Abstract
Background
Infant responses to vaccines can be impeded by maternal antibodies and immune system immaturity. It is therefore unclear whether human immunodeficiency virus type 1 (HIV-1) vaccination would elicit similar responses in adults and infants.
Method
HIV-1 Env–specific antibody responses were evaluated in 2 completed pediatric vaccine trials. In the Pediatric AIDS Clinical Trials Group (PACTG) 230 protocol, infants were vaccinated with 4 doses of Chiron rgp120 with MF59 (n = 48), VaxGen rgp120 with aluminum hydroxide (alum; n = 49), or placebo (n = 19) between 0 and 20 weeks of age. In PACTG 326, infants received 4 doses of ALVAC-HIV-1/AIDSVAX B/B with alum (n = 9) or placebo (n = 13) between 0 and 12 weeks of age.
Results
By 52 weeks of age, the majority of maternally acquired antibodies had waned and vaccine Env-specific immunoglobulin G (IgG) responses in vaccinees were higher than in placebo recipients. Chiron vaccine recipients had higher and more-durable IgG responses than VaxGen vaccine recipients or ALVAC/AIDSVAX vaccinees, with vaccine-elicited IgG responses still detectable in 56% of recipients at 2 years of age. Remarkably, at peak immunogenicity, the concentration of anti-V1V2 IgG, a response associated with a reduced risk of HIV-1 acquisition in the RV144 adult vaccine trial, was 22-fold higher in Chiron vaccine recipients, compared with RV144 vaccinees.
Conclusion
As exemplified by the Chiron vaccine regimen, vaccination of infants against HIV-1 can induce robust, durable Env-specific IgG responses, including anti-V1V2 IgG.
Keywords: HIV-1, vaccine, infants, antibodies
(See the editorial commentary by Gray and Corey on pages 501–3.)
Breast milk–mediated transmission is responsible for almost half of the 260 000 annual pediatric human immunodeficiency virus type 1 (HIV-1) infections [1, 2]. These infections mostly occur in resource-limited areas where breast-feeding is the safest nutritional option to maximize the survival of infants [3]. Although it is established that antiretroviral therapy can reduce the risk of breast milk–mediated transmission [4], this strategy faces implementation and adherence challenges that limit its effectiveness. The global plan to reduce the incidence of HIV-1 transmission to infants born to HIV-1–infected mothers to <5% by 2015 requires prevention of mother-to-child transmission services to reach >90% of HIV-1–infected mothers. However, antiretroviral prophylaxis coverage for HIV-1–infected mothers remains <65% in low- and middle-income countries [1]. Thus, an effective infant HIV-1 vaccine would significantly contribute to the efforts to eliminate pediatric HIV-1.
The ability of infants to mount immune responses following vaccination differs from that of adults owing to the presence of maternal antibodies and immaturity of the infant immune system [5]. Because of these differences, it is unclear whether HIV-1 vaccine discoveries in adults will be applicable to infants. Importantly, the RV144 vaccine trial involving Thai adults demonstrated an estimated vaccine efficacy of 31% [6]. The immune correlate analysis of this trial revealed an association between high levels of immunoglobulin G (IgG) against the Env V1V2 loops and reduced HIV-1 acquisition risk, whereas high levels of plasma Env-specific immunoglobulin A (IgA) were associated with an increased risk of HIV-1 acquisition [7–9]. To investigate whether infant vaccination can induce similar immune responses, we assessed the binding and functional antibody responses in HIV-1–exposed vaccinated infants from the historical Pediatric AIDS Clinical Trials Group (PACTG) 230 and 326, 2 of the 3 neonatal HIV-1 vaccine trials conducted to date.
MATERIALS AND METHODS
Study Population
PACTG 230 enrolled infants born to HIV-1–infected mothers in the United States to determine the safety and immunogenicity of 2 recombinant, monomeric gp120 subunit protein vaccines: rgp 120-MN adsorbed into aluminum hydroxide (alum; VaxGen) and rgp120-SF2 with MF59 adjuvant (Novartis Vaccines [formally Chiron]) [10, 11]. PACTG 326 enrolled a similar population to determine the safety and immunogenicity of ALVAC HIV-1 vaccine alone or with rgp120 boost (Table 1 and Supplementary Materials) [12]. Both studies were phase 1/2, randomized, and placebo controlled. As no vaccine-elicited antibody response was detected in infants vaccinated with ALVAC alone, only results from the ALVAC/AIDSVAX vaccinees are reported. PACTG 230/326 participants were included on the basis of sample availability in the IMPAACT repository. This retrospective study was approved by the institutional review board at Duke University.
Table 1.
Study Population and Immunization Schedules
| Group | Vaccine/Adjuvant | Subjects, No. | Immunization Schedule |
|---|---|---|---|
| PACTG 230 | |||
| VaxGen regular | rgp120 (MN) + alum | 40 | Birth and ages 4, 8, and 20 weeks |
| VaxGen accelerated/optimal | rgp120 (MN) + alum | 9 | Birth and ages 2, 8, and 20 weeks |
| Chiron regular | rgp120 (SF-2) + MF-59 | 39 | Birth and ages 4, 8, and 20 weeks |
| Chiron accelerated/optimal | rgp120 (SF-2) + MF-59 | 9 | Birth and ages 2, 8, and 20 weeks |
| Placebo | Alum | 6 | Birth and ages 4, 8, and 20 weeks |
| 1 | Birth and ages 2, 8, and 20 weeks | ||
| MF-59 | 8 | Birth and ages 4, 8, and 20 weeks | |
| 4 | Birth and ages 2, 8, and 20 weeks | ||
| PACTG 326 | |||
| ALVAC | Vcp205-HIV-1 | 13 | Birth and ages 4, 8, and 12 weeks |
| Vcp1452-HIV-1 | 8 | Birth and ages 4, 8, and 12 weeks | |
| ALVAC/AIDSVAX B/B | Vcp1452-HIV-1/rgp120 (GNE8/MN) + alum | 9 | Birth and ages 4, 8, and 12 weeks |
| Placebo | Sodium chloride (vcp205 placebo) | 5 | Birth and ages 4, 8, and 12 weeks |
| Sodium chloride (vcp1452 placebo) | 3 | Birth and ages 4, 8, and 12 weeks | |
| Sodium chloride/alum (ALVAC + AIDSVAX placebo) | 3 | Birth and ages 4, 8, and 12 weeks | |
Abbreviation: PACTG, Pediatric AIDS Clinical Trials Group.
Measurement of Env-Specific Antibody Responses by Binding Antibody Multiplex Assays
Env-specific IgG responses were measured as previously described [7, 13], using plasma/sera diluted 1:100 against a panel of antigens, including MN gp120 (vaccine strain), 92TH023 gp120 (RV144 vaccine strain), A244 gp120 (RV144 vaccine strain), B con gp140 CF (clade B consensus), and gp70 B case A V1V2 (obtained from Drs B. Haynes and H. Liao); GNE8 rgp120 Q pool (vaccine strain obtained from Dr Berman); and 4403 bmc5 gp120 (clade C postnatal transmitted/founder Env) [14]. IgG1 and IgG3 were measured against gp70 B case A V1V2 at a plasma/sera dilution of 1 in 40. For IgA measurement, samples were depleted of IgG by using protein G columns and were tested at a 1:10 dilution. The mean fluorescence intensity (MFI) of blank beads was subtracted to determine the level of antigen-specific binding, and the reporting criteria included a coefficient of variation between replicates ≤20 and a minimum of 100 beads counted per sample/antigen.
Neutralization
Neutralization assays were performed in TZM-bl cells (National Institutes of Health [NIH] AIDS Reagent Program, from J. Kappes and X. Wu) against 2 clade B HIV-1 tier 1 Env-pseudotype viruses [HIV-1 MN.3 (B. MN) and HIV-1 SF162.LS (B. SF162)] and a nonspecific retrovirus (murine leukemia virus [MLV]) as previously described [15]. The median infectious dose (ID50) was determined, and the cutoff for positivity was set at 3 times above the ID50 against an MLV background.
Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
ADCC activity was measured using the Luciferase ADCC assay as previously described [16]. CEM.NKRCCR5 target cells (NIH AIDS Reagent Program, from Dr A. Trkola) were infected with a replication-competent infectious molecular clone designed to encode the HIV-1 SF162.LS env gene in cis within an isogenic backbone that also expresses the Renilla luciferase reporter gene and preserves all viral open reading frames [17]. Effector cells were peripheral blood mononuclear cells isolated from an HIV-1–seronegative donor heterozygous for 158F/V polymorphic variants of Fcγ receptor 3A.
Statistical Analysis
Available samples from PACTG 230 and 326 were analyzed, excluding samples from 6 HIV-1–infected participants. For PACTG 230, all vaccine regimens and doses were pooled in the analysis, except for comparison of accelerated and regular regimens. The Wilcoxon rank sum test was used for comparing the magnitude of antibody responses, and the Fisher exact test was used to compare the frequency of responders between groups. All analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina), and graphs were produced using the R software. A detailed description of the statistical analyses is provided in Supplementary Materials.
RESULTS
Decline of Maternally Acquired HIV-1 Env–Specific Antibodies
To differentiate between maternally acquired and vaccine-elicited antibodies, gp41-specific IgG levels were measured over time. As expected for maternally acquired antibodies, the magnitude of anti-gp41 IgG decreased over 1 year (Figure 1A). At 52 weeks of age, these antibodies were detected in only 7%–21% of vaccine or placebo recipients, and the magnitude represented <3% of that present at birth, in accordance with previous reports [12]. Therefore, we focused the vaccine-elicited antibody response analysis on 24 (peak immunogenicity in PACTG 230) and 52 weeks of age, when the measured Env-specific IgG was primarily vaccine elicited.
Figure 1.
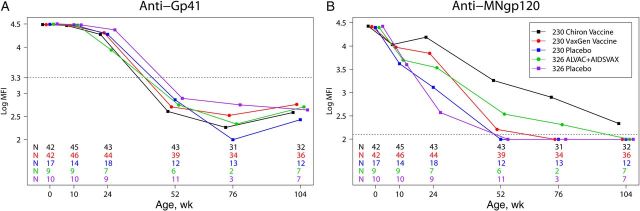
Human immunodeficiency virus type 1 (HIV-1) Env vaccination induces durable immunoglobulin G (IgG) responses in infants. A, There was no difference in the kinetics of the decline of maternal anti-gp41 IgG antibody level between vaccine and placebo recipients. B, The majority of Chiron vaccine recipients had detectable anti-gp120 IgG responses at week 104 of age, whereas the response rapidly waned in VaxGen vaccine recipients and ALVAC/AIDSVAX recipients. Symbols represent the median IgG response of a vaccine/placebo group. The dashed line represents the positivity cutoff, defined as the mean value + 3 SDs of the mean fluorescence intensity (MFI) of a panel of 60 HIV-1–negative plasma specimens. The number of infant samples available for each vaccine group at each time point is shown on the graph (colors match the legend). Abbreviations: SD, standard deviation; 230, Pediatric AIDS Clinical Trials Group 230; 326, Pediatric AIDS Clinical Trials Group 326.
Induction of Durable HIV-1 Env–Specific IgG Responses
The durability of vaccine-elicited responses in infants was examined by measuring anti-MN gp120 IgG over the first 2 years of life (Figure 1B). At 24 weeks of age, the magnitude of the response was significantly higher in recipients of the VaxGen vaccine (P < .001) and the Chiron vaccine (P < .001) than in placebo recipients. At 52 weeks of age, while anti-MN gp120 IgG was no longer detected in placebo recipients, this response was still present in 59% of VaxGen vaccine recipients and 79% of Chiron vaccine recipients. Moreover, at week 104 of age, 28% and 56% of VaxGen vaccine and Chiron vaccine recipients, respectively, had a detectable response. The half-life of anti-MN gp120 IgG was 15.1 and 21.3 weeks in recipients of the VaxGen and Chiron vaccines, respectively, and was 7 weeks in placebo recipients, suggesting that vaccine-elicited responses lasted several months after levels of maternally acquired antibodies waned. While the magnitude of the anti-MN gp120 response did not differ between ALVAC/AIDSVAX vaccinees and placebo recipients at week 24 of age, at week 52 of age, 83% of ALVAC/AIDSVAX vaccinees but only 9% of placebo recipients had a detectable response (Table 2). Only 1 of 7 ALVAC/AIDSVAX vaccinees (14%) had detectable MNgp120 IgG at 104 weeks of age. The half-life of the MN gp120 IgG response was 9.5 weeks in ALVAC/AIDSVAX vaccinees and 8.4 weeks in PACTG 326 placebo recipients. These results suggest that Env vaccination could induce IgG responses that span the duration of breast-feeding.
Table 2.
Proportion of HIV-1 Exposed Vaccinated Infants With Detectable Immunoglobulin G Responses Against a Multiclade Panel of Human Immunodeficiency Virus Envelope Constructs at Week 52 of Age
| Antigen | Clade | PACTG 230 |
P (VaxGen vs Chiron Vaccines) | PACTG 326 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 12) | VaxGen Vaccine (n = 39) | P Value | Chiron Vaccine (n = 43) | P Value | Placebo (n = 11) | ALVAC/AIDSVAX (n = 6) | P Value | |||
| MN gp120 | B | 0 (0) | 23 (59) | <.001 | 34 (79) | <.001 | .06 | 1 (9) | 5 (83) | .005 |
| GNE8 gp120 | B | 0 (0) | 7 (18) | .18 | 18 (42) | .005 | .03 | 0 (0) | 3 (50) | .03 |
| 92Th023 gp120 | AE | 0 (0) | 3 (8) | 1.00 | 11 (26) | .10 | .04 | 0 (0) | 0 (0) | |
| A244 gp120 | AE | 0 (0) | 7 (18) | .18 | 25 (58) | <.001 | <.001 | 0 (0) | 2 (33) | .11 |
| 4403 BMC5 gp120 | C | 0 (0) | 7 (18) | .18 | 24 (56) | <.001 | <.001 | 0 (0) | 1 (17) | .35 |
| B Con gp140 CF | B | 0 (0) | 1 (3) | 1.00 | 19 (44) | .004 | <.001 | 0 (0) | 0 (0) | |
Data are no. (%) of subject. P values were determined by the Fisher exact test. A P value of < .05 is considered statistically significant.
Abbreviation: PACTG, Pediatric AIDS Clinical Trials Group.
Breadth of Vaccine-Elicited Env-Specific IgG Responses
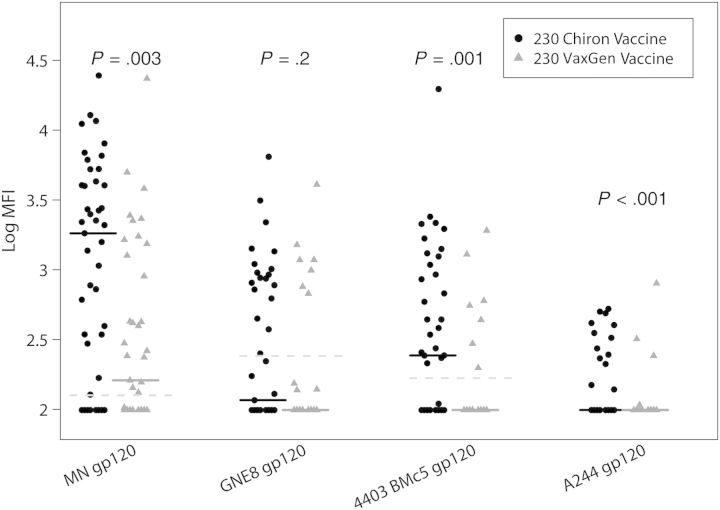
The breadth of vaccine-elicited IgG responses was measured at 52 weeks of age, using a multiclade panel of Env constructs (Table 2). The proportion of VaxGen vaccine recipients with detectable IgG responses was significantly higher than placebo recipients only for MNgp120 (0% vs 59%; P < .001), whereas more Chiron vaccine recipients had detectable IgG responses than placebo recipients for all antigens except 92Th023 gp120. The proportion of vaccinees with detectable responses against the VaxGen vaccine strain MN gp120 tended to be higher in the Chiron vaccine group than in the VaxGen vaccine group (79% vs 59%; P = .06), and more Chiron vaccine recipients than VaxGen vaccine recipients had detectable responses against the other antigens tested (Table 2). Moreover, IgG responses were significantly higher in Chiron vaccine recipients than in VaxGen vaccine recipients for MNgp120 (P = .003), A244 gp120 (P < .001), and 4403 BMC5 (P = .001; Figure 2). There were no differences in the magnitude and frequency of Env-specific IgG responses between infants vaccinated according to the regular versus the accelerated VaxGen vaccine schedules or between infants vaccinated according to the regular versus the accelerated Chiron vaccine schedules (Supplementary Table 1). A higher proportion of ALVAC/AIDSVAX vaccinees than placebo recipients had detectable responses against PACTG 326 vaccine strains (MNgp120, 83% vs 9% [P = .005]; and GNE8 gp120, 50% vs 0% [P = .003]), but differences were not significant for the other antigens tested (Table 2).
Figure 2.
Chiron vaccine induces robust human immunodeficiency virus type 1 (HIV-1) Env-specific multiclade immunoglobulin G (IgG) responses. Magnitudes of the IgG responses against multiclade HIV-1 Env gp120 proteins, including the clade B vaccine strains (MN gp120 and GNE8 gp120), a clade C postnatal transmitted/founder Env (4403 BMc5), and a clade AE Env (A244 gp120; RV144 vaccine strain), are presented. The black lines represent median values, and the dashed lines represent the positivity cutoff for each antigen. The Wilcoxon test was used to compare vaccine groups. Abbreviations: MFI, mean fluorescence intensity; 230, Pediatric AIDS Clinical Trials Group 230.
Elicitation of Anti-V1V2 IgG Responses
We next explored the ability of vaccination to induce anti-V1V2 IgG in infants, a response associated with reduced HIV-1 acquisition risk in the RV144 adult vaccine trial [7], using the same gp70 scaffold V1V2 protein as in the RV144 immune correlate analysis (Figure 3). In PACTG 230, the frequency of anti-V1V2 IgG antibodies at birth (maternally acquired) was similar between vaccinees and placebo recipients; more vaccinees than placebo recipients had detectable levels of anti-V1V2 IgG at 24 weeks of age (17% in placebo group, compared with 73% in the VaxGen vaccine group [P < .001] and 98% in the Chiron vaccine group [P < .001]; Figure 3A). The proportion of VaxGen vaccine recipients with anti-V1V2 IgG responses decreased to levels comparable to those of placebo recipients at 52 weeks of age, whereas the proportion of Chiron vaccine recipients with detectable responses remained higher than that of placebo recipients at 52 (93%; P < .001) and 104 weeks of age (63%; P < .001). Anti-V1V2 IgG levels were higher in VaxGen vaccine recipients than placebo recipients at 24 and 52 weeks of age (P < .001 for both comparisons) but were comparable by 76 weeks of age (Figure 3B). In contrast, Chiron vaccines recipients had higher anti-V1V2 IgG responses than placebo recipients at 24, 52, 76 and 104 weeks of age (P < .001 for all comparisons). Thus, receipt of the Chiron vaccine induced long lasting anti-V1V2 IgG responses in infants.
Figure 3.
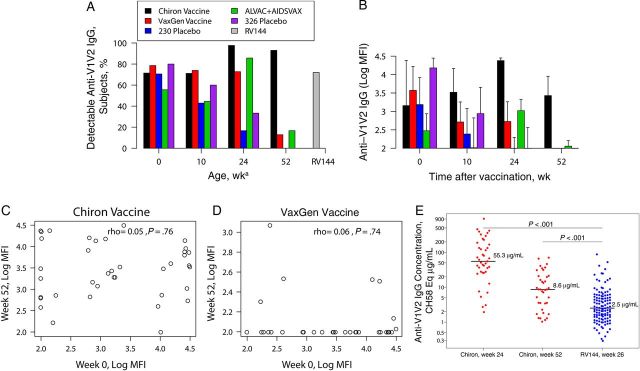
Human immunodeficiency virus type 1 (HIV-1) Env vaccination elicits high anti-V1V2 immunoglobulin G (IgG) responses in infants. A, Proportions of infants and, for the RV144 vaccine trial, adults with anti-V1V2 IgG responses. aData for RV144 vaccinees are from week 26 after vaccination (peak immunogenicity). B, Magnitudes of anti-V1V2 IgG responses in infant recipients of vaccine and placebo. C and D, Correlation between the levels of anti-V1V2 IgG at week 0 of age (maternal) and at week 52 of age (vaccine response) in infant recipients of Chiron (C) and VaxGen (D) vaccines. E, Plasma concentration of CH58 equivalent anti-V1V2 IgG responses in infant recipients of Chiron vaccine and adult recipients of RV144. The Wilcoxon test was used to compare responses between adult and infant vaccinees. Median and 75th percentile values are presented in panel B. Lines in panel E represent median values. Abbreviations: MFI, mean fluorescence intensity; 230, Pediatric AIDS Clinical Trials Group 230; 326, Pediatric AIDS Clinical Trials Group 326.
The proportion of infants with anti-V1V2 IgG antibodies at birth was comparable between PACTG 326 placebo recipients (80%) and ALVAC/AIDSVAX vaccinees (56%), but the levels of antibodies were higher in placebo recipients than in vaccinees (P = .02), potentially because of the small sample size of this trial. At 24 weeks of age, the magnitude (P = .11) and frequency (86% vs 33%; P = .06) of the anti-V1V2 IgG response trended higher in ALVAC/AIDSVAX vaccinees than in placebo recipients, although this difference did not reach statistical significance. By 52 weeks of age, the magnitude and frequency of anti-V1V2 IgG responses were comparably low between vaccine and placebo recipients (Figure 3A and 3B).
No Correlation Between the Magnitude of Maternal Antibodies and Vaccine-Elicited Responses
As maternal antibodies interfere with infant immune responses to certain vaccines [5], we sought to determine whether high levels of maternal anti-V1V2 IgG correlated with lower levels of vaccine-elicited responses (Figure 3C and 3D). There was no correlation between the levels of anti-V1V2 IgG at birth and those at 24 or 52 weeks of age in VaxGen (24 weeks of age, r = 0.03 and P = .84; 52 weeks of age, r = 0.06 and P = .74) and Chiron (24 weeks of age, r = −0.11 and P = .81; 52 weeks of age, r = 0.05 and P = .76) vaccine recipients. Thus, the magnitude of maternal antibodies did not impact the ability of infants to mount robust anti-V1V2 IgG responses.
Higher Anti-V1V2 IgG Responses in Infant Recipients of Chiron Vaccine Than in Adult Recipients of RV144
As Chiron vaccine recipients developed strong anti-V1V2 IgG responses, we compared the magnitude of this response in infant recipients of Chiron vaccine to that of adults from the RV144 vaccine trial. At 52 weeks of age, >6 months after the last vaccine dose, the proportion of vaccinated infants with anti-V1V2 IgG was higher than among adult RV144 vaccine recipients at peak immunogenicity (93% vs 72%; Figure 3A) [18]. Moreover, at peak immunogenicity, the concentration of anti-V1V2 IgG (measured in the same assay and calculated with CH58, a V2-specific monoclonal antibody isolated from an RV144 vaccine recipient as a standard [16]) was 22 times higher in infants than in adults (median concentration, 55 µg/mL vs 2.5 µg/mL of CH58 equivalent; P < .001; Figure 3E). At 52 weeks of age, the concentration of anti-V1V2 IgG remained >3 times higher in infants than adults at peak immunogenicity (median concentration, 8.6 vs 2.5 µg/mL CH58 equivalent; P < .001). Thus, infant vaccination can induce high levels of potentially protective anti-V1V2 IgG responses.
Elicitation of Anti-V1V2 IgG3 Responses
We next investigated whether receipt of the Chiron vaccine elicited anti-V1V2 IgG3 responses in infants, because this antibody subclass was associated with reduced HIV-1 acquisition in adult RV144 vaccine recipients [19]. At birth, 10% of infant vaccine recipients and 5% of placebo recipients had detectable anti-V1V2 IgG3 responses (Figure 4). The frequency of responders increased following vaccination to 42% at week 24 of age, compared with 5% among placebo recipients (P = .003). However, this response was short-lived, because by 52 weeks of age only 4% of Chiron vaccine recipients had detectable anti-V1V2 IgG3 responses, consistent with the reported short duration of Ig3 responses in adult vaccinees [19].
Figure 4.

Human immunodeficiency virus type 1 (HIV-1) Env vaccination induces anti-V1V2 immunoglobulin G 3 (IgG3) responses in infants. A and B, Frequency (A) and magnitude (B) of anti-V1V2 IgG3 responses in Chiron vaccine recipients. In panel B, the dashed line represents the cutoff, and data points below the cutoff are represented by clear circles. Abbreviations: MFI, mean fluorescence intensity; PACTG 230, Pediatric AIDS Clinical Trials Group 230.
Association Between T-Cell Responses and Antibody Response Durability
To determine whether there was an association between the durability of the vaccine-elicited IgG and T-cell responses, we compared the proportion of vaccinated infants with detectable IgG responses between Env-specific lymphoproliferation (LPA) responders and nonresponders as measured in the original study (Supplementary Table 2) [20]. At 104 weeks of age, 14 of 18 Chiron vaccine recipient LPA responders, and 3 of 9 nonresponders had detectable anti-V1V2 IgG responses (P = .04). A marginal but not statistically significant difference was also observed in the magnitude of anti-V1V2 IgG responses between the 2 groups (P = .08). However, there was no difference in the magnitude or frequency of anti-MNgp120 IgG responses between LPA responders and nonresponders. In VaxGen vaccine recipients, at 104 weeks of age, there were more anti-MNgp120 IgG responders (P = .04), and the magnitude of anti-MNgp120 IgG responses was higher (P = .003) among LPA responders than nonresponders.
Low Levels of HIV-1 Env–Specific IgA in Vaccinated Infants
RV144 vaccinees with high levels of plasma HIV-1 Env–specific IgA responses had decreased vaccine efficacy [7]. Thus, we measured HIV-1 Env–specific IgA responses at birth and after vaccination in infants. Since maternal IgA is not passively transferred to the fetus in utero, the positivity cutoff was determined as 3 SDs above the average MFI of placebo recipients at birth. For all antigens, the median MFI in uninfected vaccine and placebo recipients was below the positivity cutoff at 0 and 24 weeks of age (Supplementary Table 3). At 24 weeks of age, only 1 of 43 Chiron vaccine recipients had a detectable level of IgA against the vaccine strain MNgp120 that was slightly above the cutoff. In addition, 1 Chiron vaccine recipient and 1 VaxGen vaccine recipient had detectable anti-gp140 IgA responses. However, the same infants also had detectable IgA responses against gp41 that was not included in the vaccine, suggesting that the detected gp140 IgA responses were not vaccine elicited. Notably, high levels of IgA responses mainly directed against gp41 were detected in HIV-1–infected infants, indicating that infants are able to mount potent HIV-1 Env–specific IgA responses following infection. No Env-specific IgA was detected in ALVAC/AIDSVAX vaccinees.
Induction of Tier 1 Neutralizing Antibody Responses
Finally, we investigated the ability of infant vaccination to induce ADCC and neutralization responses. Comparable frequencies and magnitudes of ADCC responses were observed between placebo and vaccine recipients following vaccination (Table 3), indicating that the vaccine regimens did not induce potent ADCC responses in HIV-1–exposed vaccinated infants. At 24 weeks of age, the frequency of infants with detectable neutralizing responses against the tier 1 vaccine strain HIV-1 MN was higher in vaccinees (VaxGen vaccine recipients, 80% [P = .006]; Chiron vaccine recipients, 72% [P = .03]) than in placebo recipients (36%), but the magnitude of these responses was comparable between vaccine and placebo recipients (Table 3). At 52 weeks of age, neutralization titers were higher among Chiron vaccine recipients, compared with placebo recipients (P = .02). Only few infants had neutralizing responses against HIV-1 SF162 that were above the cutoff. No difference in the frequencies or magnitudes of these responses was observed between ALVAC/AIDSVAX vaccinees and 326 placebo recipients.
Table 3.
Functional Immunoglobulin G Responses in Human Immunodeficiency Virus Type 1–Exposed Vaccinated Infants
| Variable | PACTG 230 |
PACTG 326 |
||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | VaxGen Vaccine | P Value | Chiron Vaccine | P Value | Placebo | ALVAC/ AIDSVAX | P Value | |
| ADCC (B. SF162) | ||||||||
| Week 24 | ||||||||
| Subjects, no. | 14 | 40 | 36 | 9 | 6 | |||
| Median ADCC titer | 70 | ND | 49 | .06a | 49 | 61 | .95a | |
| Detectable response, subjects, % | 64 | ND | 36 | .11b | 44 | 67 | .61b | |
| Week 52 | ||||||||
| Subjects, no. | 12 | 40 | 43 | 11 | 6 | |||
| Median ADCC titer | 49 | 49 | .8a | 49 | .8a | 49 | 52 | .07a |
| Detectable response, subjects, % | 17 | 15 | 1.00b | 23 | 1.00 | 9 | 50 | .10b |
| Neutralization (B. MN) | ||||||||
| Week 24 | ||||||||
| Subjects, no. | 14 | 40 | 37 | 9 | 8 | |||
| Median ID50c | 141 | 110 | .8a | 187.5 | .49a | 76.5 | 105 | .86a |
| Response, subjects, % | 36 | 80 | .006b | 72 | .03b | 67 | 63 | 1.00 |
| Week 52 | ||||||||
| Subjects, no. | 11 | 40 | 43 | 11 | 6 | |||
| Median ID50c | 23 | 33 | .22a | 68 | .02a | ND | ND | |
| Response, subjects, % | 45 | 50 | 1.00 | 53 | .74b | 9 | 17 | |
A P value of < .05 is considered statistically significant.
Abbreviations: ADCC, antibody-dependent cell-mediated cytotoxicity; B. MN, B HIV-1 MN.3; B. SF162, B HIV-1 SF162.LS; ID50, median infectious dose; ND, not done; PACTG, Pediatric AIDS Clinical Trials Group.
By the Wilcoxon test, compared with placebo.
By the Fisher exact test, compared with placebo.
The median value was computed for responders only.
DISCUSSION
The estimated 31% vaccine efficacy achieved by immunization of heterosexual adults with vcp1521 + AIDVAX B/E in the RV144 vaccine trial has provided the first opportunity to investigate immune correlates of protection against HIV-1 acquisition in vaccinated adults [6, 7]. Analysis of vaccine-elicited immune responses in this trial identified anti-V1V2 IgG as a potentially protective immune response [7]. Because of the established difference in infant and adult immune systems, we investigated whether infant vaccination could induce this response, using samples from 2 neonatal HIV-1 vaccine trials. Anti-V1V2 IgG responses were elicited in vaccinated infants from both trials, including PACTG 326, in which infants were vaccinated using an ALVAC/AIDSVAX strategy similar to that used for RV144 (Figure 3). Surprisingly, one of the gp120 vaccines (the one developed by Chiron) induced anti-V1V2 IgG responses >20-fold higher than that elicited in RV144 vaccinees at peak immunogenicity (Figure 3E). Because Shen et al reported that vaccines can induce responses to multiple sequences within the V1V2 antigens [21], future studies should map the epitope specificity of the infant response in comparison to that for RV144 and other vaccines studied in adults. Interestingly, the Chiron vaccine also induced anti-Env IgG responses detectable up to 2 years of age in the infants, which was a considerably longer duration than that for the VaxGen vaccine or ALVAC/AIDSVAX. It is important to note that the limited differences in vaccine-elicited antibody responses in ALVAC/AIDVAX vaccinees and placebo recipients could be related to the small number of vaccinees. Differences between vaccine groups could also be related to the vaccination schedule, rgp120 proteins, or adjuvants used. Interestingly, certain vaccine regimens, including a gp120/MF-59 regimen [22, 23], have been reported to induce antibody responses that persist for years in adults. Induction of durable antibody responses is an important challenge faced by the HIV-1 vaccine field [24], so it is encouraging that infant vaccination can elicit antibody responses detectable >18 months after vaccination. This suggests that a pediatric vaccine that protects throughout the duration of breast-feeding is feasible.
In the RV144 immune correlate analysis, vaccinees with high levels of plasma Env-specific IgA responses had a higher risk of acquiring HIV-1 than those with low IgA responses [7]. These Env-specific IgA responses may have interfered with the function of IgG antibodies directed against the same epitope [25]. Our study detected only rare, very low level Env-specific IgA responses following vaccination. Because infants are able to mount potent IgA responses against other vaccines, such as the rotavirus vaccine [26], this negligible Env-specific IgA response is not due to an inherent inability of infants to mount IgA responses. Interestingly, strong IgA responses mostly directed against gp41 were detected in HIV-1–infected infants, similar to the predominance of anti-gp41 IgA responses in acutely infected adults [27]. Our results indicate a lower potential for mitigation of vaccine-elicited, protective Env-specific IgG antibody responses by nonprotective Env-specific IgA responses in infants.
Maternal antibodies interfere with infant antibody responses to some vaccines, leading to impaired vaccine responses [5]. Thus, it is critical to investigate the extent to which maternal antibodies may or may not interfere with infant responses to HIV-1 vaccines. In our study, there was no association between maternally acquired and vaccine-elicited anti-V1V2 IgG responses. This finding is important because to protect the majority of infants from breast milk–mediated HIV-1 acquisition, an effective HIV-1 vaccine would need to be administered to infants as early as possible. Importantly, it was recently reported that infant rhesus monkeys develop more rapid and potent functional antibody responses following simian/human immunodeficiency virus (SHIV) infection when suboptimal levels of neutralizing antibodies are present at the onset of infection [28, 29], suggesting that, in some circumstances, preexisting maternal antibodies could potentiate vaccine-induced responses. Because it is hypothesized that maternal antibodies inhibit infant responses by masking important epitopes [30], the fine specificity of maternal and vaccine-elicited HIV-1–specific antibody responses should be further investigated in HIV-1–exposed, vaccinated infants.
Passive immunization studies in nonhuman primates have shown that broadly neutralizing antibody responses can protect animals from SHIV challenge [31], highlighting the potentially protective role of broadly neutralizing responses. However, most of the vaccines tested to date, including RV144, have predominantly induced tier 1 HIV-1 neutralizing activity [32]. Similarly, we detected only tier 1 HIV-1 neutralizing responses in vaccinated infants (Table 3). The elicitation of this tier 1 neutralizing responses in vaccinated infants suggests that if vaccine immunogens capable of inducing broadly neutralizing responses in adults are developed, these reagents may also elicit potent responses in infants. However, we also found that vaccination of infants did not induce potent ADCC responses, although there was a trend toward a higher proportion of ADCC responders in Chiron vaccine recipients, compared with placebos recipients, at week 24 of age (Table 3). This result suggests that vaccination of infants may have elicited a short-lived ADCC response that was masked by the presence of maternal antibodies. It has also been reported that infants with vertically transmitted HIV-1 infection have delayed ADCC responses [33]. Further investigations of nonneutralizing antibody responses in the context of infant HIV-1 infection and vaccination will allow a better understanding of their role in acquisition of HIV-1 among infants.
It is important to note that while anti-V1V2 IgG antibodies were associated with lower HIV-1 acquisition risk in the RV144 vaccine trial, this response is not an established mechanism of protection in adults, and the role of anti-V1V2 IgG in mother-to-child transmission is unknown. Moreover, no vaccine efficacy trial has been conducted in infants to date. Thus, our study does not establish whether HIV-1 Env vaccination of infants would have been equally or more efficacious than HIV-1 Env vaccination of adults. Instead, our results highlight that infants are able to mount robust HIV-1 Env–specific antibody responses, including against the V1V2 epitope that was an immune correlate of risk in the only efficacious HIV-1 vaccine trial. This suggests that HIV-1 vaccine candidates should be investigated in both adults and infants. Definition of protective HIV-1 vaccine–elicited responses in infants will require inclusion of infants in future HIV-1 vaccine efficacy trials.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the participants and clinical staff of the PACTG 230, PACTG 326, and RV144 clinical trials; and Dr P. Berman, for providing GNE8 gp120.
Disclaimer. The views expressed in this article are those of the authors and should not be construed as official or as representing the views of the Department of Health and Human Services, the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, the Department of Defense, or the Department of the Army.
Financial support. This work was supported by the Duke University Center for AIDS Research (CFAR; 5P30 AI064518, funded by the National Institutes of Health [NIH]) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH (1R03-HD072796-01A1). Research reported in this publication was supported by the National Center For Advancing Translational Sciences, NIH (KL2TR001115 to G. F.); the National Institute of Allergy and Infectious Diseases (NIAID), NIH and the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (UM1-AI100645-01); the Duke University CFAR Immunology Core (P30 AI64518); and the HIV-1 Vaccine Trials Network Laboratory Center (5UO1 AI46725 to G. T.). Overall support for the IMPAACT group was provided by the NIAID, NIH (U01 AI068632); and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. The Statistical and Data Analysis Center at the Harvard School of Public Health is supported by the NIAID, NIH (cooperative agreements 5 U01 AI41110 [with the Pediatric AIDS Clinical Trials Group] and 1 U01 AI068616 [with the IMPAACT group]). Additional funding was provided by the Army Medical Research and Material Command and the NIAID, NIH (interagency agreement Y1-AI-2642-12), through the Henry M. Jackson Foundation for the Advancement of Military Medicine and the Department of Defense (cooperative agreement W81XWH-07-2-0067).
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–74. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. Global report. UNAIDS report on the global AIDS epidemics. 2013. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. Accessed 12 March 2014.
- 3.De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283:1175–82. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 4.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007;137(suppl 1):S4–9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 7.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zolla-Pazner S, de Camp AC, Cardozo T, et al. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PloS One. 2013;8:e53629. doi: 10.1371/journal.pone.0053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottardo R, Bailer RT, Korber BT, et al. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PloS One. 2013;8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham CK, Wara DW, Kang M, et al. Safety of 2 recombinant human immunodeficiency virus type 1 (HIV-1) envelope vaccines in neonates born to HIV-1-infected women. Clin Infect Dis. 2001;32:801–7. doi: 10.1086/319215. [DOI] [PubMed] [Google Scholar]
- 11.McFarland EJ, Borkowsky W, Fenton T, et al. Human immunodeficiency virus type 1 (HIV-1) gp120-specific antibodies in neonates receiving an HIV-1 recombinant gp120 vaccine. J Infect Dis. 2001;184:1331–5. doi: 10.1086/323994. [DOI] [PubMed] [Google Scholar]
- 12.McFarland EJ, Johnson DC, Muresan P, et al. HIV-1 vaccine induced immune responses in newborns of HIV-1 infected mothers. AIDS. 2006;20:1481–9. doi: 10.1097/01.aids.0000237363.33994.45. [DOI] [PubMed] [Google Scholar]
- 13.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouda GG, Mahlokozera T, Salazar-Gonzalez JF, et al. Postnatally-transmitted HIV-1 Envelope variants have similar neutralization-sensitivity and function to that of nontransmitted breast milk variants. Retrovirology. 2013;10:3. doi: 10.1186/1742-4690-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 16.Liao HX, Bonsignori M, Alam SM, et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–86. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edmonds TG, Ding H, Yuan X, et al. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010;408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zolla-Pazner S, deCamp A, Gilbert PB, et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PloS one. 2014;9:e87572. doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yates NL, Liao HX, Fong Y, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3007730. 228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borkowsky W, Wara D, Fenton T, et al. Lymphoproliferative responses to recombinant HIV-1 envelope antigens in neonates and infants receiving gp120 vaccines. AIDS Clinical Trial Group 230 Collaborators. J Infect Dis. 2000;181:890–6. doi: 10.1086/315298. [DOI] [PubMed] [Google Scholar]
- 21.Shen X, Howington R, Park H, et al. Vaccine Induced Epitope Specific Antibodies to the SIV Envelope Are Distinct from Those Induced to the HIV-1 Envelope. [abstract OA0403]. Abstract book of the AIDS Vaccine Meeting; Barcelona, Spain: 2013. p. 65. [Google Scholar]
- 22.Seaton K, Yates N, Williams W, et al. Human HIV-1 Vaccine Induced Antibody Durability and Env IgG3 Responses. [abstract P13.71 LB]. Abstract book of the AIDS Vaccine Meeting; Barcelona,. Spain: 2013. p. 306. [Google Scholar]
- 23.Spearman P, Tomaras GD, Montefiori D, et al. Rapid Development of Cross-Clade Neutralizing Antibody Responses After Clade B gp120/gp140 Protein Priming And Clade C gp140 Protein Boosting. [abstract P04.30 LB ]. Abstract book of the AIDS vaccine meetting; Boston, MA: 2012. p. 163. [Google Scholar]
- 24.Kim JH, Rerks-Ngarm S, Excler JL, Michael NL. HIV vaccines: lessons learned and the way forward. Curr Opin HIV AIDS. 2010;5:428–34. doi: 10.1097/COH.0b013e32833d17ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomaras GD, Ferrari G, Shen X, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A. 2013;110:9019–24. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armah GE, Breiman RF, Tapia MD, et al. Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine. 2012;30(suppl 1):A86–93. doi: 10.1016/j.vaccine.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Yates NL, Stacey AR, Nolen TL, et al. HIV-1 gp41 envelope IgA is frequently elicited after transmission but has an initial short response half-life. Mucosal Immunol. 2013;6:692–703. doi: 10.1038/mi.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng CT, Jaworski JP, Jayaraman P, et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med. 2010;16:1117–9. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaworski JP, Kobie J, Brower Z, et al. Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques. J Virol. 2013;87:10447–59. doi: 10.1128/JVI.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine. 2003;21:3406–12. doi: 10.1016/s0264-410x(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 31.Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 32.Montefiori DC, Karnasuta C, Huang Y, et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis. 2012;206:431–41. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pugatch D, Sullivan JL, Pikora CA, Luzuriaga K. Delayed generation of antibodies mediating human immunodeficiency virus type 1-specific antibody-dependent cellular cytotoxicity in vertically infected infants. WITS Study Group. Women and Infants Transmission Study. J Infect Dis. 1997;176:643–8. doi: 10.1086/514085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.