Although HIV-infected veterans had a higher risk of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer, diagnosis with these conditions were occurring at similar ages compared with HIV-uninfected veterans.
Keywords: HIV infection, aging, myocardial infarction, end-stage renal disease, non-AIDS-defining cancers
Abstract
Background. Although it has been shown that human immunodeficiency virus (HIV)-infected adults are at greater risk for aging-associated events, it remains unclear as to whether these events happen at similar, or younger ages, in HIV-infected compared with uninfected adults. The objective of this study was to compare the median age at, and risk of, incident diagnosis of 3 age-associated diseases in HIV-infected and demographically similar uninfected adults.
Methods. The study was nested in the clinical prospective Veterans Aging Cohort Study of HIV-infected and demographically matched uninfected veterans, from 1 April 2003 to 31 December 2010. The outcomes were validated diagnoses of myocardial infarction (MI), end-stage renal disease (ESRD), and non-AIDS-defining cancer (NADC). Differences in mean age at, and risk of, diagnosis by HIV status were estimated using multivariate linear regression models and Cox proportional hazards models, respectively.
Results. A total of 98 687 (31% HIV-infected and 69% uninfected) adults contributed >450 000 person-years and 689 MI, 1135 ESRD, and 4179 NADC incident diagnoses. Mean age at MI (adjusted mean difference, −0.11; 95% confidence interval [CI], −.59 to .37 years) and NADC (adjusted mean difference, −0.10 [95% CI, −.30 to .10] years) did not differ by HIV status. HIV-infected adults were diagnosed with ESRD at an average age of 5.5 months younger than uninfected adults (adjusted mean difference, −0.46 [95% CI, −.86 to −.07] years). HIV-infected adults had a greater risk of all 3 outcomes compared with uninfected adults after accounting for important confounders.
Conclusions. HIV-infected adults had a higher risk of these age-associated events, but they occurred at similar ages than those without HIV.
The widespread use of antiretroviral therapy (ART) has transformed human immunodeficiency virus (HIV) infection into a chronic disease [1, 2]. Life expectancy with HIV after ART initiation has greatly improved over time and is approaching that of the general population in the United States [3]. As HIV-infected adults attain older ages, the appropriate management of these patients is of major concern [4].
Whether HIV-infected adults are diagnosed with aging-associated comorbidities more frequently than uninfected adults is an actively debated question [5]. A complete picture relies on 2 concepts: (1) accelerated aging, reflecting whether the age at diagnosis of the comorbid condition is younger than uninfected adults; and (2) accentuated aging, reflecting whether the risk of comorbidities is elevated at every age relative to uninfected adults [6]. These characteristics are complementary; HIV-infected adults may experience a greater burden of aging-associated diseases, but these events may or may not occur at similar ages. An increased relative risk does not mandate a younger age at diagnosis.
The objective of this study was 2-fold. First, we compared the mean age at diagnosis of myocardial infarction (MI), end-stage renal disease (ESRD), and non-AIDS-defining cancer (NADC) in HIV-infected and uninfected adults. Second, we determined if there was an increased risk among HIV-infected compared with uninfected adults.
METHODS
Study Population
The Veterans Aging Cohort Study Virtual Cohort (VACS) was established in 1998 as a prospective, longitudinal cohort study of HIV-infected and uninfected veterans matched by age, race/ethnicity, and clinical site using the United States Department of Veterans Affairs (VA) national electronic medical record system [7]. Data for this cohort were extracted from the VA Immunology Case Registry, the Decision Support System, the National Patient Care Database, Medicare, and the VA electronic medical record health factor table. The data were de-identified and the institutional review boards at Yale University and the VA Connecticut Healthcare System approved this study.
For this study, we identified participants from the VACS who were alive and enrolled between 1 April 2003 and 31 December 2010 to allow for investigation of patterns of outcomes occurring during the modern ART era. Baseline was defined as participant's first clinical encounter on or after 1 April 2003. Individuals with prevalent cardiovascular disease (defined as International Classification of Diseases, Ninth Revision [ICD-9] codes for MI, unstable angina, cardiovascular revascularization, stroke or transient ischemic attack, peripheral vascular disease or heart failure), ESRD, and cancer occurring at or within 6 months after baseline were excluded from their respective analyses as prevalent events.
Outcomes: MI, ESRD, and NADC
Acute MIs were validated as part of the VA-wide Ischemic Heart Disease Quality Enhancement Research Initiative; events treated at non-VA hospitals or fatal events solely noted on the death certificate were not included. All participants with acute MI identified via discharge summary were reviewed using data collected by trained abstractors from the VA External Peer Review program [8]. Clinical validation of MI events required documentation in the discharge summary followed by a review of the physician's notes and medical chart. An MI was defined as evidence of elevated serum markers of myocardial damage (including elevated troponin I, troponin T, or creatinine kinase–muscle brain) and supporting evidence of electrocardiographic findings (ST segment elevation of 1 mV or higher in ≥2 contiguous leads and/or left bundle branch block).
ESRD was defined as the initiation of long-term hemodialysis or peritoneal dialysis or receipt of kidney transplant. In the United States, renal replacement therapies are covered by Medicare. ESRD was identified using data from the Centers for Medicare and Medicaid Services (outpatient file: revenue center codes 0800–0804, 0809, 0820–0825, 0829, 0841, 0851, 0800–0889; carrier file outpatient provider dialysis services using claims with BETOS P9A or P9B).
To obtain validated NADC diagnoses, VACS was linked to the VA Central Cancer Registry (VACCR), which maintains a national database of cancer cases diagnosed or treated at VA facilities, aggregated from local VA medical center registries. VACCR, which seeks complete and accurate identification of all cancer cases diagnosed or treated in the VA, adheres to North American Association of Central Cancer Registries standards. The following NADCs were included in our study: anal, bladder, brain and nervous system, breast, colon and rectum, esophageal, Hodgkin lymphoma, kidney and renal pelvis, larynx, leukemia (lymphoid and myeloid), liver, lung, melanoma, myeloma, oral cavity and pharynx, pancreatic, penis, prostate, soft tissue (including heart), stomach, testicular, and thyroid cancer; there were no cases of vulvar cancer, and the single case of ovarian cancer was omitted. As demonstrated in previous studies and reflected in data from our study population, anal, oral cavity and pharynx, penis, Hodgkin lymphoma, liver, and lung cancer occur at an increased rate in HIV-infected compared with uninfected adults; these cancers were termed “HIV-associated cancers” and examined as a subgroup in analyses [9–13]. The selection of certain cancers as “HIV-associated cancers” is somewhat subjective, as other cancers such as renal, melanoma, and vaginal cancer have also been shown to occur at an increased rate in HIV-infected compared with uninfected adults [11, 12].
Exposures and Potential Confounders
HIV-infected adults were identified using a validated algorithm with ≥1 inpatient and/or ≥2 outpatient ICD-9 codes for HIV infection at baseline [7]. Age was a time-varying covariate.
Confounders were measured consistently, regardless of HIV status. Sex and race were determined using administrative records. Other potential confounders were defined using outpatient and clinical laboratory data collected closest to the participants' baseline date. Body mass index (BMI) and cigarette smoking (current, past, or never) were collected via standardized electronic forms that providers are routinely required to complete for all patients [14]. The occurrence of major depression or an alcohol- or cocaine-related diagnosis was measured using ICD-9 codes [15]. Hepatitis C (HCV) infection was defined as a positive HCV antibody test or ≥1 inpatient and/or ≥2 outpatient ICD-9 codes for HCV diagnosis [16].
Diabetes was diagnosed using a previously validated metric that considers glucose measurements, use of antidiabetic agents, and/or ≥1 inpatient and/or ≥2 outpatient ICD-9 codes for this diagnosis [17]. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Modification of Diet in Renal Disease equation [18]. Hyperlipidemia was defined as triglycerides >200 mg/dL; and/or serum total cholesterol >240 mg/dL measured on 2 separate occasions. Hypertension was defined using ICD-9 codes [19]. Antidiabetic, lipid-lowering, and antihypertensive medication use was measured using pharmacy data.
Statistical Analysis
To investigate evidence of accelerated aging, Student t tests and multivariate linear regression models were used to determine differences in crude and adjusted mean age, at diagnosis of each outcome and 95% confidence intervals (CIs), respectively. To correct for small differences in the age distribution of the HIV-infected and uninfected adults that resulted from differential survival in the early years of the VACS cohort, we used outcome-specific weighed multivariate linear regression models; details of this method and results can be found in the Supplementary Data.
To investigate evidence of accentuated aging, time-to-event analyses were conducted. Participants were followed from their baseline date (ie, a participant's first clinical encounter on or after 1 April 2003) to the first occurrence of the outcome of interest, death, date of last follow-up, or 31 December 2010. To describe the rate of MI, ESRD, and NADC occurrence, crude and age-specific incidence rates (IRs) and 95% CIs were estimated. To describe the relative risk of MI, ESRD, and NADC in HIV-infected vs uninfected adults, crude (HR) and adjusted hazard ratios (aHRs) and 95% CIs were estimated using Cox proportional hazards models. To test if the proportional hazards by HIV infection assumption held, we examined log-minus-log–transformed Kaplan–Meier estimates of the survival function and conducted tests for proportionality using Schoenfeld residuals; neither method indicated a deviation of the proportionality of the hazards by HIV status. To test our a priori hypothesis that HIV modifies the effect of age on our outcomes of interest, likelihood ratio tests from nested models were used to determine if the model with the interaction term provided a better fit to the data.
Separate analyses were conducted for each of the 3 outcomes of interest (MI, ESRD, and NADC). Participants could contribute data to all 3 analyses. The following variables were evaluated for each outcome: HIV status, age, race, sex, HCV infection, major depression, alcohol-related diagnosis, cocaine-related diagnosis, cigarette smoking, BMI, diabetes, hyperlipidemia, hypertension, lipid-lowering medications, antihypertensive medications, eGFR, and year of diagnosis. Multivariate models were constructed based on univariate relationships with the outcomes and prior knowledge. Analyses were conducted using Stata software version 12.0 (College Station, Texas), and a P value <.05 guided statistical interpretation.
RESULTS
From 1 April 2003 to 31 December 2010, 98 687 adults (30 564 [31%] HIV-infected and 68 123 [69%] uninfected) contributed to the analysis of at least 1 outcome. Participants with prevalent cardiovascular disease, ESRD, and NADC were excluded from the analysis of the respective outcome, resulting in total study populations of 83 527, 97 922, and 96 417 adults for our MI, ESRD, and NADC studies, respectively. A total of 689 MI, 1135 ESRD, and 4179 NADC events occurred; the distribution of age at diagnosis, by HIV status, can be found in the Supplementary Data.
The study population for each outcome had similar age distributions by HIV status due to the matched nature of the VACS (Supplementary Data). Table 1 shows baseline characteristics, by HIV status, for each outcome. Overall, greater proportions of HIV-infected adults who were infected with HCV had a cocaine-related diagnosis, were current smokers, and had an eGFR < 60 mL/minute/1.73 m2 compared with uninfected adults. In contrast, HIV-infected adults had a lower burden of other relevant comorbidities, including diabetes, hyperlipidemia, hypertension, and obesity.
Table 1.
Characteristics of HIV-Infected and Age-, Race-, and Geographical Location-Matched Uninfected Adults in the Veterans Aging Cohort Study Virtual Cohort, April 2003–December 2010
| Characteristic | MI (n=83 527) |
ESRD (n = 97 922) |
NADC (n = 96 417) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-Uninfected |
HIV-Infected |
HIV-Uninfected |
HIV-Infected |
HIV-Uninfected |
HIV-Infected |
|||||||
| n = 56 274 | n = 27 253 | n = 67 679 | n = 30 243 | n = 66 531 | n = 29 886 | |||||||
| Age | ||||||||||||
| <40 y | 7975 | 14% | 4533 | 17% | 8227 | 12% | 4599 | 15% | 8233 | 12% | 4595 | 15% |
| 40–49 y | 22 124 | 39% | 10 676 | 39% | 24 387 | 36% | 11 286 | 37% | 24 318 | 37% | 11 293 | 38% |
| 50–59 y | 20 371 | 36% | 9302 | 34% | 25 747 | 38% | 10 689 | 35% | 25 228 | 38% | 10 535 | 35% |
| 60–69 y | 4451 | 8% | 2111 | 8% | 6670 | 10% | 2676 | 9% | 6314 | 9% | 2555 | 9% |
| ≥70 y | 1353 | 2% | 631 | 2% | 2648 | 4% | 993 | 3% | 2438 | 4% | 908 | 3% |
| Race | ||||||||||||
| White | 21 316 | 38% | 10 347 | 38% | 26 486 | 39% | 11 665 | 39% | 26 004 | 39% | 11 440 | 38% |
| Black | 26 882 | 48% | 13 101 | 48% | 31 983 | 47% | 14 494 | 48% | 31 410 | 47% | 14 388 | 48% |
| Other | 8076 | 14% | 3805 | 14% | 9210 | 14% | 4084 | 14% | 9117 | 14% | 4058 | 14% |
| Male | 54 721 | 97% | 26 509 | 97% | 66 008 | 98% | 29 445 | 97% | 64 879 | 98% | 29 096 | 97% |
| Hepatitis C infectiona | 8665 | 15% | 9427 | 35% | 10 247 | 15% | 10 541 | 35% | 10 128 | 15% | 10 458 | 35% |
| Cocaine-related diagnosisb | 4016 | 7% | 3069 | 11% | 4642 | 7% | 3494 | 12% | 4599 | 7% | 3462 | 12% |
| Alcohol-related diagnosisc | 7299 | 13% | 3814 | 14% | 8654 | 13% | 4331 | 14% | 8478 | 13% | 4268 | 14% |
| Cigarette smoking | ||||||||||||
| Never | 15 533 | 28% | 6831 | 25% | 18 250 | 27% | 7427 | 25% | 17 997 | 27% | 7349 | 25% |
| Current | 27 978 | 50% | 15 342 | 56% | 33 513 | 50% | 16 972 | 56% | 32 937 | 50% | 16 790 | 56% |
| Past | 8412 | 15% | 3398 | 12% | 10 920 | 16% | 3981 | 13% | 10 659 | 16% | 3893 | 13% |
| Depressiond | 3321 | 6% | 2137 | 8% | 4167 | 6% | 2448 | 8% | 4085 | 6% | 2428 | 8% |
| BMI | ||||||||||||
| <19 kg/m2 (underweight) | 928 | 2% | 1325 | 5% | 1141 | 2% | 1476 | 5% | 1082 | 2% | 1437 | 5% |
| 19–29 kg/m2 (normal/overweight) | 32 390 | 58% | 21 582 | 79% | 38 341 | 57% | 23 783 | 79% | 37 647 | 57% | 23 510 | 79% |
| ≥30 kg/m2 (obese) | 21 368 | 38% | 3895 | 14% | 26 522 | 39% | 4497 | 15% | 26 117 | 39% | 4449 | 15% |
| Diabetese | 11 739 | 21% | 3873 | 14% | 16 872 | 25% | 4989 | 16% | 16 563 | 25% | 4889 | 16% |
| Hyperlipidemiaf | 21 645 | 38% | 9139 | 34% | 29 410 | 43% | 10 884 | 36% | 28 778 | 43% | 10 690 | 36% |
| Hypertensiong | 17 918 | 32% | 5918 | 22% | 25 390 | 38% | 7494 | 25% | 24 765 | 37% | 7411 | 25% |
| Renal impairment (eGFR <60 mL/min/1.73 m3)h | 2102 | 4% | 1611 | 6% | 3574 | 5% | 1993 | 7% | 3602 | 5% | 2105 | 7% |
| Polymorbidityi | 23 099 | 41% | 7157 | 26% | 31 563 | 47% | 8840 | 30% | 30 935 | 46% | 8840 | 30% |
| HIV+ participants | ||||||||||||
| CD4 count <200 cells/µL | … | … | 5105 | 19% | … | … | 5627 | 19% | … | … | 5553 | 19% |
| Detectable HIV RNA (≥500 copies/mL) | … | … | 10 632 | 39% | … | … | 11 628 | 38% | … | … | 11 493 | 38% |
| Clinical AIDS diagnosisj | … | … | 6803 | 25% | … | … | 7772 | 26% | … | … | 7621 | 26% |
| Prescribed ART | 12 324 | 45% | 13 838 | 46% | 13 618 | 46% | ||||||
| ART (among those prescribed) | ||||||||||||
| PI-based regimen | … | … | 5539 | 45% | … | … | 6232 | 45% | … | … | 6144 | 45% |
| NNRTI-based regimen | … | … | 5959 | 48% | … | … | 6692 | 48% | … | … | 6577 | 48% |
| Other | … | … | 826 | 7% | … | … | 914 | 7% | … | … | 897 | 7% |
Data are presented as No. (%). Characteristics measured at, or closest to, baseline. The χ2 test statistics for differences in characteristics by HIV status were all P < .001 with the exception of race (MI: P = .31; ESRD: P = .15; cancer: P = .024) and sex (MI: P = .81; ESRD: P = .12, cancer: P = .14). Comparison of CD4 cell count, HIV RNA or ART use by HIV status was not possible because these data were not collected among uninfected subjects. The characteristics with missing data, and the proportion of individuals with missing data, were as follows: cigarette smoking (MI: HIV– 8%, HIV+ 6%; ESRD: HIV– 7%, HIV+ 6%; cancer: HIV–7%, HIV+ 6%), BMI (MI: HIV– 3%, HIV+ 2%; ESRD: HIV– 2%, HIV+ 2%; cancer: HIV– 3%, HIV+ 2%), eGFR (MI: HIV– 32%, HIV+ 15%; ESRD: HIV– 30%, HIV+ 15%; cancer: HIV– 30%, HIV+ 15%), CD4 count (HIV+ only: MI 34%; ESRD 35%; cancer 35%), detectable HIV RNA (HIV+ only: MI 28%; ESRD 28%; cancer 28%).
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HCV, Hepatitis C virus; HIV, human immunodeficiency virus; MI, myocardial infarction; NADC, non-AIDS-defining cancer; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
a Hepatitis C infection was defined as a positive HCV antibody test or ≥1 inpatient and/or ≥2 outpatient International Classification of Diseases, Ninth Revision (ICD-9) codes for HCV diagnosis.
b Cocaine-related diagnosis was defined as ≥1 inpatient or outpatient ICD-9 codes for cocaine-related diagnosis.
c Alcohol-related diagnosis was defined as ≥1 inpatient or outpatient ICD-9 codes for alcohol-related diagnoses.
d Depression was defined as ≥1 inpatient or outpatient ICD-9 codes for major depression diagnoses.
e Diabetes measured as (a) glucose ≥200 mg/dL measured on 2 separate occasions, (b) ≥2 outpatient or ≥1 inpatient ICD-9 codes for diabetes and treatment with an oral hypoglycemic or insulin for ≥30 days, (c) ≥2 outpatient or ≥1 inpatient ICD-9 codes for diabetes and glucose ≥126 mg/dL measured on 2 separate occasions, or (d) glucose ≥200 mg/dL measured once and treatment with an oral hypoglycemic or insulin for ≥30 days.
f Hyperlipidemia was defined as (a) triglycerides >200 mg/dL; and/or (b) serum total cholesterol >240 mg/dL measured on 2 separate occasions.
g Hypertension was defined as ≥1 inpatient or outpatient ICD-9 codes for hypertension.
h eGFR calculated using the Modification of Diet in Renal Disease study equation.
i Polymorbidity was defined by counting the number of chronic conditions (hepatitis C, depression, BMI, diabetes, hyperlipidemia, hypertension, renal impairment measured as eGFR <60 mL/min/1.73 m3) measured within each individual.
j Clinical AIDS diagnoses was defined by ICD-9 codes for AIDS-defining illnesses, including pneumocystis pneumonia, tuberculosis, mycobacterium, cytomegalovirus, HIV wasting, HIV dementia, candidiasis, cryptococcosis, toxoplasmosis of the brain, coccidiomycosis, histoplasmosis, isosporiasis, herpes zoster, herpes simplex, bacterial pneumonia, or Kaposi sarcoma.
Results for the Outcome of MI
A total of 83 527 adults contributed 453 982 person-years of follow-up to the analysis of MI (median follow-up, 5.3 and 5.5 years in HIV-infected and uninfected adults). There were 291 and 398 MI events in HIV-infected and uninfected adults, with a mean age at diagnosis of 56.2 and 56.0 years, respectively (P = .84). After adjustment for confounders, there continued to be no difference in mean age at MI diagnosis, with an adjusted mean difference of −0.11 (95% CI, −.59 to .37) years in HIV-infected compared with uninfected adults. The lack of a difference persisted when weighted linear regression models were used to correct for the small difference in the age distributions by HIV status (Supplementary Data).
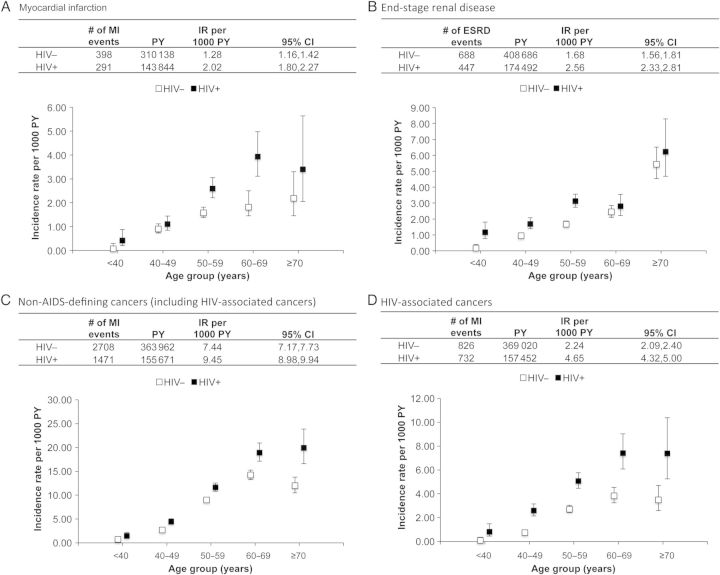
The crude IR of MI was 2.02 (95% CI, 1.80–2.27) and 1.28 (95% CI, 1.16–1.42) per 1000 person-years in HIV-infected and uninfected adults, respectively. Comparing HIV-infected with uninfected adults, the IRs were similar at age <50 years, but higher in HIV-infected adults among those age ≥50 years (Figure 1A).
Figure 1.
Overall and age-specific incidence rates (IRs) (and 95% confidence intervals [CIs]) for myocardial infarction (MI) (A), end-stage renal disease (ESRD) (B), non-AIDS-defining cancers (including human immunodeficiency virus [HIV]-associated cancers) (C), and HIV-associated cancers (D), by HIV status, Veterans Aging Cohort Study Virtual Cohort, April 2003–December 2010. HIV-associated cancers included anal, oral, penis, Hodgkin lymphoma, liver, and lung cancers. To estimate the person-years (PY) denominator for the IRs, participants were followed from their baseline date (ie, a participant's first clinical encounter on or after 1 April 2003) to the first occurrence of the outcome of interest, death, date of last follow-up, or 31 December 2010. Non-AIDS-defining cancers included anal, bladder, brain and nervous system, breast, colorectal, esophageal, Hodgkin lymphoma, kidney, larynx, leukemia (lymphoid and myeloid), liver, lung, melanoma, myeloma, oral cavity and pharynx, pancreatic, penis, prostate, soft tissue, stomach, testicular, thyroid (there were no cases of vulvar cancer).
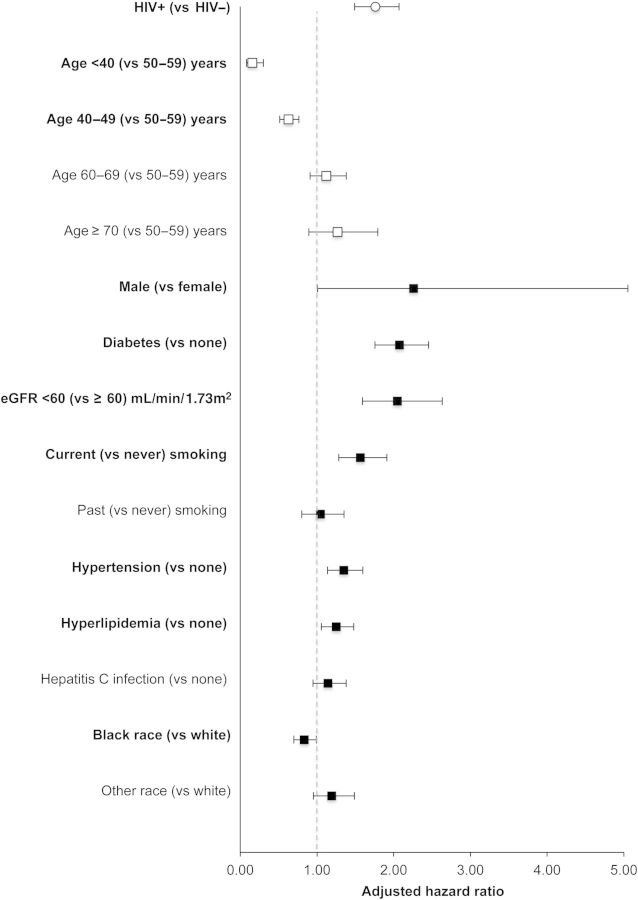
The risk of MI was also significantly higher in HIV-infected compared with uninfected adults after adjustment for confounders (aHR, 1.76 [95% CI, 1.49–2.07]) (Figure 2; see Supplementary Data for point estimates and 95% CIs). The interaction of age and HIV was not statistically significant in multivariate Cox proportional hazards models (P = .086). Factors with a greater magnitude of association than HIV included male sex, diabetes, hyperlipidemia, and eGFR <60 mL/minute/1.73 m2.
Figure 2.
Adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for myocardial infarction, Veterans Aging Cohort Study Virtual Cohort, April 2003–December 2010. Bold signifies statistically significant relationships with the outcome. aHRs and 95% CIs were estimated using Cox proportional hazards models. See Table 1 for definitions and measurement descriptions of the confounders depicted in the figure. Confounders with missing data were modeled with a separate category for missing. See the Supplementary Data for enumerated univariate (not shown) and multivariate (shown in this figure) point estimates and 95% CIs. Abbreviations: eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus.
Results for the Outcome of ESRD
A total of 97 922 adults contributed 583 178 person-years of follow-up to the analysis of ESRD (median follow-up, 5.3 and 5.5 years in HIV-infected and uninfected adults). There were 447 and 688 ESRD events in HIV-infected and uninfected adults, with a mean age at diagnosis of 56.0 years and 59.4 years, respectively (P < .001; Table 2). After adjustment for confounders, HIV-infected adults were diagnosed with ESRD at a mean age that was 5.5 months younger than uninfected adults (adjusted mean difference, −0.46 [95% CI, −.86 to −.07] years. The difference in age at diagnosis persisted when weighted linear regression models were used to correct for the small difference in the age distributions by HIV status (Supplementary Data).
Table 2.
Crude Mean Age and Adjusted Mean Difference in Age at Diagnosis—Veterans Aging Cohort Study Virtual Cohort, April 2003–December 2010
| Event | No. of Adults | No. of Diagnoses | Crude Mean Age, y, at Diagnosis | Crude Mean Difference in Age, y, at Diagnosis | P Valuea | Adjusted Difference in Mean Age, y, at Diagnosisb | 95% CI |
|---|---|---|---|---|---|---|---|
| Myocardial infarction | |||||||
| HIV− | 56 274 | 398 | 56.0 | 0.2 | .84 | … | … |
| HIV+ | 27 253 | 291 | 56.2 | −0.11 | –.59 to .37 | ||
| End-stage renal disease | |||||||
| HIV− | 67 679 | 688 | 59.4 | −3.4 | <.001 | … | … |
| HIV+ | 30 243 | 447 | 56.0 | −0.46 | –.86 to –.07 | ||
| Non-AIDS defining cancersc | |||||||
| HIV− | 66 531 | 2708 | 58.9 | −1.1 | <.001 | … | … |
| HIV+ | 29 886 | 1471 | 57.8 | −0.10 | –.30 to .10 | ||
| HIV-associated (anal, oral, penis, Hodgkin lymphoma, liver, lung cancers) | |||||||
| HIV− | 66 531 | 826 | 58.6 | −2.0 | <.001 | … | … |
| HIV+ | 29 886 | 732 | 56.6 | −0.22 | –.52 to .08 | ||
Bold signifies statistically significant relationships with the outcome.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
a P values for the difference in crude mean age at diagnosis were estimated using the Student t test for a difference in means.
b Adjusted difference in mean age (in years) at diagnosis was estimated using linear regression models. For myocardial infarction and end-stage renal disease, models were adjusted for race, sex, hepatitis C infection, body mass index (BMI), alcohol use, smoking, anemia, diabetes, hyperlipidemia, hypertension, antihypertensive medications, and statin use. Linear regression models for age at cancer was adjusted for race, sex, hepatitis C infection, BMI, alcohol use, smoking, anemia, diabetes, and year of cancer diagnosis. Confounders with missing data had a category for missing and were included in the models. Please see Table 1 for definitions and measurement descriptions of the confounders.
c Non-AIDS-defining cancers included anal, bladder, brain and nervous system, breast, colorectal, esophageal, Hodgkin lymphoma, kidney, larynx, leukemia (lymphoid and myeloid), liver, lung, melanoma, myeloma, oral cavity and pharynx, pancreatic, penis, prostate, soft tissue, stomach, testicular, thyroid. There were no cases of vulvar cancer.
The crude IR of ESRD was 2.56 (95% CI, 2.33–2.81) per 1000 person-years in HIV-infected adults and 1.68 (95% CI, 1.56–1.81) per 1000 person-years in uninfected adults. Age-stratified crude IRs showed higher rates of ESRD among HIV-infected participants at all ages (Figure 1B).
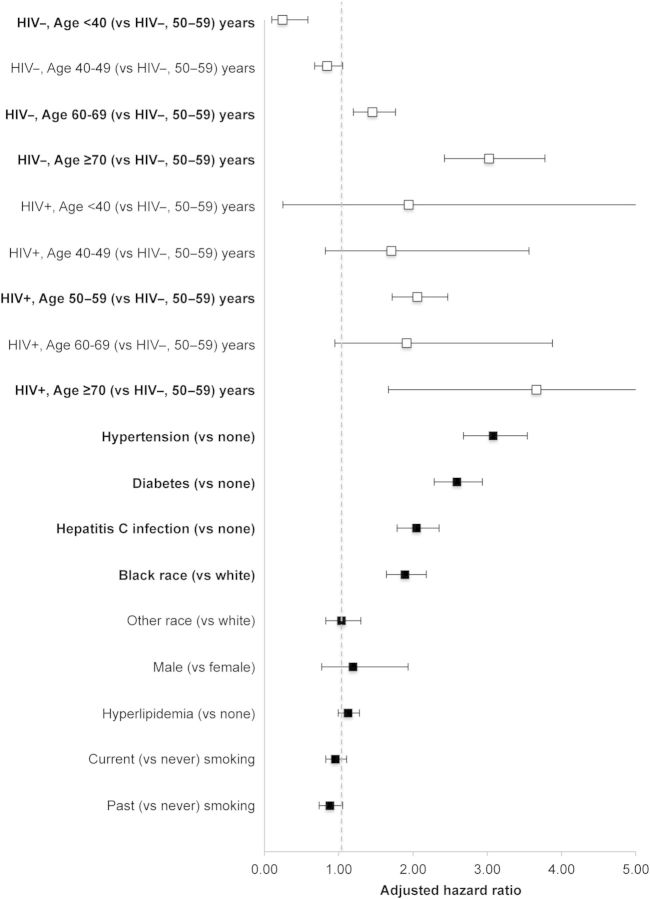
In multivariate Cox proportional hazards models, HIV modified the effect of age on ESRD (P for interaction = .0002, Figure 3; see Supplementary Data for stratified analyses). Factors with ≥2-fold increase in risk of ESRD (other than HIV and age) included HCV infection, diabetes, and hypertension.
Figure 3.
Adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for end-stage renal disease, by human immunodeficiency virus (HIV) status, Veterans Aging Cohort Study Virtual Cohort, April 2003–December 2010. Bold signifies statistically significant relationships with the outcome. aHRs and 95% CIs were estimated using Cox proportional hazards models. See Table 1 for definitions and measurement descriptions of the confounders depicted in the figure. Confounders with missing data were modeled with a separate category for missing. See the Supplementary Data for enumerated univariate (not shown) and multivariate (shown in this figure) point estimates and 95% CIs.
Results for the Endpoint of NADC
A total of 96 417 adults contributed 519 632 person-years of follow-up to the analysis of NADC (median follow-up, 5.2 and 5.5 years in HIV-infected and uninfected adults). There were 1471 and 2708 NADC events in HIV-infected and uninfected adults, respectively. The most commonly diagnosed cancer was prostate cancer (n = 1365), followed by lung (n = 802), liver (n = 336), colorectal (n = 308), oral cavity and pharynx (n = 232), kidney (n = 179), anal (n = 123), pancreatic (n = 108), and larynx (n = 102); all other cancer types had <100 diagnoses each (Supplementary Data). The mean age at diagnosis was 57.8 years in HIV-infected and 58.9 years in uninfected adults (P < .001; Table 2). After adjustment for confounders, there was no statistically significant difference in mean age at NADC diagnosis, with an adjusted mean difference of −0.10 (95% CI, −.30 to .10) years. The difference was similar when weighted linear regression models were used to correct for the small differences in the age distributions by HIV status (Supplementary Data).
The crude NADC IR per 1000 person-years was 9.45 (95% CI, 8.98–9.94) in HIV-infected and 7.44 (95% CI, 7.17–7.73) in uninfected adults; rates of HIV-associated cancers were 4.65 (95% CI, 4.32–5.00) and 2.24 (95% CI, 2.09–2.40) per 1000 person-years, respectively. Similar to MI IRs, NADC rates show a greater rate among HIV-infected adults at all ages, with more pronounced differences in older ages (Figure 1C); this was driven by the HIV-associated cancers (Figure 1D).
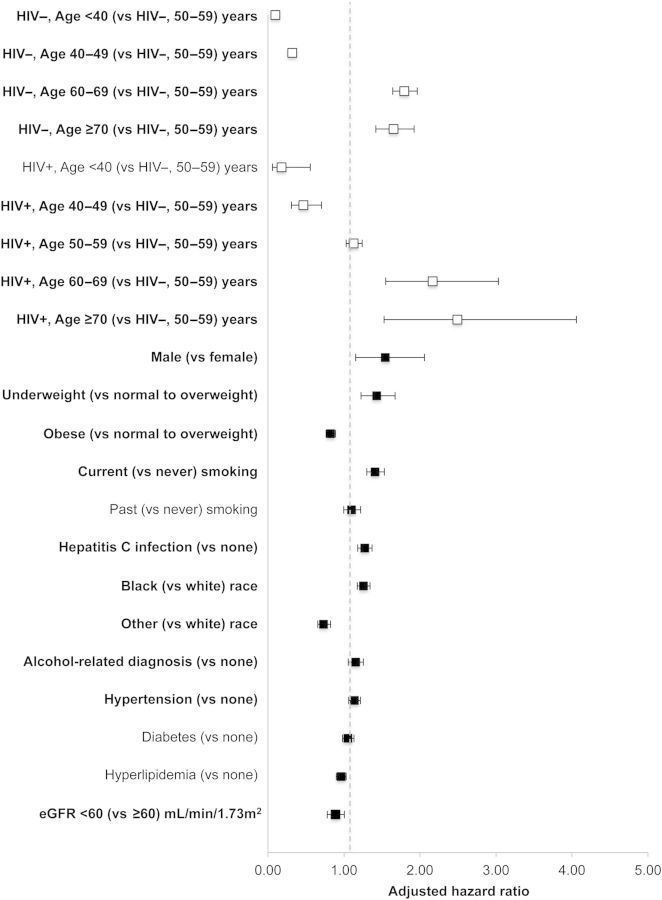
In multivariate Cox proportional hazards models, HIV modified the effect of age on NADC (P for interaction = .034; Figure 4; see Supplementary Data for stratified analyses). Factors with a similar magnitude of effect as HIV and age included male sex, current smoking status, and BMI.
Figure 4.
Adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for non-AIDS-defining cancers (including human immunodeficiency virus [HIV]-associated cancers), by HIV status, Veterans Aging Cohort Study Virtual Cohort, April 2003–December 2010. Bold signifies statistically significant relationships with the outcome. See Table 1 for definitions and measurement descriptions of the confounders depicted in the figure. Confounders with missing data were modeled with a separate category for missing. aHRs and 95% CIs were estimated using Cox proportional hazards models. See the Supplementary Data for enumerated univariate (not shown) and multivariate (shown in this figure) point estimates and 95% CIs. Abbreviation: eGFR, estimated glomerular filtration rate.
Sensitivity analyses included removing lung cancer from HIV-associated cancers and investigating type-specific NADCs; these analyses supported the inferences of the grouped NADC outcome.
DISCUSSION
This study answers 2 important questions in the field of aging with HIV: compared with demographically and behaviorally similar uninfected adults, are those with HIV (1) likely to develop aging-associated diseases at younger ages and (2) at greater risk for these diseases? Contrary to some previous studies concluding a dramatically younger age at diagnosis for MI and cancer, we found no clinically meaningful difference in the age at diagnoses for MI, ESRD, and NADC after controlling for differences in age distribution by HIV status and other important risk factors. Our study does, however, support previous findings of an increased risk of MI, ESRD, and NADC in HIV-infected compared to uninfected adults, although our estimates are attenuated compared to previous estimates. Our data show that HIV is a risk factor for these outcomes; however, many traditional risk factors have a similar or greater magnitude of relationship with these outcomes. With respect to the validated, clinically relevant diagnoses of MI, ESRD, and NADC, our results support accentuated, not accelerated, aging in HIV-infected adults compared with similar uninfected adults after employing a consistent analytic approach and appropriate adjustment for confounding.
The hypothesis of accelerated aging has been contentiously debated in the field of aging with HIV [5, 6, 20–22]. Our findings within an age-matched population demonstrate that HIV-infected adults are diagnosed with ESRD at ages 5.5 months younger than uninfected adults, but no clinically significant differences in mean age at diagnosis of MI, ESRD, or NADC by HIV status after accounting for important traditional risk factors.
Our estimates of the relationships of HIV with ESRD and NADC were attenuated compared with previous studies [11–13, 23, 24]. This attenuation may be attributed to the richer data allowing for more complete adjustment of many important confounders that were omitted from other studies. The risk of MI in HIV-infected and uninfected adults continues to be challenging to estimate, with recent estimates ranging from a relative risk of 1.4 (95% CI, 1.3–1.6) among those with access to care to 1.75 (95% CI, 1.51–2.02) in a healthcare system–based cohort [25, 26]. A previously published study of MI in the VACS that had a shorter follow-up time and a similar (but not the same) definition of MI showed relative risk of MI ranging from 1.48 (95% CI, 1.27–1.72) to 1.72 (95% CI, 1.49–1.97) depending upon the confounders considered in the multivariate models [27].
There have been a number of hypotheses suggested to explain the increased risk of aging-associated disease among HIV infected adults, one of them being higher prevalence of traditional risk factors such as cigarette smoking [28], high blood pressure [29], dyslipidemia [30], oncogenic viral infections [31–33], male sex, black race, and lower socioeconomic status [34] compared with the general population. Our findings suggest that traditional risk factors do play an important and independent role in aging-associated diseases. Many traditional risk factors were associated with a greater magnitude of risk compared with HIV in multivariate models. Targeting traditional risk factors may help decrease the excess burden of aging-related diseases among HIV-infected adults. Studies to estimate the proportion of these outcomes avoided with reduction of these risk factors, and intervention studies that estimate the reduction of these outcomes due to reduction of these risk factors, are needed to inform interventions.
Other causes of comorbidities in HIV-infected adults include HIV itself or ART. We did not attempt to differentiate a treated vs nontreated HIV relationship in this study; however, univariate relationships of ART regimen classes used at baseline were statistically significantly associated with the outcomes of interest (Supplementary Data).
Whether increased risk of these age-associated diseases should lead to increased screening among HIV-infected adults is not a simple determination. The decision to screen and treat must balance potential benefits from early diagnosis and treatment and potential harms from diagnostic tests and treatment toxicity. Whereas increased risk of a condition often increases the potential benefit to screening and treatment, it only does so when risks from screening and potential toxicity from additional treatment are not also proportionately increased [35]. However, we find no conclusive evidence to suggest that screening for these aging-associated outcomes should occur at younger ages in HIV-infected compared with uninfected adults.
This study has strong internal validity, which is essential to determining the relationship of HIV and these aging-associated outcomes. The internal validity of the study is founded on a comparison to a demographically and behaviorally similar uninfected group of age-matched adults with similar access to care (as opposed to comparison with the general population of presumed uninfected adults with differential disease detection) and reinforced with rich data, allowing for more complete adjustment for traditional risk factors not included in prior studies. Another strength includes the identification of outcomes of interest using clinical measures (MI), Medicare data (ESRD), and cancer registry data (NADC), rather than ICD-9 diagnostic codes. Finally, the study population size allowed for capture of a large number of incident events.
Our study is not without limitations. Most important, we address the mean age at which individual age-related diseases occurred. Prior research has demonstrated that those aging with HIV may be susceptible to geriatric syndromes, including multimorbidity, frailty, and polypharmacy, at earlier ages [36–38]. Second, generalizability of our findings may be limited to men and to individuals without significant barriers to healthcare. Third, by combining NADC into a single composite outcome, we may be masking differences among individual cancers. Sensitivity analyses of type-specific cancers supported the inferences of the NADC outcome. Fourth, we were not able to adjust for time since HIV infection, which is typically not captured in HIV clinical cohorts. Finally, only 9 MI, 26 ESRD, 45 NADC, and 17 HIV-associated NADC and 44 000 person-years (9% of total person-time) were observed in the <40-year-old age group. Although we have no reason to believe there is differential capture of events by age group or by HIV status, differential incomplete capture of events in this youngest group compared to the older groups could mask an accelerated aging.
Our findings show no clinically meaningful difference in the age at diagnosis for MI, ESRD, and NADC in HIV-infected compared with similar uninfected adults. Compared with previous estimates of the increased risk of these aging-associated conditions previously associated with HIV, our findings show an attenuated HIV relationship after careful consideration of age and other important risk factors. These findings should reassure HIV-infected patients that they are unlikely to experience these conditions decades earlier than those aging without HIV. Continued investigations of the mechanisms of the HIV effect on age-associated conditions, and impact of lifestyle interventions on aging-associated disease risk, are needed to inform clinical management of adults aging with HIV.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. K. A. M. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Financial support. The Veterans Aging Cohort Study is supported by the National Institutes of Health: National Institute on Alcohol Abuse and Alcoholism (grant numbers U24-AA020794, U01-AA020790, U01-AA020795, U01-AA020799, U24-AA022001, U24 AA022007, U10 AA013566-completed); National Heart, Lung, and Blood Institute (grant numbers R01-HL095136; R01-HL090342); National Institute of Allergy and Infectious Diseases (NIAID) (grant number U01-A1069918); National Institute of Mental Health (grant number P30-MH062294); National Institute on Drug Abuse (grant number R01DA035616); National Cancer Institute (NCI) (grant number R01 CA173754); Agency for Healthcare Research and Quality (grant number R01-HS018372); and the Veterans Health Administration Office of Research and Development (grant number VA REA 08-266, VA IRR Merit Award) and Office of Academic Affiliations (Medical Informatics Fellowship). Additional funding for this work includes K01-AI093197 funded by the NIAID (to K. N. A.), F31-CA180775 funded by the NCI (to L. S. P.), P01-DK056492 funded by the National Institute of Diabetes and Digestive and Kidney Diseases (to C. M. W.), VA I01 RX000667 funded by the Department of Veterans Affairs, Veterans Health Administration (to K. K. O.), and the Intramural Research Program of the National Cancer Institute (to M. S. S.).
Potential conflicts of interest. C. M. W. reports consulting for BMS. S. J. G. reports serving on a data and safety monitoring board that is financed by Merck. K. A. G. reports receiving grant funding from Tibotec and consulting for Tibotec and BMS. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.May MT, Ingle SM. Life expectancy of HIV-positive adults: a review. Sexual Health. 2011;8:526–33. doi: 10.1071/SH11046. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The HIV and Aging Consensus Project. American Academy of HIV Medicine; Recommended treatment strategies for clinicians managing older patients with HIV. Available at: http://aahivm.org/Upload_Module/upload/HIV%20and%20Aging/Aging%20report%20working%20document%20FINAL%2012.1.pdf. Accessed 20 October 2014. [Google Scholar]
- 5.Martin J, Volberding P. HIV and premature aging: a field still in its infancy. Ann Intern Med. 2010;153:477–9. doi: 10.7326/0003-4819-153-7-201010050-00013. [DOI] [PubMed] [Google Scholar]
- 6.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69:833–42. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 8.Every NR, Fihn SD, Sales AE, Keane A, Ritchie JR. Quality Enhancement Research Initiative in ischemic heart disease: a quality initiative from the Department of Veterans Affairs. Med Care. 2000;38(6 suppl 1):I49–59. [PubMed] [Google Scholar]
- 9.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 10.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24:1383–8. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 11.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;148:728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 12.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–22. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–45. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–9. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraemer KL, McGinnis KA, Skanderson M, et al. Alcohol problems and health care services use in human immunodeficiency virus (HIV)-infected and HIV-uninfected veterans. Med Care. 2006;44(8 suppl 2):S44–51. doi: 10.1097/01.mlr.0000223703.91275.78. [DOI] [PubMed] [Google Scholar]
- 16.Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;19(suppl 3):S99–105. doi: 10.1097/01.aids.0000192077.11067.e5. [DOI] [PubMed] [Google Scholar]
- 17.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004;40:115–9. doi: 10.1002/hep.20289. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–33. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Justice AC, Braithwaite RS. Lessons learned from the first wave of aging with HIV. AIDS. 2012;26(suppl 1):S11–8. doi: 10.1097/QAD.0b013e3283558500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–9. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas GM, Mehta SH, Atta MG, et al. End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS. 2007;21:2435–43. doi: 10.1097/QAD.0b013e32827038ad. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65:160–6. doi: 10.1097/QAI.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 26.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824–35. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan RC, Kingsley LA, Sharrett AR, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis. 2007;45:1074–81. doi: 10.1086/521935. [DOI] [PubMed] [Google Scholar]
- 30.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients—association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 31.Beachler DC, Weber KM, Margolick JB, et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev. 2012;21:122–33. doi: 10.1158/1055-9965.EPI-11-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Baarle D, Hovenkamp E, Dukers NHTM, et al. High prevalence of Epstein-Barr virus type 2 among homosexual men is caused by sexual transmission. J Infect Dis. 2000;181:2045–9. doi: 10.1086/315521. [DOI] [PubMed] [Google Scholar]
- 33.Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49(5 suppl):S138–45. doi: 10.1002/hep.22883. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. HIV surveillance report. 2012;Vol. 24 Available at: http://www.cdc.gov/hiv/library/reports/surveillance/ Published November 2014. Accessed 11 November 2014. [Google Scholar]
- 35.Sigel K, Dubrow R, Silverberg M, Crothers K, Braithwaite RS, Justice AC. Cancer screening in patients infected with HIV. Curr HIV AIDS Rep. 2011;8:142–52. doi: 10.1007/s11904-011-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45:1593–601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onen NF, Overton ET. A review of premature frailty in HIV-infected persons; another manifestation of HIV-related accelerated aging. Curr Aging Sci. 2011;4:33–41. [PubMed] [Google Scholar]
- 38.Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging. 2013;30:613–28. doi: 10.1007/s40266-013-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.