Protective antibody levels persist long-term in a majority of healthcare workers after initial immunization. Those without protective levels have a rapid and robust response to a booster vaccine, suggesting that immunologic memory is long-lasting and booster vaccination is probably unnecessary.
Keywords: hepatitis B vaccine, healthcare workers, booster vaccination, chronic hepatitis B
Abstract
Background. Follow-up studies of recipients of hepatitis B vaccine from endemic areas have reported loss of antibody to hepatitis B surface antigen (anti-HBs) in a high proportion of persons vaccinated at birth. In contrast, the long-term durability of antibody in persons vaccinated as adults in nonendemic areas is not well defined. We aimed to assess the durability of anti-HBs among healthcare workers (HCWs) vaccinated as adults and response to a booster among those without protective levels of antibody.
Methods. Adult HCWs aged 18–60 at the time of initial vaccination were recruited. All were tested for hepatitis B surface antigen (HBsAg), antibody to hepatitis B core antigen (anti-HBc), and anti-HBs level. HCWs with anti-HBs <12 mIU/mL were offered a booster and levels were measured 1, 7, and 21 days afterward.
Results. Anti-HBs levels were <12 mIU/mL in 9 of 50 (18%), 13 of 50 (26%), and 14 of 59 (24%) HCWs 10–15, 16–20, and >20 years postvaccination, respectively, (P = ns). Four HCWs were anti-HBc positive; none had HBsAg. By logistic regression, older age at vaccination was the only predictor of inadequate anti-HBs level (P = .0005). Thirty-four of 36 subjects with inadequate anti-HBs levels received a booster and 32 (94%) developed levels >12 mIU/mL within 3 weeks.
Conclusions. Anti-HBs levels decrease after 10–31 years and fall below a level considered protective in approximately 25% of cases. The rapid and robust response to a booster vaccine suggests a long-lasting amnestic response. Hepatitis B vaccination provides long-term protection against hepatitis B and booster vaccination does not appear to be necessary in HCWs.
Clinical Trials Registration. NCT01182311.
The implementation of vaccination programs worldwide against hepatitis B virus (HBV) has reduced the morbidity and mortality of acute and chronic HBV infection and the incidence of hepatocellular carcinoma, particularly in endemic regions [1–3]. Vaccination against HBV consists of 3 or 4 intramuscular injections of recombinant hepatitis B surface antigen (HBsAg) at varying schedules [4]. Response rates to primary vaccination are high, with 85%–100% of vaccinees developing antibody to HBsAg (anti-HBs) ≥10 mIU/mL [5], a level that is considered protective [5–9]. Factors found to be associated with nonresponse include male sex, increasing age at vaccination (>40 years old), obesity, alcoholism, smoking, and genetic factors [10–12].
Asymptomatic breakthrough infections (detected by the presence of antibody to hepatitis B core antigen [anti-HBc] or HBV DNA in serum) have been reported in vaccinated persons with a documented initial antibody response [13, 14]. Long-term follow-up studies of persons who were vaccinated as infants have reported absence of anti-HBs in 50%–70% of persons 15–30 years later [13, 15–18]. In contrast, data on the longevity of immunity afforded by hepatitis B vaccine in a healthy adult population are scarce. The few available studies in young adults who initially responded to a past primary vaccine series with antibody concentrations of ≥10 mIU/mL reported that 17%–50% have low or undetectable anti-HBs (reflecting anti-HBs loss) 10–15 years after vaccination [14, 19]. Whether low or undetectable levels of anti-HBs predispose to subsequent infection is unknown. Moreover, whether individuals may respond to a hepatitis B vaccine booster to maintain long-term protection is unknown. Current guidelines do not recommend booster doses, but the duration of long-term protection is unknown [4, 20].
Healthcare workers (HCWs) in the United States are mandated to receive hepatitis B vaccine and are at risk for hepatitis B through occupational exposure. Therefore, they would be an ideal population to assess durability of antibody response and long-term (≥10 years) vaccine protection and to determine response to a booster dose in those who did not maintain the immune response to primary vaccination as adults.
METHODS
Subjects
All HCWs from the Clinical Center, National Institutes of Health (NIH) and Suburban Hospital, Bethesda, Maryland, were invited to participate in the study. Eligible subjects were those who received a 3-dose series of either plasma-derived or recombinant HBV vaccine within a 6-month period between the ages of 18 and 60 years and could provide documentation of the dates of vaccination. In the absence of such documentation, subjects were requested to obtain a note from their physician or to sign a written affidavit indicating the dates. HBV serological status before and after vaccination was not a requirement for enrollment but was available on a subset of individuals (n = 36) who received their initial immunization at the Clinical Center, NIH. Exclusion criteria included chronic hepatitis B, known nonresponse to an adequate series of HBV vaccination, receipt of a booster dose of HBV vaccine, recent use of immunosuppressive medications or their use at the time of vaccination, renal failure requiring dialysis, chronic hepatitis C, hematopoietic cell or solid organ transplant, known immunodeficiency, and human immunodeficiency virus (HIV) infection.
Study Design
Eligible HCWs were stratified based on the time interval since the first dose of hepatitis B vaccine: group 1 (10–15 years after vaccination), group 2 (16–20 years after vaccination), and group 3 (>20 years after vaccination). Subjects provided written informed consent and were remunerated for their participation. They were evaluated in the outpatient clinic and completed a questionnaire (Supplementary Table 1) to assess risk factors for HBV exposure and compromised immunostatus. The study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH (ClinicalTrials.gov identifier NCT01182311).
Testing
Serological testing for HBV status was performed for HBsAg, anti-HBs, and anti-HBc using commercial assays (VITROS, Ortho Clinical Diagnostics, Raritan, New Jersey). Other serologic testing included enzyme-linked immunosorbent assay for antibody to hepatitis C virus (anti-HCV), antibody to hepatitis A virus (anti-HAV), and anti-HIV (Ortho Clinical Diagnostics, Raritan, New Jersey). Serum HBV DNA levels were measured by COBAS AmpliPrep/COBAS TaqMan HBV Test, version 2.0 (lower limit of detection, 29 IU/mL; Roche Molecular Diagnostics, Branchburg, New Jersey) in subjects who tested positive for anti-HBc and/or HBsAg.
Definitions
According to the product label for the VITROS anti-HBs assay, anti-HBs levels <5 mIU/mL were considered negative, levels between 5 and 12 mIU/mL were considered indeterminate, and levels >12 mIU/mL were considered protective. These definitions were used in this analysis. Subjects with negative and indeterminate levels were offered a booster dose of HBV vaccine.
Exposure Risk Score
Risk of HBV exposure, that is, occupational risk (needle-stick injury and mucocutaneous exposure to blood or body fluids), blood transfusion, and intimate contact with a known HBV carrier, was determined by a questionnaire. HCWs without risk factors were classified at low risk, those with 1 risk factor at intermediate risk, and those with ≥2 risk factors at high risk for exposure.
Booster Dose and Follow-up
Booster vaccination consisted of a single intramuscular (deltoid) injection of 20 µg recombinant hepatitis B vaccine (Engerix-B, GlaxoSmithKline Biologicals, Rixensart, Belgium). Anti-HBs levels were measured at days 1, 7, and 21 after the booster. Subjects whose anti-HBs levels rose above 12 mIU/mL were considered responders.
Statistical Analysis
Baseline demographic, clinical, and laboratory characteristics were compared using measures of central tendency. Anti-HBs levels were compared among the 3 groups using analysis of variance. The role of age, age at vaccination, race, sex, body mass index, duration since vaccination, risk of exposure to HBV, alcohol use, and smoking history with a negative anti-HBs level was explored using multivariate logistic regression analysis. Statistical analysis was carried out using SAS software version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Baseline Characteristics of the Study Group
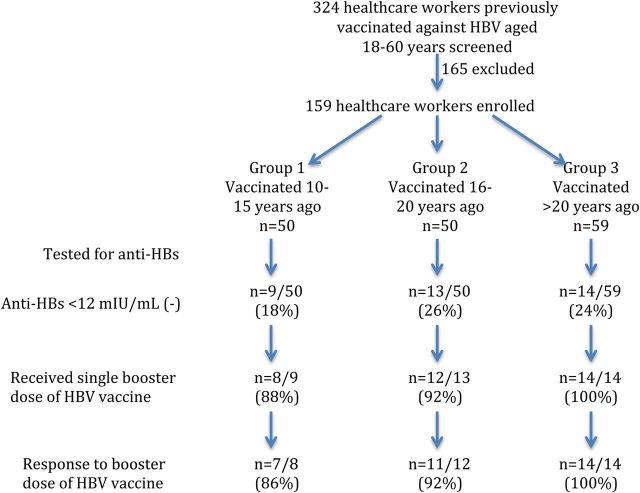
Between September 2010 and March 2012, 324 HCWs were screened for enrollment, of whom 165 were excluded. The reasons for exclusion were absence of vaccination record (n = 90), vaccination before the age of 18 years (n = 30), receipt of a booster dose (n = 28), incomplete vaccination (n = 5), study participation refusal (n = 12), and receipt of immunosuppressive agents (n = 8). Of the 159 who were recruited, 142 (89%) provided documentation indicating the dates of vaccination, 11 (7%) subjects provided a note from their physicians, and 6 (4%) signed a written affidavit indicating the dates of vaccination. Among the 159 enrolled subjects, 50 belonged to group 1 (10–15 years after vaccination), 50 to group 2 (16–20 years), and 59 to group 3 (>20 years). The average duration since vaccination was 18 years with a range of 10–31 years (group 1, 12 years; group 2, 17 years; and group 3, 24 years). The average age at vaccination among the 3 groups was 27, 28, and 30 years, respectively, and was not significantly different (P = .11). Baseline characteristics were similar among the 3 groups with the exception of a higher rate of needle-stick injury (47%) and having ever smoked cigarettes (32%) among subjects in group 3 (Table 1). All subjects tested negative for anti-HIV and anti-HCV.
Table 1.
Baseline Characteristics of the Cohort
| Features | Group 1 (10–15 y Postvaccination) (n = 50) | Group 2 (16–20 y Postvaccination) (n = 50) | Group 3 (>20 y Postvaccination) (n = 59) | P Valuea |
|---|---|---|---|---|
| Age at visit, y, average ± SD | 41 ± 10 | 46 ± 9 | 54 ± 7 | <.0001 |
| Age at vaccination, y, average ± SD | 27 ± 11 | 28 ± 8 | 30 ± 7 | .11 |
| Sex, female | 34 (68%) | 36 (72%) | 48 (81%) | .26 |
| Race, white | 30 (60%) | 33 (66%) | 47 (80%) | .08 |
| BMI, kg/m2 | 26 ± 5 | 26 ± 5 | 28 ± 6 | .04 |
| Handled HBV samples | 23 (46%) | 20 (40%) | 35 (59%) | .36 |
| Needle-stick | 10 (20%) | 13 (26%) | 28 (47%) | .005 |
| Mucocutaneous contact | 7 (14%) | 3 (6%) | 5 (8%) | .39 |
| Household HBV contact | 1 (2%) | 2 (4%) | 0 (0%) | .33 |
| HBV exposure risk score | .14 | |||
| Low | 21 (42%) | 25 (50%) | 17 (29%) | |
| Intermediate | 13 (26%) | 10 (20%) | 13 (22%) | |
| High | 16 (32%) | 15 (30%) | 29 (49%) | |
| Anti-HBc positive | 2 (4%) | 1 (2%) | 1 (2%) | |
| Diabetes mellitus | 2 (4%) | 2 (4%) | 3 (5%) | .94 |
| Hypertension | 9 (18%) | 6 (12%) | 14 (24%) | .28 |
| Chronic kidney disease | 0 (0%) | 0 (0%) | 1 (1%) | .42 |
| Smoker, past or current | 8 (16%) | 6 (12%) | 19 (32%) | .02 |
| Alcohol use | 34 (68%) | 42 (84%) | 50 (85%) | .06 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: anti-HBc, antibody to hepatitis B core antigen; BMI, body mass index; HBV, hepatitis B virus; SD, standard deviation.
aP values were calculated by t test (continuous variables) and χ2 test (frequency and categorical variables).
Antibody Status Among the 3 Study Groups 10–31 Years After Vaccination
Protective anti-HBs levels (>12 mIU/mL) were present in 123 subjects (77%): 56 with a level of 12–100 mIU/mL, 34 with a level of 101–500 mIU/mL, 17 with a level of 501–1000 mIU/mL, and 16 with a level of >1000 mIU/mL. The proportion with protective anti-HBs levels was similar between women (75%) and men (83%) (P = .32) as well as in the 3 groups (group 1, 82%; group 2, 74%; and group 3, 76%; Figure 1) (P = .59). In addition, the average anti-HBs levels did not differ among the 3 groups (group 1, 811 mIU/mL; group 2, 321 mIU/mL; and group 3, 371 mIU/mL, P = .1) (Figure 2A). Thirty-six (23%) subjects had anti-HBs levels <12 mIU/mL, including 25 with levels <5 mIU/mL (negative) and 11 with levels between 5 and <12 mIU/mL (indeterminate).
Figure 1.
Study design and major findings. Flowchart indicates number of subjects screened and included in the study. Thirty-six of 159 subjects had antibody to hepatitis B surface antigen (anti-HBs) levels <12 mIU/mL (group 1, 9/50 [18%]; group 2, 13/50 [26%]; and group 3, 14/59 [24%]). Thirty-four of 36 received a booster vaccine (group 1, 8/9 [88%]; group 2, 12/13 [92%]; and group 3, 14/14 [100%]). Thirty-two of 34 developed protective levels of anti-HBs 3 weeks after the booster vaccine (group 1, 7/8 [86%]; group 2, 11/12 [92%]; and group 3, 14/14 [100%]). Abbreviation: HBV, hepatitis B virus.
Figure 2.
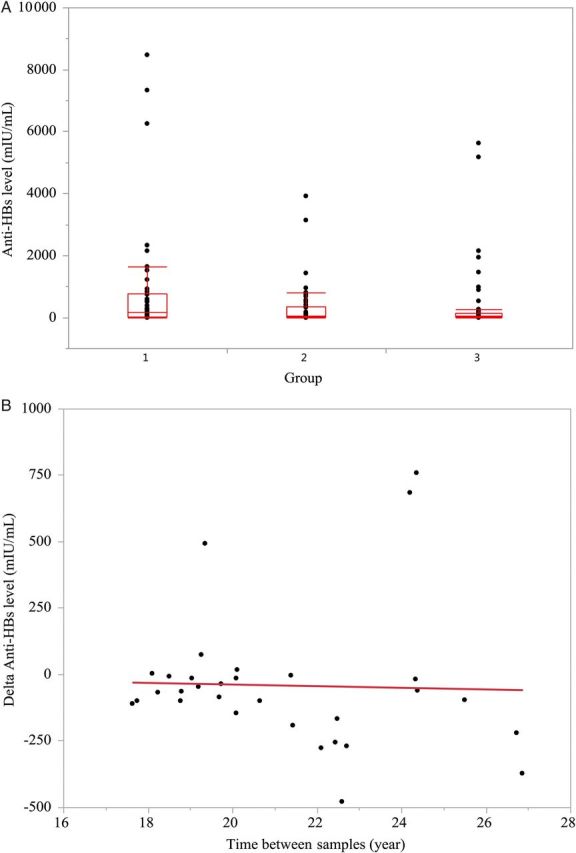
A, Box plots showing distribution of antibody to hepatitis B surface antigen (anti-HBs) levels among subjects in the 3 groups. There was no difference in anti-HBs levels among the 3 groups: group 1 (10–15 years since vaccination), 811 mIU/mL; group 2 (16–20 years since vaccination), 321 mIU/mL; and group 3 (>20 years since vaccination), 371 mIU/mL (P = .1). Average, 25th and 75th percentiles, and range are shown. B, Change in anti-HBs levels over time since initial vaccination. There was no correlation between the magnitude of change in anti-HBs level over time (r2 = 0.0013, P = .85). Change was calculated based on last anti-HBs level minus initial anti-HBs level usually obtained 9–12 months after first vaccine dose (n = 36).
Four subjects tested positive for anti-HBc. All were negative for HBsAg, hepatitis B e antigen (HBeAg), antibody to HBeAg, and HBV DNA. Anti-HBs levels were >12 mIU/mL in 3 subjects (22, 665, and 7340 mIU/mL) and were <12 mIU/mL in 1 subject (2.7 mIU/mL). None of the 4 reported a history of jaundice, hepatitis, or known HBV exposure, but 3 were born outside the United States in areas of high HBV endemicity.
Subset Analysis Examining Rates of Initial Response to Vaccination and Decline in Anti-HBs Levels Among 36 Subjects With Available Pre- And Postvaccination Anti-HBs Levels
Pre- and postvaccination anti-HBs levels were available on a subgroup of 36 subjects who received their initial course of HBV vaccine at the Clinical Center, NIH. All were anti-HBs negative before vaccination. After the standard 3-dose immunization schedule administered at 0, 1, and 6 months, 35 (97%) developed an initial response to vaccination with anti-HBs levels ranging from 13 to 497 mIU/mL. The single subject without anti-HBs after primary vaccination tested anti-HBs negative again 20 years later while participating in the current study. This subject responded to a single booster vaccination with an anti-HBs level of 685 mIU/mL at 3 weeks.
In follow-up 10–27 years (average 22 years) after the primary vaccination, 11 of the 35 vaccinees who had an initial antibody response become anti-HBs negative. Analysis of the change in anti-HBs levels and time to follow-up assessment showed no correlation (Figure 2B).
Predictors of an Inadequate Anti-HBs Level 10–31 Years After Vaccination
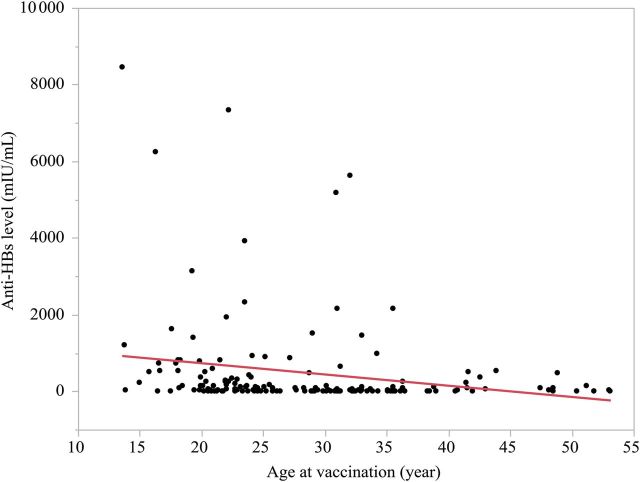
To identify factors associated with loss of anti-HBs after initial vaccination, the 36 subjects with inadequate anti-HBs levels at the time of follow-up assessment were compared to the 123 subjects with protective levels. HCWs with inadequate anti-HBs levels were older at the time of vaccination (average age, 36 vs 32 years) (P < .02), and less likely to ever have smoked (8% vs 24%) (P < .04; Table 2). There were no significant differences in sex, race, body mass index, risk factors, concurrent chronic medical conditions, or alcohol use between subjects with adequate vs inadequate anti-HBs levels. In multivariate logistic regression analysis, only age at vaccination remained a significant predictor of an inadequate anti-HBs level 10–31 years later (P = .0008; Figure 3). Age at vaccination was then tested for correlation with inadequate or negative anti-HBs levels among the individual groups, and identified as a significant predictor of anti-HBs level in groups 1 and 2 but not group 3.
Table 2.
Comparison Between Subjects With Adequate Antibody to Hepatitis B Surface Antigen (Anti-HBs) Versus Those With Inadequate Anti-HBs
| Feature | Anti-HBs Positive (>12 mIU/mL) (n = 123) | Anti-HBs Negative (≤12 mIU/mL) (n = 36) | P Valuea |
|---|---|---|---|
| Age at visit, y, average ± SD | 47 ± 10 | 51 ± 10 | .04 |
| Age at vaccination, y, average ± SD | 32 ± 9 | 36 ± 9 | .02 |
| Time since vaccination, y, average ± SD | 15 ± 5 | 15 ± 4 | .93 |
| Sex, female | 89 (72%) | 29 (81%) | .32 |
| Race, white | 82 (67%) | 28 (78%) | .57 |
| BMI, kg/m2 | 26 ± 4 | 27 ± 5 | |
| Handled HBV samples | 62 (50%) | 16 (44%) | .47 |
| HBV risk exposure score | .59 | ||
| Low | 16 (44%) | 47 (38%) | |
| Intermediate | 6 (17%) | 30 (24%) | |
| High | 14 (39%) | 46 (37%) | |
| Needle-stick | 38 (31%) | 13 (36%) | .55 |
| Mucocutaneous contact | 12 (10%) | 3 (8%) | .83 |
| Household HBV contact | 1 (1%) | 2 (6%) | .16 |
| Diabetes mellitus | 5 (4%) | 2 (6%) | .70 |
| Hypertension | 21 (17%) | 8 (22%) | .48 |
| Chronic kidney disease | 1 (1%) | 0 (0%) | .58 |
| Smoker, past or current | 30 (24%) | 3 (8%) | .04 |
| Alcohol use | 97 (79%) | 29 (81%) | .82 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: anti-HBs, antibody to hepatitis B surface antigen; BMI, body mass index; HBV, hepatitis B virus; SD, standard deviation.
aP values were calculated using t test for continuous variables and χ2 test for frequency or categorical variables.
Figure 3.
Correlation between antibody to hepatitis B surface antigen (anti-HBs) levels and age at vaccination. There was an inverse correlation between anti-HBs levels and age at vaccination. Older age at vaccination is a significant predictor of inadequate anti-HBs level (r2 = 0.069, P = .0008).
Response to HBV Booster Vaccination
All 36 subjects with inadequate anti-HBs levels were offered a single dose of HBV booster vaccine and 34 (94%) agreed and received the booster injection. The remaining 2 subjects had relocated and could no longer participate in the study. At 1 day after booster, only 1 of 28 (3.6%) subjects who returned for the visit developed an anti-HBs level ≥12 mIU/mL (average, 3.6; range, 0–14.4 mIU/mL). However, by day 7, 18 of 34 (53%) subjects had anti-HBs levels ≥12 mIU/mL (average, 40; range, 0–428 mIU/mL), and by day 21, 32 of 34 (94%) subjects had adequate levels (average, 3298; range, 0 to >20 000 mIU/mL). Thus, only 2 (6%) subjects still had inadequate anti-HBs titers 21 days after receiving a booster dose of HBV vaccine.
DISCUSSION
This study describes the durability of antibody response and the response to a booster dose of HBV vaccine among HCWs who were vaccinated as adults. Antibody levels were found to be adequate (≥12 mIU/mL) in 123 of 159 (77%) of previously vaccinated subjects. An inadequate antibody level was associated with older age at the time of vaccination, but not with sex, race, body weight, comorbidities such as diabetes or hypertension, time after vaccination, or history of ongoing risk factors for exposure. Among subjects with inadequate anti-HBs levels, no subject tested HBsAg or HBV DNA positive and almost all had a rapid anamnestic anti-HBs response to a single booster dose of vaccine, suggesting that immunologic memory to HBsAg remains intact for up to 28 years after primary immunization.
Maintenance of a long-term antibody response against HBsAg is important for protective immunity against infection with HBV, particularly among HCWs. At present, there are limited data on the durability of protective antibody in vaccinated persons from nonendemic regions at intermediate risk for HBV exposure. In the current study of HCWs vaccinated as adults, 36 of 159 (23%) had inadequate antibody levels, a mean of 18 years after vaccination. Previous studies in HCWs with shorter duration of follow-up (3–12 years postvaccination) reported inadequate anti-HBs levels in 24%–48% of subjects [21–25]. The maintenance of antibody levels decades after vaccination in the majority of our study group and the positive response to booster vaccination in those who had lost anti-HBs antibodies is remarkable for 2 reasons. First, HBsAg is a protein vaccine and 1 study of durability of other protein vaccines (tetanus and diphtheria) demonstrated a rapid decrease in antibody levels with estimated half-lives of 11–19 years. In that study, only antiviral antibody responses after live viral infections were stable with a half-life of ≥50 years [26]. Second, as many as 50%–70% of persons who were vaccinated with HBsAg as infants or in early childhood in endemic regions have inadequate antibody levels 15–30 years after vaccination [13, 15–17]. This high rate of antibody loss is notable because the natural booster effect of intermittent exposure would have been expected to maintain antibody levels. Collectively, these data suggest that anti-HBs levels decline less frequently among persons vaccinated as adults who are at risk for HBV infection through occupational exposure compared with infant/childhood vaccination from endemic regions. The detection of HBV core and polymerase-specific T-cell responses in our subjects suggests that occupational exposure may have occurred [27].
Previous studies have reported that antibody level after vaccination was related to age at vaccination and to the peak antibody response achieved [7, 28, 29]. Indeed, in this study we demonstrated that older age at vaccination (40 years) was an independent predictor of an inadequate anti-HBs level among HCWs. Furthermore, durability of anti-HBs was not related to duration since vaccination. In the group of subjects with the longest duration of follow-up since vaccination (21–31 years), 14 of 59 (24%) had inadequate antibody levels, a rate that was not different from that in subjects 15–20 years since vaccination (13 of 50 [26%]). Taken together, the existing long-term data on recipients of HBV vaccination suggest that vaccination at infancy or at an older age, approximately 40 years, is associated with higher rate of antibody loss.
Among persons with an inadequate anti-HBs level, we demonstrated a rapid and robust response to a booster dose of HBV vaccine. Fifty-three percent of subjects developed a protective anti-HBs level by day 7 and 94% by day 21, with an average 100-fold increase in antibody level noted between day 7 and day 21. These data suggest that it is possible to mount an anamnestic response to HBV vaccination up to 28 years after initial vaccination and are in keeping with other reports, albeit with shorter duration of follow-up documenting response to a booster dose of vaccine in HCWs and other populations [21, 30–32]. The absence of any cases of chronic infection as evidenced by lack of HBsAg and the positive response to a single booster dose argue against the need for a routine booster dose of HBV vaccine for HCWs. Because HBV infection has an incubation period of 6 weeks to 6 months, stimulation of memory B cells by HBV should permit development of an adequate antibody response to neutralize virus before active infection can be established. Thus, these data support current recommendations by the Advisory Committee on Immunization Practices (ACIP) and European Consensus Group that a booster dose of HBV is not required for HCWs [33, 34]. Immunologic memory is only reliable in healthy persons; therefore, booster doses are still recommended for persons receiving hemodialysis or otherwise immunocompromised if anti-HBs levels decline to <12 mIU/mL [33]. Whether immunologic memory is lifelong will require further follow-up studies.
During the conduct of this study, we noted that more than half of the vaccinees did not possess a vaccination record. The ACIP guidance on general vaccination recommends that both providers and recipients maintain a permanent vaccination record, and The National Vaccine Advisory Committee strongly encourages development of community- or state-based immunization information systems and recommends that vaccination providers participate in these systems whenever possible [35]. Having a vaccination record would be helpful in management of postexposure cases.
A limitation of our study was the lack of information about antibody response to original HBV vaccination in approximately three-fourths of study participants. Prior studies have reported that approximately 5% of persons vaccinated as adults do not develop an antibody response after an initial course of HBV vaccine. In the subset of subjects in whom postvaccination anti-HBs status was available, 35 of 36 (97%) had an initial antibody response. Thus, if we assume a nonresponse rate to initial vaccination of 3%–5%, then the observed rate of persons without anti-HBs in this study may be closer to 18%–20% (observed rate of negative anti-HBs [23%] minus initial nonresponse rate [3%–5%]). We were unable to distinguish between prior infection before vaccination or vaccine failure (with subsequent recovery) after vaccination in the 4 anti-HBc positive cases due to absence of prevaccination serological data. Prior infection was suspected, as 3 of the 4 were from an endemic region. Finally, as the majority of participants were white females, these data may not be generalizable to females of other races or to males, although we did not observe any difference in the rates of anti-HBs loss between males and females.
In summary, HBV vaccine protected against chronic HBV infection over a mean follow-up period of 18 years in a population of HCWs vaccinated as adults. Seventy-seven percent of the study group maintained an antibody level that is considered protective or adequate. The rapid and robust response to booster among HCWs with absent or inadequate anti-HBs levels suggests that even these persons would be protected upon HBV reexposure. These data support current guidelines that booster vaccination is not necessary in otherwise healthy HCWs [33]. Longer follow-up of persons at higher risk for anti-HBs loss (persons vaccinated as infants or >40 years) is necessary to establish the long-term protection afforded by hepatitis B vaccine.
Supplementary Data
Supplementary materials. are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Author contributions. Study concept and design: N. G., A. A., B. R., M. G. G.; acquisition of data: N. G., A. A., E. R., M. G. G.; analysis/interpretation: N. G., A. A., X. Z., J. M. W., J. H. H., T. J. L., B. R., M. G. G.; drafting: N. G., M. G. G.; critical revision: M. G. G., B. R., T. J. L., J. H. H.
Acknowledgments. The authors thank all healthcare workers who participated in this study. We also wish to thank the nursing staff of the National Institutes of Health (NIH) Clinical Center Outpatient Clinic 9 for their efforts in conducting the study.
Financial support. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, Intramural Research Program, NIH. J. M. W. was supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (We-4675/1-1).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 2.Chang MH, You SL, Chen CJ, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–55. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 3.Chiang CJ, Yang YW, You SL, et al. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA. 2013;310:974–6. doi: 10.1001/jama.2013.276701. [DOI] [PubMed] [Google Scholar]
- 4.Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part II: immunization of adults. MMWR Recomm Rep. 2006;55:1–33. quiz CE1-4. [PubMed] [Google Scholar]
- 5.Jack AD, Hall AJ, Maine N, et al. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–92. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- 6.Francis DP, Hadler SC, Thompson SE, et al. The prevention of hepatitis B with vaccine. Report of the centers for disease control multi-center efficacy trial among homosexual men. Ann Intern Med. 1982;97:362–6. doi: 10.7326/0003-4819-97-3-362. [DOI] [PubMed] [Google Scholar]
- 7.Hadler SC, Francis DP, Maynard JE, et al. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Engl J Med. 1986;315:209–14. doi: 10.1056/NEJM198607243150401. [DOI] [PubMed] [Google Scholar]
- 8.Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine in medical staff of hemodialysis units: efficacy and subtype cross-protection. N Engl J Med. 1982;307:1481–6. doi: 10.1056/NEJM198212093072403. [DOI] [PubMed] [Google Scholar]
- 9.Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303:833–41. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 10.Yu AS, Cheung RC, Keeffe EB. Hepatitis B vaccines. Infect Dis Clin North Am. 2006;20:27–45. doi: 10.1016/j.idc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Degos F, Duhamel G, Brechot C, et al. Hepatitis B vaccination in chronic alcoholics. J Hepatol. 1986;2:402–9. doi: 10.1016/s0168-8278(86)80051-4. [DOI] [PubMed] [Google Scholar]
- 12.Hennig BJ, Fielding K, Broxholme J, et al. Host genetic factors and vaccine-induced immunity to hepatitis B virus infection. PLoS One. 2008;3:e1898. doi: 10.1371/journal.pone.0001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu CY, Chiang BL, Chi WK, et al. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology. 2004;40:1415–20. doi: 10.1002/hep.20490. [DOI] [PubMed] [Google Scholar]
- 14.McMahon BJ, Bruden DL, Petersen KM, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med. 2005;142:333–41. doi: 10.7326/0003-4819-142-5-200503010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Hammitt LL, Hennessy TW, Fiore AE, et al. Hepatitis B immunity in children vaccinated with recombinant hepatitis B vaccine beginning at birth: a follow-up study at 15 years. Vaccine. 2007;25:6958–64. doi: 10.1016/j.vaccine.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 16.Ni YH, Huang LM, Chang MH, et al. Two decades of universal hepatitis B vaccination in Taiwan: impact and implication for future strategies. Gastroenterology. 2007;132:1287–93. doi: 10.1053/j.gastro.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 17.Huang LM, Chiang BL, Lee CY, et al. Long-term response to hepatitis B vaccination and response to booster in children born to mothers with hepatitis B e antigen. Hepatology. 1999;29:954–9. doi: 10.1002/hep.510290349. [DOI] [PubMed] [Google Scholar]
- 18.Lin AW, Wong KH. Long-term protection of neonatal hepatitis B vaccination in a 30-year cohort in Hong Kong. J Hepatol. 2013;59:1363–4. doi: 10.1016/j.jhep.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Stevens CE, Toy PT, Taylor PE, et al. Prospects for control of hepatitis B virus infection: implications of childhood vaccination and long-term protection. Pediatrics. 1992;90:170–3. [PubMed] [Google Scholar]
- 20.Banatvala JE, Van Damme P. Hepatitis B vaccine—do we need boosters? J Viral Hepat. 2003;10:1–6. doi: 10.1046/j.1365-2893.2003.00400.x. [DOI] [PubMed] [Google Scholar]
- 21.Durlach R, Laugas S, Freuler CB, et al. Ten-year persistence of antibody to hepatitis B surface antigen in healthcare workers vaccinated against hepatitis B virus, and response to booster vaccination. Infect Control Hosp Epidemiol. 2003;24:773–6. doi: 10.1086/502132. [DOI] [PubMed] [Google Scholar]
- 22.Floreani A, Baldo V, Cristofoletti M, et al. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine. 2004;22:607–10. doi: 10.1016/j.vaccine.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Pasko MT, Beam TR., Jr Persistence of anti-HBs among health care personnel immunized with hepatitis B vaccine. Am J Public Health. 1990;80:590–3. doi: 10.2105/ajph.80.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peces R, Laures AS. Persistence of immunologic memory in long-term hemodialysis patients and healthcare workers given hepatitis B vaccine: role of a booster dose on antibody response. Nephron. 2001;89:172–6. doi: 10.1159/000046064. [DOI] [PubMed] [Google Scholar]
- 25.Barash C, Conn MI, DiMarino AJ, Jr, et al. Serologic hepatitis B immunity in vaccinated health care workers. Arch Intern Med. 1999;159:1481–3. doi: 10.1001/archinte.159.13.1481. [DOI] [PubMed] [Google Scholar]
- 26.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 27.Werner JM, Abdalla A, Gara N, et al. The hepatitis B vaccine protects re-exposed health care workers, but does not provide sterilizing immunity. Gastroenterology. 2013;145:1026–34. doi: 10.1053/j.gastro.2013.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jilg W, Schmidt M, Deinhardt F, et al. Hepatitis B vaccination: how long does protection last? Lancet. 1984;2:458. doi: 10.1016/s0140-6736(84)92926-x. [DOI] [PubMed] [Google Scholar]
- 29.Jilg W, Schmidt M, Deinhardt F. Persistence of specific antibodies after hepatitis B vaccination. J Hepatol. 1988;6:201–7. doi: 10.1016/s0168-8278(88)80032-1. [DOI] [PubMed] [Google Scholar]
- 30.Williams JL, Christensen CJ, McMahon BJ, et al. Evaluation of the response to a booster dose of hepatitis B vaccine in previously immunized healthcare workers. Vaccine. 2001;19:4081–5. doi: 10.1016/s0264-410x(01)00112-8. [DOI] [PubMed] [Google Scholar]
- 31.Su FH, Chu FY, Bai CH, et al. Efficacy of hepatitis B vaccine boosters among neonatally vaccinated university freshmen in Taiwan. J Hepatol. 2013;58:684–9. doi: 10.1016/j.jhep.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 32.Zanetti AR, Mariano A, Romano L, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366:1379–84. doi: 10.1016/S0140-6736(05)67568-X. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–45. [PubMed] [Google Scholar]
- 34.Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355:561–5. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Immunization information system progress—United States, 2004. MMWR Morb Mortal Wkly Rep. 2005;54:1156–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.