in vivo. PCr is normally depleted during submaximal exercise, and its recovery is determined oxidatively (12). Therefore, recovery of PCr after challenging with exercise reflects oxidative function of the mitochondria (13). In addition, acute treatment with recombinant human GH (rhGH) via 14 hours of continuous infusion in healthy subjects increases mitochondrial oxidative capacity and the mitochondrial transcription factor TFAM (14), and 6-month treatment of obese men with rhGH improves PCr recovery by 31P-MRS (15). Furthermore, we recently demonstrated that improvements in IGF-1 after a 12-month treatment with a GHRH analog are associated with improvements in PCr recovery (16).
These previous studies suggest a potentially physiologically significant relationship between GH/IGF-1 and skeletal muscle mitochondrial function in humans. We hypothesized that rhGH treatment of obese patients exhibiting reduced GH secretion would improve skeletal muscle mitochondrial function through a transcriptional mechanism via local GH/IGF signaling. To test this hypothesis, we recently completed a 12-week interventional physiology study with in vivo assessment of skeletal muscle mitochondrial function, utilizing 31P-MRS and in vitro assessment of mitochondrial gene expression in muscle tissue obtained with percutaneous biopsy techniques. We measured mRNA expression levels of IGF-1 and of several mitochondrial genes, including PGC-1α, PPARγ, PPARα, NRF-1, and TFAM. This is the first study to assess in vivo skeletal muscle mitochondrial function utilizing 31P-MRS and mitochondrial gene expression in response to rhGH in humans.
Subjects and Methods
Study subjects
Fifteen abdominally obese male subjects with reduced GH secretion were recruited at the Massachusetts General Hospital for a 12-week, open-label, interventional, physiological study. Eighteen- to 60-year-old men with body mass index (BMI) ≥ 30 kg/m2, waist circumference ≥ 102 cm, and peak stimulated GH ≤ 4.2 μg/L after a GHRH-arginine stimulation test were included. All participants were otherwise healthy without known endocrine dysfunction. Participants who had a known diagnosis of diabetes mellitus, fasting blood glucose > 125 mg/dL, or use of antidiabetes medications were excluded. In addition, subjects with hemoglobin < 9 g/dL and creatinine > 1.5 mg/dL, as well as subjects using hormonal medication including T, glucocorticoids, anabolic steroids, GHRH, GH, or IGF-1 within 3 months of enrollment, were excluded. The study was approved by the Partners Institutional Review Board. Written informed consent was obtained from all participants.
Study design
Subjects were initially prescribed rhGH at a dose of 0.4 mg/d by daily sc injections. Doses of rhGH were titrated up to 0.6 mg/d, and then 0.8 mg/d every 4 weeks based on serum IGF-1 levels to reach the upper-normal ranges for each subject according to their age. Subjects were evaluated for PCr recovery using 31P-MRS and skeletal muscle gene expression by percutaneous muscle biopsy at baseline and again after 12 weeks of treatment.
31P-MRS protocol
In vivo skeletal muscle mitochondrial function was determined using 31P-MRS to assess PCr recovery after submaximal exercise as previously reported (17). Briefly, the magnetic resonance acquisition was performed after an overnight fast on a 3.0 T whole body magnetic resonance scanner (Trio; Siemens Medical Systems). Subjects were placed supine and feet first in the bore with their right lower leg inside a custom built device designed for dynamic plantar flexion exercise. A 13-cm-diameter custom-built single-tuned 31P surface coil was placed in contact with the posterior calf muscles. 31P spectra were acquired every 2 seconds over a 10-minute period, during which time subjects underwent 2 minutes of rest, 3 minutes of plantar flexion exercise at 40% of the maximal voluntary contraction (MVC) with a frequency of 0.5 Hz, followed by 5 minutes of recovery. MVC was determined before the protocol using a dynamometer (microFet2; Hoggan Health Industries). Maximum plantar flexion force was measured in triplicate at each visit, and the mean value was used for MVC calculation.
Concentrations of PCr, inorganic phosphate, and ATP resonances were fitted in the frequency domain using an in-house MATLAB-based software. Intracellular pH was estimated based on the chemical shift difference between PCr and inorganic phosphate resonances. Mitochondrial function was determined by plotting the PCr peak integrated area vs time during exercise recovery and fitting the recovery curve to a mono-exponential function to determine the recovery time constant (τPCr). The initial rate of PCr recovery (ViPCr) was determined from τPCr and PCr depletion using the following equation: ViPCr = (60/τPCr) × PCr depletion (18). ViPCr was chosen as the primary end-point because it normalizes PCr recovery based on participant effort and is insensitive to end-of-exercise metabolic conditions such as intracellular acidosis (18). Greater ViPCr represents relatively better mitochondrial function. Technically successful 31P- MRS scans for baseline and 12-week visits were available in 13 subjects.
Muscle biopsy
Percutaneous muscle needle biopsy was performed according to standard procedures adapted for the lateral gastrocnemius muscle after an overnight fast (19). The muscle biopsy was performed on a separate day from the MRS experiment. Subjects were instructed to lie in the prone position. The right calf was cleaned using alcohol and chlorhexidine in the lateral muscle belly area, and local anesthesia was provided using 1% lidocaine administered intradermally and sc. A 2-cm stab incision was made in the skin and soft tissue overlying the lateral gastrocnemius muscle, and a Bergstrom core biopsy needle (20) was inserted into the muscle in a 90° angle. Muscle biopsy was performed using manual suction to increase the yield of muscle tissue obtained (21). Approximately 50–100 mg of muscle tissue was obtained from each subject. Samples were immediately flash-frozen in liquid nitrogen and stored at −80°C until ready for use. Paired muscle tissue was obtained from 10 subjects at both baseline and after 12 weeks of treatment.
The MRS, biopsy, and remainder of baseline procedures were performed on separate days.
Gene expression studies
Total mRNA was extracted from the muscle biopsy samples using TRIzol (Invitrogen) following the manufacturer's instructions in one batch at the conclusion of the study. SuperScript III First-Strand Synthesis SuperMix (Invitrogen) was used for the generation of cDNA for all samples. Expression of IGF-1, PGC-1α, PPARγ, PPARα, NRF-1, and TFAM mRNA was determined by quantitative real time PCR using a Mastercycler realplex machine (Eppendorf) with iQ SYBR Green Supermix Kit (Bio-Rad) as per standard protocol. Primers for IGF-1 were chosen to assess the mRNA expression levels of all four isoforms. Expression of target-gene mRNA was normalized with glyceraldehyde-3-phosphate dehydrogenase mRNA expression. The primer sequences used for amplifying target-gene mRNA are available upon request.
Biochemical assessment
Clinically standard GHRH-arginine stimulation testing was performed after an overnight fast as previously described (22). Serum GH was measured using the Beckman Access Ultrasensitive human GH assay, a paramagnetic particle, and chemiluminescent immunoassay (Beckman Coulter). Serum IGF-1 was measured using the IMMULITE 2000 assay (Siemens Diagnostics). A 75-g oral glucose tolerance test was performed at baseline and at the 3-month visit. Plasma glucose was assessed by the commercial laboratory LabCorp, Inc, and serum insulin was assessed using a chemiluminescence immunoassay (Access Immunoassay System; Beckman Coulter). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin using the following equation: HOMA-IR = (fasting glucose (mmol/L) × fasting insulin (mIU/L))/22.5 (23). Levels of high-sensitivity C-reactive protein (hsCRP) were assessed at the Massachusetts General Hospital clinical laboratory.
Body composition
Height and body weight were measured after an overnight fast. Total lean body mass (LBM) and fat mass were determined by dual-energy x-ray absorptiometry (DXA) (Discovery A; Hologic, Inc). Measurements of regional lean mass, specifically the right leg lean mass, using DXA were standardized (1995 User's Guide; Hologic, Inc). The technique has a precision error (1 SD) of 1.5% for lean mass (24).
Indirect calorimetry
Resting energy expenditure (REE) was assessed by determining oxygen consumption (VO2) and carbon dioxide production (VCO2) for 20 minutes using a calibrated calorimeter (VMAX29N; Sensormedics) after a 20-minute rest. REE was normalized to LBM as determined by DXA.
Physical activity assessment
The level of physical activity was assessed by self-reported regular structured exercise during direct interview and expressed as total hours of activity per week (metabolic equivalents) and hours of TV watching per day.
Statistical analysis
Baseline variables are presented as mean ± SEM. Univariate regression analysis using Pearson's correlation coefficient was performed at baseline to determine the relationship between serum IGF-1 and skeletal muscle mRNA expression of IGF-1 to PCr recovery parameters before initiating treatment. In addition, further univariate regression analyses were performed relating serum IGF-1 and skeletal muscle IGF-1 mRNA expression to the expression levels of various metabolic genes within skeletal muscle at baseline. The effects of rhGH over 12 weeks were determined by paired Student's t test. Additional univariate regression analyses with Pearson's correlation coefficient were performed relating changes in circulating IGF-1 and skeletal muscle IGF-1 mRNA expression to changes in PCr recovery parameters as well as body composition and metabolic parameters. Statistical analysis was performed using JMP Statistical Database Software (version 10.0.0; SAS Institute, Inc). Statistical significance was determined as a value of P < .05.
Results
Baseline clinical characteristics of study subjects
The clinical characteristics of the subjects are presented in Table 1. Fifteen men were enrolled in the study. The subjects were on average 47.9 ± 2.2 years old (range, 31–59 y), with a mean BMI of 37.8 ± 5.9 kg/m2, waist circumference of 124 ± 3 cm, peak stimulated serum GH of 2.7 ± 0.4 μg/L, and serum IGF-1 of 142 ± 9 μg/L. All subjects had a normal ankle-brachial index (ABI) (all > 0.90).
Table 1.
Baseline Characteristics of Study Subjects
| No. of subjects | 15 |
| Age, y | 47.9 ± 2.2 |
| Race (Caucasian), n (%) | 10 (66.7) |
| Ethnicity (Hispanic), n (%) | 1 (6.7) |
| Tobacco use, pack years | 2.5 ± 1.6 |
| Pulse | 74 ± 3 |
| Systolic blood pressure, mm Hg | 131 ± 3 |
| Diastolic blood pressure, mm Hg | 82 ± 2 |
| ABI | 1.2 ± 0.05 |
| BMI, kg/m2 | 37.8 ± 5.9 |
| Waist circumference, cm | 124 ± 3 |
| Fasting GH, μg/L | 0.06 ± 0.01 |
| Peak stimulated GH on GHRH-arginine, μg/L | 2.7 ± 0.4 |
| IGF-1, μg/L | 142 ± 9 |
| Hours of TV watching/d | 3.03 ± 0.77 |
| Metabolic activity/d | 54.3 ± 18.0 |
| ViPCr, mm/min | 17.65 ± 1.52 |
| PCr depletion, % | 30.85 ± 2.08 |
Data are expressed as mean ± SEM, unless otherwise specified.
Baseline relationship between serum IGF-1 and skeletal muscle IGF-1 mRNA to PCr recovery
At baseline, before intervention with rhGH, serum IGF-1 was not associated with the PCr recovery parameter ViPCr (r = −0.16; P = .60). In contrast, skeletal muscle IGF-1 mRNA expression level was significantly associated with ViPCr (r = 0.79; P = .01) such that greater skeletal muscle IGF-1 mRNA expression was associated with better PCr recovery, a marker for better mitochondrial function (Table 2). Neither serum IGF-1 nor IGF-1 mRNA expression in muscle was associated with resting [PCr], pH, or [ADP] (all P > .10).
Table 2.
Baseline Relationship Between Serum IGF-1 and Skeletal Muscle IGF-1 mRNA Expression to MRS and Gene Expression Parameters
| Serum IGF-1 |
Skeletal Muscle IGF-1 mRNA |
|||
|---|---|---|---|---|
| r | P | r | P | |
| ViPCr | −0.16 | .60 | 0.79 | 0.01 |
| [PCr] at rest | −0.08 | .79 | 0.20 | 0.58 |
| pH at rest | −0.14 | .63 | −0.42 | .23 |
| [ADP] at rest | 0.004 | .99 | −0.28 | .44 |
| PGC-1α mRNA | 0.20 | .59 | 0.59 | .07 |
| PPARγ mRNA | 0.23 | .52 | 0.72 | .02 |
| PPARα mRNA | 0.35 | .32 | 0.75 | .01 |
| NRF-1 mRNA | 0.07 | .86 | 0.87 | .001 |
| TFAM mRNA | 0.30 | .39 | 0.86 | .001 |
Statistical analyses were performed with Pearson's univariate regression analyses.
In addition, skeletal muscle IGF-1 mRNA expression was significantly associated with mRNA expression levels of PPARγ (r = 0.72; P = .02), PPARα (r = 0.75; P = .01), NRF-1 (r = 0.87; P = .001), and TFAM (r = 0.86; P = .001) and trended to an association with PGC-1α mRNA (r = 0.59; P = .07) (Supplemental Figure 1), whereas serum IGF-1 was not associated with PPARγ, PPARα, NRF-1, TFAM, or PGC-1α expression levels in skeletal muscle (all P > .10) (Table 2). Neither serum IGF-1 nor IGF-1 mRNA expression in muscle was significantly associated with BMI, waist circumference, or homeostatic model assessment (HOMA) at baseline (all P > .10).
Effects of rhGH treatment
rhGH treatment for 12 weeks resulted in increased serum IGF-1 (baseline, 142 ± 9; 12-week, 359 ± 29; change, 218 ± 29 μg/L; P < .0001) and IGF-1 mRNA expression in skeletal muscle (baseline, 1 ± 0.2; 12-week, 1.9 ± 0.3; fold change, 2.1 ± 0.3; P = .002) (Figure 1, A and B).
Figure 1.
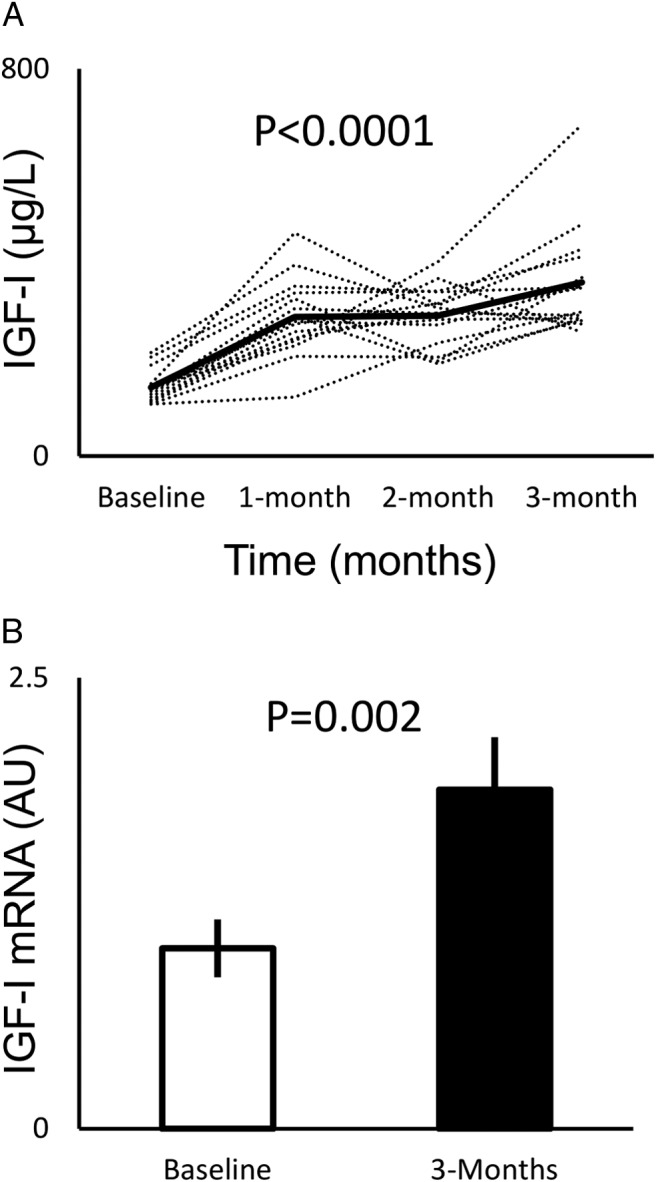
A, Effects of rhGH on serum IGF-1 levels over 12 weeks, with mean values indicated by the solid line. Statistical significance was tested by paired Student's t test comparing baseline to 12-week IGF-1 levels. B, Effects of rhGH on skeletal muscle IGF-1 mRNA expression measured by quantitative RT-PCR and normalized to baseline. Error bars represent SEM. Significance was tested by paired Student's t test.
rhGH treatment decreased waist circumference (change, −3 ± 1 cm; P = .03) but did not change body weight or BMI (both P > .05). rhGH decreased hsCRP (change, −1.78 ± 0.57 mg/L; P = .008). Fasting glucose (change, 6 ± 3 mg/dL; P = .04), fasting insulin (change, 6.9 ± 2.7 μIU/mL; P = .02), and HOMA (change, 1.9 ± 0.8; P = .03) increased during the study, but 2-hour glucose did not change (P > .10). The 12-week treatment with rhGH also increased REE (change, 166 ± 51 kcal/d; P = .006) as well as REE/LBM (change, 2.30 ± 0.77 kcal/d/kg; P = .01), VO2 (change, 0.03 ± 0.008 L/min; P = .005), and VCO2 (change, 0.02 ± 0.008 L/m; P = .05) (Table 3).
Table 3.
Effects of 12-Week Treatment With rhGH
| Baseline | 3 mo | Change | P | |
|---|---|---|---|---|
| Weight, kg | 116.3 ± 4.7 | 116.1 ± 4.9 | −0.2 ± 0.6 | .69 |
| BMI, kg/m2 | 37.8 ± 1.5 | 37.7 ± 1.6 | −0.1 ± 0.2 | .52 |
| Waist circumference, cm | 124 ± 3 | 121 ± 3 | −3 ± 1 | .03 |
| Total fat mass, kg | 44.4 ± 3.1 | 43.9 ± 3.1 | −0.5 ± 0.7 | .47 |
| Total LBM, kg | 70.3 ± 1.8 | 70.7 ± 2 | 0.4 ± 0.7 | .56 |
| Right lower extremity lean mass, kg | 12.0 ± 0.4 | 12.0 ± 0.4 | −0.05 ± 0.1 | .73 |
| Fasting glucose, mg/dL | 88 ± 2 | 94 ± 4 | 6 ± 3 | .04 |
| 2-h Glucose, mg/dL | 120 ± 9 | 134 ± 12 | 14 ± 10 | .16 |
| Fasting insulin, μIU/mL | 8.9 ± 1.3 | 15.8 ± 2.8 | 6.9 ± 2.7 | .02 |
| HOMA | 2 ± 0.3 | 3.9 ± 0.8 | 1.9 ± 0.8 | .03 |
| hsCRP, mg/L | 3.53 ± 0.44 | 1.74 ± 0.40 | −1.78 ± 0.57 | .008 |
| REE, kcal/d | 1757 ± 65 | 1922 ± 55 | 166 ± 51 | .006 |
| REE/LBM, kcal/d/kg | 24.98 ± 0.71 | 27.28 ± 0.68 | 2.30 ± 0.77 | .01 |
| Respiratory quotient | 0.88 ± 0.01 | 0.86 ± 0.02 | −0.02 ± 0.02 | .29 |
| VO2, L/min | 0.25 ± 0.009 | 0.27 ± 0.008 | 0.03 ± 0.008 | .005 |
| VCO2, L/min | 0.22 ± 0.009 | 0.24 ± 0.009 | 0.02 ± 0.008 | .05 |
Statistical analyses were performed with paired Student's t test.
rhGH treatment had no effect on the PCr recovery parameter ViPCr (P > .10). Effect of rhGH on skeletal muscle gene expression is reported in Supplemental Figure 2.
There was no change in ABI or physical activity during the study as determined by metabolic equivalents of self-reported activity or hours of TV watching (all P > .10).
Relationship between changes in serum IGF-1, skeletal muscle IGF-1 mRNA expression, and changes in metabolic parameters using univariate regression
Univariate regression analysis among all available subjects demonstrated that change in serum IGF-1 was not significantly associated with change in skeletal muscle IGF-1 mRNA expression (r = 0.23; P = .53).
Increase in serum IGF-1 was significantly associated with decreases in waist circumference (r = −0.82; P = .0002), total fat mass (r = −0.53; P = .04), and trunk fat mass (r = −0.55; P = .03) and increases in fat-free mass (r = 0.58; P = .02). In contrast, change in skeletal muscle IGF-1 expression was not related to changes in any body composition parameters (Table 4).
Table 4.
Relationship Between Changes in Serum IGF-1 and Changes in Skeletal Muscle IGF-1 mRNA Expression Level to Changes in Metabolic Parameters
| Change in Serum IGF-1 |
Change in Muscle IGF-1 mRNA |
|||
|---|---|---|---|---|
| r | P | r | P | |
| Body weight | 0.03 | .91 | 0.17 | .64 |
| BMI | −0.18 | .52 | 0.24 | .50 |
| Waist circumference | −0.82 | .0002 | −0.06 | .87 |
| Total LBM | 0.58 | .02 | 0.20 | .59 |
| Total fat mass | −0.53 | .04 | −0.07 | .85 |
| Trunk fat mass | −0.55 | .03 | 0.12 | .75 |
| REE | 0.15 | .60 | 0.54 | .11 |
| REE/LBM | 0.052 | .85 | 0.47 | .17 |
| VO2 | 0.15 | .61 | 0.60 | .07 |
| VCO2 | 0.096 | .73 | 0.26 | .48 |
Statistical analyses were performed with Pearson's univariate regression analyses.
Changes in serum IGF-1 were not related to ViPCr (P > .10). However, changes in skeletal muscle IGF-1 mRNA expression were significantly related to improvements in ViPCr (r = 0.74; P = .02), such that increased IGF-1 mRNA expression associated with better PCr recovery, a marker for better mitochondrial function (Figure 2, A and B).
Figure 2.
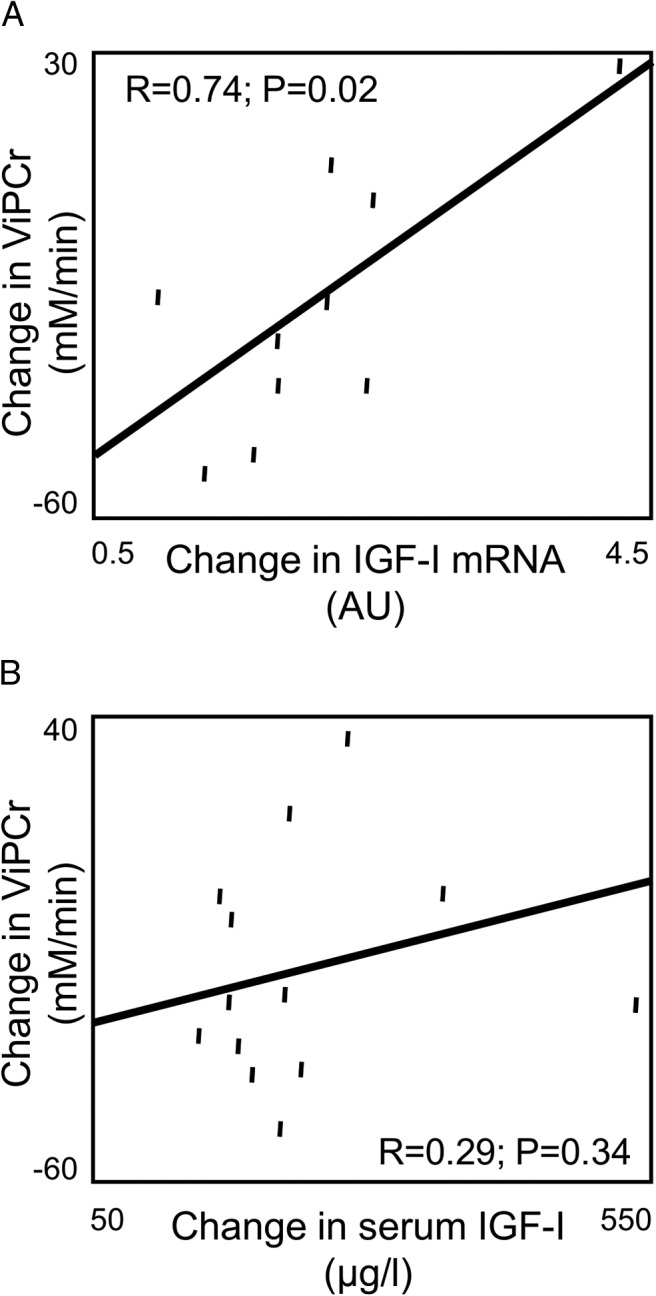
Univariate regression analyses relating change in skeletal muscle IGF-1 mRNA expression to change in PCr recovery parameter, ViPCr (A), and change in serum IGF-1 to change in PCr recovery parameter ViPCr (B).
After a 12-week treatment with rhGH, increases in skeletal muscle IGF-1 mRNA expression trended to an association with increases in VO2 (r = 0.60; P = .07), whereas serum IGF-1 was not significantly associated with changes in REE, REE/LBM, VO2, or VCO2 (all P > .50).
Discussion
This study demonstrates, for the first time, that skeletal muscle IGF-1 mRNA expression, but not serum IGF-1, is significantly related to skeletal muscle mitochondrial function. Skeletal muscle IGF-1 mRNA expression at baseline was related to the PCr recovery parameter ViPCr, as well as to PGC-1α, PPARγ, PPARα, NRF-1, and TFAM mRNA expression levels. In addition, increases in skeletal muscle IGF-1 mRNA expression with rhGH treatment were significantly related to improvements in PCr recovery, suggestive of improving mitochondrial function in the muscle. In contrast, serum IGF-1 was related to neither PCr recovery nor mitochondrial gene expression levels at baseline, and changes in serum IGF-1 were not related to improvements in PCr recovery.
These results suggest a possible role for local paracrine effects of skeletal muscle IGF-1 on skeletal muscle mitochondrial function. Although significant attention is focused on the endocrine effects of IGF-1 produced by the liver, GH also stimulates local or autocrine/paracrine synthesis of IGF-1 in almost all tissues, including skeletal muscle (25, 26). Local or paracrine IGF-1 signaling appears to play a significant role in growth and development because liver-specific IGF-1 knockout mice display normal body weight, length, and development despite having significantly reduced circulating IGF-1 levels (27). In a transgenic mouse model of IGF-1 overexpression in cardiac myocytes, animals were protected against reactive oxygen species, mitochondrial damage, and cardiac malfunction caused by high-fat diet (28). Because plasma IGF-1 also increased in these transgenic mice, we cannot rule out a possible contribution of circulating IGF-1 in these metabolic effects. Nonetheless, this result suggests a significant role for local IGF-1 in metabolic homeostasis in the heart. Consistently, our results suggest for the first time that local IGF-1 signaling improves mitochondrial function in skeletal muscle as well in human subjects.
An alternative interpretation of our data is a direct and independent effect of GH on skeletal muscle, liver, and adipose tissue—specifically, a direct effect of GH to increase skeletal muscle mitochondrial gene expression and function. This is consistent with the known anabolic effects of GH on muscle. The increase in skeletal muscle IGF-1 mRNA expression in this instance may represent GH action in this particular tissue, but it does not necessarily indicate an effect mediated through IGF-1 as opposed to direct GH actions. A direct effect of GH on muscle mitochondria is consistent with the significant relationship between IGF-1 mRNA and mitochondrial function and gene expression observed at baseline, before administration of the rhGH. Regardless of whether changes in skeletal muscle mitochondria are caused directly by GH or mediated by IGF-1, our results indicate that the effects of rhGH on skeletal muscle mitochondria may occur independently of its effects on other tissues (ie, independent from the effects of rhGH to increase IGF-1 synthesis and secretion from the liver, because changes in serum IGF-1 were not related to changes in skeletal muscle mitochondrial function).
rhGH appears to have a physiological benefit on skeletal muscle contraction, relaxation, and muscle mass in a mouse model of congestive heart failure created by monocrotaline treatment (29), potentially through a PGC-1α-mediated process (30). As discussed previously, Short et al (14) demonstrated that acute rhGH infusion stimulates the expression of nuclear-encoded mitochondrial gene TFAM in healthy subjects. In this study, we confirm this relationship between GH/IGF-1- and PGC-1α-mediated transcription factors within skeletal muscle, with significant baseline association between IGF-1 mRNA and TFAM, as well as NRF-1, PPARγ, and PPARα (and a trend to association with PGC-1α).
Our study shows that rhGH increases serum IGF-1 and skeletal muscle IGF-1 mRNA levels as expected, demonstrating subject compliance with the rhGH treatment during the study. However, changes in serum IGF-1 were not significantly associated with changes in skeletal muscle IGF-1 mRNA expression. Although this differential effect is difficult to explore further in this small study, future investigations distinguishing between endocrine effects of circulating IGF-1 and paracrine effects of locally expressed IGF-1 are warranted.
Our study also confirms the effects of rhGH on reducing waist circumference and improving REE, as previously demonstrated in obese subjects (31–34). However, we were unable to demonstrate an effect of rhGH on fat mass or LBM as previously reported in a large meta-analysis of 539 obese subjects (35). Because this was a small physiology study whose primary end-point was not a change in body composition, this may simply reflect a lack of power in our study to assess this particular end-point. Despite the lack of significant effects on fat mass and lean mass, interestingly, increases in serum IGF-1 in response to rhGH were significantly associated with improvements in body composition (decreasing body fat, trunk fat, and improving fat-free mass). Therefore, the greater the increase in serum IGF-1, the greater the improvements in body composition. This finding also suggests that the lack of statistical effect of rhGH on body composition parameters may be due to those subjects whose serum IGF-1 levels did not increase enough and therefore did not have the expected improvements in body composition. In contrast, a change in skeletal muscle IGF-1 mRNA was not associated with changes in body composition, further highlighting the differential effects between serum IGF-1 and IGF-1 mRNA expression in skeletal muscle. Conversely, change in serum IGF-1 was not associated with improvements in REE, whereas change in skeletal muscle IGF-1 mRNA expression trended to an association with improving resting VO2. This suggests that the effects of rhGH on VO2 and REE may be mediated by improved skeletal muscle mitochondrial function, effected through local IGF-1 effects. This is consistent with prior work from Zurlo et al (36), who demonstrated that skeletal muscle metabolism accounts for significant variance in basal and sleeping metabolic rate.
Short-term treatment with rhGH decreased insulin sensitivity, consistent with prior studies (14, 37, 38). In addition to its well-known anabolic effects, GH is also known to be a counter-regulatory hormone to insulin with the ability to raise plasma glucose. GH can lead to insulin resistance through its effects to promote lipolysis from adipocytes, promote gluconeogenesis in the liver, and inhibit peripheral glucose disposal in the muscle (39, 40). Previous human studies of rhGH in obese subjects have demonstrated a negative effect of rhGH on insulin sensitivity in the short term. However, in a longer term study lasting 9 months, Johannsson et al (31) demonstrated a beneficial effect of rhGH on insulin sensitivity as determined by the glucose disposal rate on a hyperinsulinemic-euglycemic clamp. This improvement in glucose disposal rate was attributed to the effect of rhGH to reduce visceral adipose tissue, which is thought to be detrimental to metabolic health. However, 80% of insulin-stimulated glucose uptake occurs in the muscle (41); therefore, the improvement in glucose disposal rate determined via the hyperinsulinemic-euglycemic clamp mostly indicates an improvement in skeletal muscle insulin sensitivity. The current study may not have been long enough to demonstrate a concomitant improvement in insulin sensitivity with rhGH treatment. Nonetheless, we may also need to consider the possible beneficial effects of rhGH on skeletal muscle mitochondrial function as a contributor to the improved glucose disposal rate after longer term treatment with rhGH in obese subjects. The long-term effect of rhGH on insulin sensitivity is most likely determined by the sum of its effects on various tissues, including muscle, adipose, and liver.
In this study, rhGH improved the inflammatory marker hsCRP. This is consistent with previous interventional studies utilizing rhGH (15, 42) and our own work utilizing a synthetic GHRH analog in obesity (43) that showed that treatment with rhGH or GHRH resulted in a significant reduction in hsCRP.
Although we did not see a significant effect of rhGH on PCr recovery parameters after 12 weeks of treatment, this was most likely due to a lack of sufficient power from the small sample size (n = 15). This was a pilot and feasibility study designed with detailed and invasive experimental techniques by intention. A larger study may be needed to demonstrate a statistically significant effect of rhGH on PCr recovery. Subjects may also have had variable compliance throughout the study based on the large variability in the subjects' individual IGF-1 response. In addition, the dose of rhGH was chosen to target IGF-1 levels in the upper normal range adjusted for age. However, this strategy resulted in exposure to different doses of rhGH at different times for each individual, which may be a source of variability. Furthermore, the optimal dosing of rhGH in this context is not yet known. Moreover, whereas previous studies by Bredella et al (15) and Makimura et al (16) were 6 and 12 months long, this was a 12-week study. The ideal duration for treatment is unknown, and additional studies are needed to identify the differences in short-term and long-term GH treatment on mitochondrial function in the skeletal muscle. The subjects' physical activity was assessed by self-reported questionnaires, which may be less accurate among obese subjects (44) who tend to over-report their activity (45, 46). Lastly, rhGH treatment results in a nonpulsatile, nonphysiological increase in GH, and the role of physiological pulsatile GH secretion in this regard has also not been investigated in great detail.
In summary, this is the first study to demonstrate a significant association between skeletal muscle IGF-1 mRNA expression and the PCr recovery parameter ViPCr, as well as between muscle IGF-1 mRNA expression and expression of mitochondrial genes PGC-1α, PPARγ, PPARα, NRF-1, and TFAM. This study suggests differential effects of endocrine IGF-1 on body composition and local paracrine effects of skeletal muscle IGF-1 on muscle mitochondrial function and potentially on REE. These observations have important implications with regard to the physiological relationship between GH/IGF-1 and mitochondrial function in obese men, and future studies will be necessary to examine the mechanistic basis of this relationship in more detail. Further studies will also be necessary to investigate ways to optimize GH/IGF-1 and whether such strategies may be able to improve skeletal muscle mitochondrial function while minimizing its adverse effects.
Acknowledgments
We thank the nursing and bionutrition staff of the Massachusetts General Hospital Clinical Research Center for their dedicated patient care and all of the research subjects who participated in the study.
This study, including the provision of the study drug, was supported by an investigator-initiated research grant from Pfizer, Inc (to H.M.). H.M. also receives support from National Institutes of Health Grant K23DK087857. The project was supported by the National Institutes of Health Grants 1UL1RR025758-04 and 8UL1TR000170-05, the Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Clinical Trials Registration: NCT 01421589.
Disclosure Summary: H.M. has received investigator initiated research support from Pfizer, Inc, for this study as above. H.M. has served on the Scientific Advisory Board to Novo Nordisk, Inc, and as a consultant to Endosphere, Inc, both unrelated to this manuscript. The other authors have nothing to declare.
Footnotes
- ABI
- ankle-brachial index
- BMI
- body mass index
- DXA
- dual-energy x-ray absorptiometry
- HOMA
- homeostatic model assessment
- HOMA-IR
- HOMA of insulin resistance
- hsCRP
- high-sensitivity C-reactive protein
- LBM
- lean body mass
- MRS
- magnetic resonance spectroscopy
- MVC
- maximal voluntary contraction
- NRF-1
- nuclear respiratory factor-1
- PCr
- phosphocreatine
- τPCr
- recovery time constant
- PGC-1α
- PPARγ coactivator 1-α
- 31P-MRS
- 31P-magnetic resonance spectroscopy
- PPAR
- peroxisome proliferator-activated receptor
- REE
- resting energy expenditure
- rhGH
- recombinant human GH
- TFAM
- mitochondrial transcription factor A
- VCO2
- carbon dioxide production
- ViPCr
- initial rate of PCr recovery
- VO2
- oxygen consumption.
References
- 1. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. [DOI] [PubMed] [Google Scholar]
- 2. Fleischman A, Johnsen S, Systrom DM, et al. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol Endocrinol Metab. 2007;292:E1666–E1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. [DOI] [PubMed] [Google Scholar]
- 5. Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1 α (PGC-1 α): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. [DOI] [PubMed] [Google Scholar]
- 6. Kang C, Li Ji L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann NY Acad Sci. 2012;1271:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheah JS, Chua SP, Ho CL. Ultrastructure of the skeletal muscles in acromegaly–before and after hypophysectomy. Am J Med Sci. 1975;269:183–187. [DOI] [PubMed] [Google Scholar]
- 8. Makimura H, Stanley TL, Sun N, Hrovat MI, Systrom DM, Grinspoon SK. The association of growth hormone parameters with skeletal muscle phosphocreatine recovery in adult men. J Clin Endocrinol Metab. 2011;96:817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Befroy DE, Shulman GI. Magnetic resonance spectroscopy studies of human metabolism. Diabetes. 2011;60:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lanza IR, Bhagra S, Nair KS, Port JD. Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements. J Magn Reson Imaging. 2011;34:1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larson-Meyer DE, Newcomer BR, Hunter GR, Joanisse DR, Weinsier RL, Bamman MM. Relation between in vivo and in vitro measurements of skeletal muscle oxidative metabolism. Muscle Nerve. 2001;24:1665–1676. [DOI] [PubMed] [Google Scholar]
- 12. Sahlin K, Harris RC, Hultman E. Resynthesis of creatine phosphate in human muscle after exercise in relation to intramuscular pH and availability of oxygen. Scand J Clin Lab Invest. 1979;39:551–558. [DOI] [PubMed] [Google Scholar]
- 13. Kemp GJ, Thompson CH, Barnes PR, Radda GK. Comparisons of ATP turnover in human muscle during ischemic and aerobic exercise using 31P magnetic resonance spectroscopy. Magn Reson Med. 1994;31:248–258. [DOI] [PubMed] [Google Scholar]
- 14. Short KR, Moller N, Bigelow ML, Coenen-Schimke J, Nair KS. Enhancement of muscle mitochondrial function by growth hormone. J Clin Endocrinol Metab. 2008;93:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bredella MA, Gerweck AV, Lin E, et al. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab. 2013;98:3864–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makimura H, Murphy CA, Feldpausch MN, Grinspoon SK. The effects of tesamorelin on phosphocreatine recovery in obese subjects with reduced GH. J Clin Endocrinol Metab. 2014;99:338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosseini Ghomi R, Bredella MA, Thomas BJ, Miller KK, Torriani M. Modular MR-compatible lower leg exercise device for whole-body scanners. Skeletal Radiol. 2011;40:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roussel M, Bendahan D, Mattei JP, Le Fur Y, Cozzone PJ. 31P magnetic resonance spectroscopy study of phosphocreatine recovery kinetics in skeletal muscle: the issue of intersubject variability. Biochim Biophys Acta. 2000;1457:18–26. [DOI] [PubMed] [Google Scholar]
- 19. Dietrichson P, Coakley J, Smith PE, Griffiths RD, Helliwell TR, Edwards RH. Conchotome and needle percutaneous biopsy of skeletal muscle. J Neurol Neurosurg Psychiatry. 1987;50:1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- 21. Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14:101–102. [PubMed] [Google Scholar]
- 22. Makimura H, Stanley T, Mun D, You SM, Grinspoon S. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab. 2008;93:4254–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 24. Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. [DOI] [PubMed] [Google Scholar]
- 25. Butler AA, LeRoith D. Minireview: tissue-specific versus generalized gene targeting of the igf1 and igf1r genes and their roles in insulin-like growth factor physiology. Endocrinology. 2001;142:1685–1688. [DOI] [PubMed] [Google Scholar]
- 26. Yakar S, Wu Y, Setser J, Rosen CJ. The role of circulating IGF-I: lessons from human and animal models. Endocrine. 2002;19:239–248. [DOI] [PubMed] [Google Scholar]
- 27. Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Yuan M, Bradley KM, Dong F, Anversa P, Ren J. Insulin-like growth factor 1 alleviates high-fat diet-induced myocardial contractile dysfunction: role of insulin signaling and mitochondrial function. Hypertension. 2012;59:680–693. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Dalla Libera L, Ravara B, Volterrani M, et al. Beneficial effects of GH/IGF-1 on skeletal muscle atrophy and function in experimental heart failure. Am J Physiol Cell Physiol. 2004;286:C138–C144. [DOI] [PubMed] [Google Scholar]
- 30. Vescovo G, Ravara B, Gobbo V, Angelini A, Dalla Libera L. Skeletal muscle fibres synthesis in heart failure: role of PGC-1α, calcineurin and GH. Int J Cardiol. 2005;104:298–306. [DOI] [PubMed] [Google Scholar]
- 31. Johannsson G, Mårin P, Lönn L, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab. 1997;82:727–734. [DOI] [PubMed] [Google Scholar]
- 32. Karlsson C, Stenlöf K, Johannsson G, et al. Effects of growth hormone treatment on the leptin system and on energy expenditure in abdominally obese men. Eur J Endocrinol. 1998;138:408–414. [DOI] [PubMed] [Google Scholar]
- 33. Franco C, Brandberg J, Lönn L, Andersson B, Bengtsson BA, Johannsson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:1466–1474. [DOI] [PubMed] [Google Scholar]
- 34. Pasarica M, Zachwieja JJ, Dejonge L, Redman S, Smith SR. Effect of growth hormone on body composition and visceral adiposity in middle-aged men with visceral obesity. J Clin Endocrinol Metab. 2007;92:4265–4270. [DOI] [PubMed] [Google Scholar]
- 35. Mekala KC, Tritos NA. Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis. J Clin Endocrinol Metab. 2009;94:130–137. [DOI] [PubMed] [Google Scholar]
- 36. Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Møller N, Schmitz O, Pørksen N, Møller J, Jørgensen JO. Dose-response studies on the metabolic effects of a growth hormone pulse in humans. Metabolism. 1992;41:172–175. [DOI] [PubMed] [Google Scholar]
- 38. Møller N, Møller J, Jørgensen JO, et al. Impact of 2 weeks high dose growth hormone treatment on basal and insulin stimulated substrate metabolism in humans. Clin Endocrinol (Oxf). 1993;39:577–581. [DOI] [PubMed] [Google Scholar]
- 39. Davidson MB. Effect of growth hormone on carbohydrate and lipid metabolism. Endocr Rev. 1987;8:115–131. [DOI] [PubMed] [Google Scholar]
- 40. Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–177. [DOI] [PubMed] [Google Scholar]
- 41. Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. [DOI] [PubMed] [Google Scholar]
- 42. Franco C, Andersson B, Lönn L, Bengtsson BA, Svensson J, Johannsson G. Growth hormone reduces inflammation in postmenopausal women with abdominal obesity: a 12-month, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2007;92:2644–2647. [DOI] [PubMed] [Google Scholar]
- 43. Makimura H, Feldpausch MN, Rope AM, et al. Metabolic effects of a growth hormone-releasing factor in obese subjects with reduced growth hormone secretion: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97:4769–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Warner ET, Wolin KY, Duncan DT, Heil DP, Askew S, Bennett GG. Differential accuracy of physical activity self-report by body mass index. Am J Health Behav. 2012;36:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Slootmaker SM, Schuit AJ, Chinapaw MJ, Seidell JC, van Mechelen W. Disagreement in physical activity assessed by accelerometer and self-report in subgroups of age, gender, education and weight status. Int J Behav Nutr Phys Act. 2009;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McMurray RG, Ward DS, Elder JP, et al. Do overweight girls overreport physical activity? Am J Health Behav. 2008;32:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]


