Abstract
Background
Defining mucosal immune responses and inflammation to candidate human immunodeficiency virus type 1 (HIV-1) vaccines represents a current research priority for the HIV-1 vaccine field. In particular, it is unclear whether intramuscular immunization can elicit immune responses at mucosal surfaces in humans.
Methods
In this double-blind, randomized, placebo-controlled clinical trial, we evaluated systemic and mucosal immune responses to a candidate adenovirus serotype 26 (Ad26) vectored HIV-1 envelop (Env) vaccine in baseline Ad26-seronegative and Ad26-seropositive healthy volunteers. Systematic mucosal sampling with rectal Weck-Cel sponges and rectal biopsies were performed.
Results
Intramuscular immunization elicited both systemic and mucosal Env-specific humoral and cellular immune responses in the majority of subjects. Individuals with preexisting Ad26-specific neutralizing antibodies had vaccine-elicited immune responses comparable to those of subjects who were Ad26 seronegative. We also observed no increase in activated total or vector-specific mucosal CD4+ T lymphocytes following vaccination by either histopathology or flow cytometry.
Conclusions
These data demonstrate that a single intramuscular administration of this Ad26-vectored HIV-1 Env vaccine elicited both systemic and mucosal immune responses in humans. Induction of antigen-specific humoral and cellular mucosal immunity was not accompanied by a detectable increase in mucosal inflammation.
Clinical Trials Registration
Keywords: HIV, vaccine, adenovirus, mucosal immunity
There has been substantial progress in approaches to prevent and treat human immunodeficiency virus type 1 (HIV-1) infection, but the need for a safe and effective HIV-1 vaccine remains a high global health priority [1–4]. However, very few HIV-1 vaccine trials have evaluated mucosal immune responses elicited by candidate vaccines in humans. In particular, it remains unclear whether intramuscular immunization can induce mucosal immune responses in humans. Mucosal sites represent the portal of entry for the majority of HIV-1 infections, and thus it would presumably be desirable to induce mucosal HIV-1–specific immune responses that may be able to block or attenuate infection.
Limited data are currently available regarding the magnitude and phenotype of mucosal immune responses and immune activation following HIV-1 vaccination in humans [5]. This reflects logistic challenges associated with mucosal sampling and obtaining mucosal biopsies in the context of clinical vaccine studies as well as the immunologic challenges associated with accurately evaluating mucosal immune responses in humans.
We have recently reported the first-in-human immunogenicity of a novel adenovirus serotype 26 (Ad26) vector expressing HIV-1 envelop (Env) [6, 7]. Ad26 exhibits lower seroprevalence and neutralizing antibody (NAb) titers compared with adenovirus serotype 5 (Ad5), particularly in the developing world [8, 9]. Moreover, Ad26 and Ad5 differ in terms of their basic virology, innate immune profiles, and adaptive immune phenotypes [10–14]. In addition, Ad26-based vaccine regimens have shown improved protection compared with Ad5 and DNA/Ad5 regimens in stringent SIVmac251 (simian immunodeficiency virus) challenge models in rhesus monkeys [15, 16].
In the present study, we explored mucosal immune responses and inflammation in humans following immunization with an Ad26 vector expressing clade A Env glycoprotein (gp) 140 (Ad26-EnvA) [6, 7] in baseline Ad26-seronegative and Ad26-seropositive subjects.
MATERIALS AND METHODS
Participants and Study Design
This is a phase 1, double-blind, randomized, placebo-controlled clinical trial to assess the mucosal immune responses to a single intramuscular injection by needle of Ad26-EnvA vaccine at a dose of 5 × 1010 viral particles in HIV-1–uninfected healthy adults. Twenty-four subjects were enrolled: 16 Ad26– in group 1, and 8 Ad26+ in group 2. In a block-randomized manner, subjects were allocated to vaccine or placebo in a 3:1 ratio. Subjects were healthy, were HIV-1 and -2 uninfected, were between 18 and 50 years of age, completed an assessment of understanding questionnaire for participation, and were at low risk for HIV acquisition as per standard criteria. This study was approved by the Partners Institutional Review Board and Institutional Biosafety Committee, and written informed consent was obtained from all subjects. The trial was registered at ClinicalTrials.gov (NCT01103687).
Vaccine
Ad26.ENVA.01 (Ad26-EnvA) is an E1/E3-deleted recombinant adenovirus serotype 26 vector vaccine expressing a modified gp140 HIV-1 clade A Env glycoprotein (strain 92rw020) and manufactured in HER96 cells by Crucell, and it has been previously described [6]. The placebo was adenovirus final formulation buffer.
Safety, Reactogenicity, and Adverse Event Evaluation
Subjects were observed for at least 30 minutes after immunization. In addition, subjects maintained a diary for the 7 days after immunization to monitor for reactogenicity. All reactogenicity symptoms were followed until resolution and graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, version 1.0, December 2004, Clarification August 2009 (http://rcc.tech-res-intl.com; see Supplementary Appendix for further details). Safety laboratory assessments were obtained at baseline and days 14 and 168 after vaccination and included complete blood count with differential, prothrombin time, partial thromboplastin time, creatinine, liver function tests (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and total bilirubin). A urine analysis was performed at baseline and day 168.
Clinical Specimens
Blood for immunogenicity assessments was collected at baseline and on days 1, 3, 7, 14, 28, 61, 168, and 365 after vaccination. Mucosal sampling was conducted under direct visual inspection with a Weck-Cel sponge (Ultracell, Aspen Surgical, Caledonia, Michigan) placed against the rectal mucosa and held in place for at least 5 minutes (and then repeated). Rectal biopsies were obtained under direct visualization with approximately 8–10 biopsies obtained at each of 3 time points (day −14 for baseline, day 14 to assess acute postvaccine mucosal response, and day 168 as a remote time point). All subjects were required to refrain from anal trauma before and after the procedure and to also avoid any antiplatelet agents during this time (eg, aspirin, nonsteroidal anti-inflammatory drugs).
Enzyme-Linked Immunosorbent Assays
Binding antibody titers were determined by direct enzyme-linked immunosorbent assays (ELISAs) using a 92RW020 gp140 antigen matched to the vaccine strain and isotype-specific immunoglobulin G (IgG) or immunoglobulin A (IgA) secondary antibodies, as previously described [6]. Mucosal antibody responses were assessed using antibodies extracted from Weck-Cel sponges and corrected for dilution [15]. The threshold for positivity for the ELISA is a titer of >30.
Intracellular Cytokine Staining Assays
Lymphocytes were isolated from peripheral blood by Ficoll density gradient sedimentation. Mucosal lymphocytes were isolated from tissues essentially as previously described [17]. Intracellular cytokine staining (ICS) assays were performed essentially as described previously [18, 19] and in the Supplementary Appendix. The ICS assays utilized monoclonal antibodies specific for Ki67-fluorescein isothiocyanate (Ki67-FITC), interleukin-2 phycoerythrin (IL-2-PE), CD45RO-ECD, CD8-PERCP-cyanine 5.5 (Cy5.5), interferon (IFN) γ–PE-cyanine 7 (Cy7), CD107a-Pacific Blue, Live/Dead-AmCyan, CD57-QD565, CD3-QD605, CD4-QD655, CCR5-allophycocyanin (APC), tumor necrosis factor (TNF)-α–Alexa700, and CD27-allophycocyanin cyanine 7 (APC-Cy7). Background subtracted responses are shown, with positivity defined as ≥0.05% and 3-fold background.
Virus Neutralization Assays
Ad26-specific NAb titers using serum samples were assessed by luciferase-based virus neutralization assays as described previously [20] and in the Supplementary Appendix. Neutralization titers were defined as the maximum serum dilution that neutralized 90% of luciferase activity.
Pathology
Histopathology
Per previous experience with monkey tissues, a protocol for human colon was adapted for histology, immunohistochemistry, and image analysis [21]. In brief, tissue biopsies were fixed in 10% neutral buffered formalin, processed per routine, and embedded in paraffin for sectioning. Sections cut included 1 section at 5 µm for hematoxylin and eosin staining and 10 unstained sections at 5 µm for immunohistochemistry. Standard clinical pathologic criteria were utilized to assess for inflammation or any other potential pathology such as acute inflammation (neutrophils), foamy macrophage response, granulomata, abscess, lymphoid hyperplasia, and/or increased plasma cells in combination with (or without) edema, hemorrhage, crypt distortion (chronic), or ulceration (acute).
Immunohistochemistry
Unstained sections were heated overnight at 60°C, deparaffinized in xylene, and rehydrated prior to staining. A summary of antibodies, retrieval, and staining conditions are given in Supplementary Table 1. All primary antibody incubations (postretrieval) were 1 hour in a moisture chamber. Staining was visualized with addition of diaminobenzidine and counterstained with hematoxylin.
Objective Scoring and Quantitative Image Analysis
Assessment of tissue for adequacy was performed on each sample using hematoxylin and eosin slides by the primary pathologist (D. A. M.). The quantitative assessment of staining for each antibody in epithelium and lamina propria was performed using 400× images from an Olympus BX41 microscope equipped with a DP25 camera. All cases had at least ten 400× fields photographed and stored in a shared file. Individual pathologists (M. D. S., B. J. C., K. L. G., K. L. V.) blindly reviewed and counted images for each antibody stain. The primary pathologist reviewed 10% of all images for secondary check of accuracy and counts. All counts were converted to stained cells per 10 high-powered fields.
Statistical Analyses
All subjects received the single planned vaccination, thus all analyses presented are intent-to-treat. All clinical and laboratory assessments were conducted blinded to treatment allocation. Summaries of response rates are presented with geometric mean titers (GMTs) for the Ad NAb and HIV-1 ELISA and means for the HIV-1 enzyme-linked immunospot (ELISpot) assays and ICS, all with associated 95% confidence intervals. Analysis of variance (ANOVA) for NAb and ELISA are on a log scale. Nonparametric tests were used when the assumptions of ANOVA were not met and as confirmatory analyses where ANOVA was appropriate.
RESULTS
Subject Characteristics, Design, and Safety
Twenty-four healthy HIV-1–uninfected subjects were enrolled into 2 groups. Sixteen subjects who were Ad26 seronegative (Ad26−) were enrolled into group 1 (12 vaccinees, 4 placebo recipients). Eight subjects who were Ad26 seropositive (Ad26+) were enrolled into group 2 (6 vaccinees, 2 placebo recipients). In both groups, a 3:1 randomization of vaccinees:placebo recipients was utilized. Of the 24 enrolled subjects, 12 (50%) were female, 11 (46%) were ≤30 years of age (range, 20–49 years), 7 (29%) were African American, and 4 (17%) were Latino. Of the 8 subjects in group 2, the baseline Ad26 NAb titer was between 18 and 2526 (median, 47). All 24 subjects received the single immunization with 5 × 1010 viral particles Ad26-EnvA or placebo by the intramuscular injection route with a needle in the deltoid.
The vaccination was generally well tolerated and comparable to previous experience with this vaccine vector [6, 7]. Overall, reactogenicity was similar between Ad26− and Ad26+ subjects (See Supplementary Data for details of the safety assessments).
Immunogenicity
Antibody Responses
Peripheral Ad26-Specific NAb Responses
Ad26-specific NAb titers were assessed by luciferase-based virus neutralization assays [20]. As seen in Figure 1A, 11 of 12 Ad26− subjects developed detectable Ad26-specific NAbs by 2 weeks, and the remaining subject developed detectable Ad26-specific NAbs by 8 weeks. The GMT in Ad26– subjects at baseline was undetectable and significantly increased by 2 weeks and persisted at 24 weeks after vaccination (Table 1). A similar pattern was seen in the Ad26+ group (Table 1). The Ad26 NAb titers in the 4 placebo recipients in the Ad26– group remained negative and in the 2 placebo recipients in Ad26+ group remained unchanged during the study period. These data show that a single Ad26-EnvA vaccination elicited Ad26 vector-specific NAbs as expected.
Figure 1.

Antibody responses. A, Individual adenovirus serotype 26 (Ad26) peripheral neutralizing antibody (NAb) responses from subjects by week and group are shown. Dots show individual responses at a given time point. Red horizontal lines show the mean values at a given time point for the group. The dashed horizontal line represents threshold for assay positivity. Solid circles = placebo Ad26− subjects and open circles = placebo Ad26+ subjects at baseline. B and C, Individual envelop A (EnvA) enzyme-linked immunosorbent assay responses for the peripheral and mucosal compartments from subjects by week and group are shown for immunoglobulin G (IgG; B) and immunoglobulin A (IgA; C). Dots show individual responses at a given time point. Red horizontal lines show the mean values at a given time point for the group. The dashed horizontal line represents threshold for assay positivity. B, Peripheral group 1 vs group 2: P = .9 (week 2), P = .7 (week 8), and P = .7 (week 24); mucosal group 1 vs group 2: P = .6 (week 2), P = 1 (week 24).
Table 1.
Peripheral and Mucosal Antibody Response for Adenovirus Serotype 26 Neutralizing Antibodies and EnvA Enzyme-Linked Immunosorbent Assay
| Assay | Weeks After Vaccination |
|||
|---|---|---|---|---|
| 0 | 2 | 8 | 24 | |
| Peripheral | ||||
| Ad26 NAb | ||||
| Ad26− | ≤16 | 675 | 300 | 250 |
| Ad26+ | 89 | 3166 | 2373 | 1088 |
| Placebo Ad26− | ≤16 | ≤16 | ≤16 | ≤16 |
| Placebo Ad26+ | 229 | 374 | 363 | 239 |
| EnvA | ||||
| Ad26− | ≤30 | 340 | 338 | 158 |
| Ad26+ | ≤30 | 373 | 305 | 144 |
| Placebo | ≤30 | ≤30 | ≤30 | ≤30 |
| Mucosal | ||||
| EnvA | ||||
| Ad26− | ≤30 | 100 | ND | 92 |
| Ad26+ | ≤30 | 132 | ND | 90 |
| Placebo | ≤30 | ≤30 | ND | ≤30 |
Geometric mean titers are shown.
Abbreviations: Ad26, adenovirus serotype 26; EnvA, envelop A; NAb, neutralizing antibody; ND, not measured at this time point.
Peripheral EnvA-Specific IgG ELISA Responses
EnvA-specific binding antibody titers were determined using a direct ELISA with a 92RW020 gp140 antigen matched to the vaccine strain [6, 7]. All 24 subjects had no detectable peripheral EnvA-specific IgG ELISA responses at baseline, and placebo recipients had no detectable peripheral responses throughout the course of the study (Figure 1B). In Ad26− subjects, 11 of 12 had a detectable peripheral EnvA IgG ELISA titer by 2 weeks with persistence in most subjects at 24 weeks. In Ad26+ subjects, a similar pattern was observed with 5 of 6 subjects having a detectable titer at week 2 and all by week 8 with persistence in most subjects at week 24 (Table 1). Overall, the EnvA IgG GMTs were not significantly different between Ad26− and Ad26+ subjects, but both groups were significantly greater than placebo recipients (P < .01 for Ad26−, P < .05 for Ad26+).
Mucosal EnvA-Specific IgG ELISA Responses
Mucosal antibodies were extracted from colorectal Weck-Cel sponges, which had collected mucosal secretions [15]. Total mucosal IgG was extracted from sponges and was found to be comparable among samples. Mucosal EnvA-specific IgG titers were determined by a direct ELISA using extracted antibodies and corrected for dilution volume. All 24 subjects had no detectable mucosal EnvA-specific IgG ELISA titers at baseline, and all placebo recipients had no detectable mucosal responses throughout the course of the study (Figure 1B). Mucosal EnvA-specific IgG ELISA responses were detected 2 weeks postvaccination in 6 of 12 Ad26− subjects and in 5 of 6 Ad26+ subjects. This response rate increased slightly at 24 weeks, with 8 of 11 Ad26− subjects and 5 of 6 Ad26+ subjects having a detectable response. No significant differences were observed between Ad26− and Ad26+ subjects. Responses were significantly different between both groups as compared with placebo recipients (P < .05). Overall, the pattern of mucosal EnvA-specific IgG was similar in the mucosa compared with the serum (P = .004; Spearman rank correlation of vaccinees postvaccination), although antibody titers trended a half-log lower. These data show that intramuscular Ad26-EnvA vaccination elicited clearly detectable mucosal EnvA-specific antibody responses.
Peripheral EnvA-Specific IgA ELISA Responses
All 24 subjects had no detectable peripheral EnvA-specific IgA ELISA responses at baseline, and all placebo recipients had no detectable peripheral responses throughout the course of the study (Figure 1C). Overall, IgA responses were lower than IgG responses. Peripheral IgA responses were detected in 8 of 12 Ad26− subjects by week 2. By week 8, this response had declined to 1 of 12 subjects with a detectable titer. The GMT in Ad26− subjects at baseline, 2 weeks, 8 weeks, and 24 weeks were 11, 70, 18, and 18, respectively. Ad26+ subjects showed a similar pattern with 4 of 6 subjects having a response at week 2. The GMT in Ad26+ subjects at baseline, 2 weeks, 8 weeks, and 24 weeks were 11, 108, 26, and 22, respectively. The median titers at week 2 were similar between Ad26− and Ad26+ subjects.
Mucosal EnvA-Specific IgA ELISA Responses
Mucosal EnvA-specific IgA responses were limited with titers detected in 4 subjects (2 in each group). The GMTs at baseline, 2 weeks, and 24 weeks were 11, 20, and 23 in Ad26− subjects and 11, 19, and 37 in Ad26+ subjects, respectively (Figure 1C).
Cellular Immune Responses
Peripheral Ad26 Vector-Specific ICS Responses
Ad26 vector-specific IFN-γ T-cell responses were assessed by multiparameter flow cytometry using peripheral blood mononuclear cells (PBMCs) and whole Ad26 virus stimulation as we have previously described [6, 7]. Similar responses were observed using Ad26 hexon peptide pools (data not shown). Ad26-specific CD8+ T-cell responses were observed at baseline and at weeks 2 and 24 in subjects in all 3 groups with mean responses of 1.99%, 4.83%, and 3.51% at baseline in Ad26− subjects, Ad26+ subjects, and placebo recipients, respectively. The Ad26-specific T-cell responses in Ad26-seronegative individuals suggest T-cell cross-reactivity among Ad serotypes, consistent with prior observations [22–24]. These responses were 4.67%, 4.52%, and 2.52% at week 2 and 3.09%, 3.42%, and 4.91% at week 24 for Ad26− subjects, Ad26+ subjects, and placebo recipients, respectively. Ad26-specific IFN-γ CD4+ T-cell responses were detectable at baseline in all groups with means of 1.24%, 6.68%, and 3.30%. These baseline responses to Ad26 did not change significantly following vaccination with mean responses of 3.14%, 4.33%, and 1.42% at week 2 and 2.10%, 3.60%, and 1.75% at week 24 for Ad26− subjects, Ad26+ subjects, and placebo recipients, respectively (Figure 2A and Supplementary Table 2). These data suggest that peripheral Ad26 vector-specific CD4+ T-cell responses were not substantially augmented following Ad26-EnvA vaccination.
Figure 2.
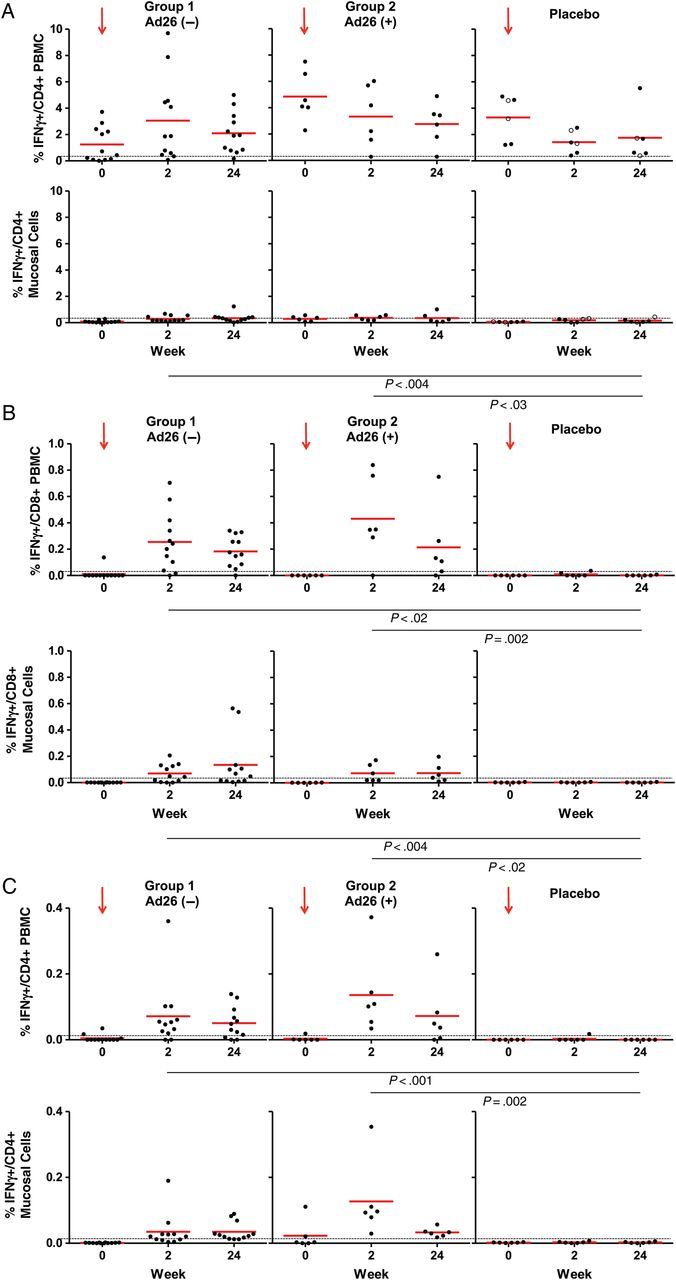
Antigen and vector intracellular cytokine staining (ICS) responses. A, Peripheral and mucosal adenovirus serotype 26 (Ad26) CD4+ ICS responses are shown as percentage of interferon (IFN) γ-positive cells. Dots represent individual responses at a given time point. The red horizontal lines show the mean values at a given time point for the group. The dashed horizontal line represents threshold for assay positivity. Group 1 vs group 2: P = not significant (weeks 2 and 24) for both peripheral and mucosal. Solid circles = placebo Ad26− subjects and open circles = placebo Ad26+ subjects at baseline. B, Peripheral and mucosal envelop A (EnvA) CD8+ ICS responses are shown as percentage of IFN-γ–positive cells. Dots represent individual responses at a given time point. The red horizontal lines show the mean values at a given time point for the group. The dashed horizontal line represents threshold for assay positivity. Peripheral group 1 vs group 2: P = .2 (week 2) and P = .6 (week 24); mucosal group 1 vs group 2: P = .8 (week 2) and P = .6 (week 24). C, Peripheral and mucosal EnvA CD4+ ICS responses are shown as percentage of IFN-γ–positive cells. Dots represent individual responses at a given time point. The red horizontal lines show the mean values at a given time point for the group. The dashed horizontal line represents threshold for assay positivity. Peripheral group 1 vs group 2: P = .08 (week 2), P = 1 (week 24); mucosal group 1 vs group 2: P = .003 (week 2), P = .4 (week 24). Abbreviation: PBMC, peripheral blood mononuclear cell.
Peripheral EnvA-Specific ICS Responses
EnvA-specific IFN-γ T-cell responses were assessed by multiparameter flow cytometry using PBMCs and a pool of overlapping EnvA peptides [6, 7]. EnvA-specific CD8+ T-cell responses were undetectable at baseline and remained undetectable in placebo recipients at all time points. In Ad26− and Ad26+ subjects, the mean responses increased postvaccination to 0.25% and 0.43%, respectively, at 2 weeks (Figure 2B and Supplementary Table 3) and 0.18% and 0.26%, respectively, at 24 weeks. A similar pattern was observed with EnvA CD4+ T-cell responses, with no responses detected at baseline or in placebo recipients at all time points (Figure 2C). Mean responses in Ad26− subjects were 0.07% and 0.05% and in Ad26+ subjects were 0.14% and 0.07% at 2 weeks and 24 weeks postvaccination, respectively.
Mucosal Ad26 Vector-Specific ICS Responses
Mucosal Ad26 vector-specific IFN-γ T-cell responses were assessed by multiparameter flow cytometry using cells extracted from colorectal biopsies and stimulated with whole Ad26 virus as described [10, 17]. Similar responses were observed using Ad26 hexon peptide pools (data not shown). Mucosal Ad26-specific CD4+ T-cell responses were detected and comparable in all groups but were approximately 10-fold lower than peripheral Ad26-specific CD4+ T-cell responses. Mean responses by group (Ad26−, Ad26+, placebo Ad26−, and placebo Ad26+) were 0.09%, 0.29%, 0.06%, and 0.06% at baseline; 0.30%, 0.37%, 0.13%, and 0.29% at 2 weeks; and 0.35%, 0.52%, 0.12%, and 0.24% at 24 weeks (Figure 2A and Supplementary Table 2). These data suggest that mucosal Ad26 vector-specific CD4+ T-cell responses were not substantially augmented following Ad26-EnvA vaccination.
Mucosal EnvA-Specific ICS Responses
Mucosal EnvA-specific IFN-γ T-cell responses were also assessed by multiparameter flow cytometry using cells extracted from colorectal biopsies and stimulated with overlapping EnvA peptides [10, 17]. Mucosal EnvA-specific IFN-γ CD8+ T-cell responses were undetectable at baseline in all 3 groups and remained undetectable in the placebo group at all time points. In Ad26− subjects, responses were detected in half of subjects at 2 weeks and 24 weeks with mean responses of 0.07% and 0.13% at 2 and 24 weeks, respectively. In Ad26+ subjects, similar mean responses of 0.05% and 0.06% at 2 weeks and 24 weeks, respectively, were observed (Figure 2B and Supplementary Table 3). Mucosal EnvA-specific IFN-γ CD4+ T-cell responses were 0%, 0.02%, and 0% at baseline; 0.03%, 0.14%, and 0% at 2 weeks; and 0.03%, 0.03%, and 0% at 24 weeks for Ad26−, Ad26+, and placebo, respectively (Figure 2C and Supplementary Table 3). These data show that intramuscular Ad26-EnvA vaccination elicited detectable mucosal EnvA-specific T-cell responses in a subset of volunteers.
Peripheral IFN-γ EnvA-Specific ELISpot Responses
All subjects had no detectable ELISpot responses at baseline and all placebo recipients had no detectable responses throughout the study period. Eight of 12 group 1 subjects and 4 of 6 group 2 subjects had detectable responses by week 2, and 11 of 12 subjects in the Ad26− group and 5 of 6 subjects in the Ad26+ group had a detectable response by week 24. The mean responses by group were 75, 460, and 669 spot-forming cells (SFC)/106 PBMCs in the Ad26− group and 163, 85, and 77 SFC/106 PBMCs in the Ad26+ group at weeks 2 (P = .6, Ad26– vs Ad26+), 8 (P = .05), and 24 (P = .1), respectively. Both the Ad26+ and Ad26– subjects had responses significantly greater than placebo recipients (P < .05) at all time points except 2 weeks in the Ad26+ group (P = .1). At week 24, detectable responses were observed in 10 of 12 Ad26− and 3 of 6 Ad26+ subjects, but did not significantly differ by group (P = .3).
Mucosal Pathology
Mucosal Histopathology
We next assessed the degree of inflammation in colorectal mucosa by histopathology. A blinded review of colorectal tissue sections were evaluated by independent board-certified pathologists. Histological review from all cases showed no evidence of acute or chronic colitis. Resident lymphoid cells were present but not conspicuous. There were no abscesses, granulomata, or evidence of other inflammatory processes. Normal histology was observed in 71 of 71 biopsies. No increases in CD4, CD8, CD3, or CD25 cells were observed in colorectal mucosa following Ad26 vaccination. Moreover, no increased expression of activation markers Ki67 or human leukocyte antigen DR (HLA-DR) were observed in colorectal mucosa following Ad26 vaccination (Figures 3A–C and 4). These data show that no overall inflammation or cellular activation was evident by histopathology and immunohistochemistry in colorectal mucosa in humans following Ad26-EnvA vaccination of both Ad26– and Ad26+ subjects.
Figure 3.

Colorectal histopathology. Colorectal histopathology evaluation showing cellular subsets by group and week after vaccination: CD4 and CD8 (A), CD3 and CD25 (B), and Ki67 and human leukocyte antigen DR (HLA-DR) activation markers (C). Dots represent individual responses at a given time point as number of cells per 10 high-power fields (hpf). The red horizontal lines show the mean values at a given time point for the group. A–C, Group 1 vs group 2: P = not significant (week 2 and 24) for CD4 and CD8 (A), CD3 and CD25 (B), and Ki67 and HLA-DR (C). Abbreviation: Ad26, adenovirus serotype 26.
Figure 4.

Mucosal pathology. Representative images of immunohistochemistry from rectal mucosal biopsy specimens including CD3 (A), CD4 (B), CD8 (C), human leukocyte antigen DR (D), and Ki67 (E). All images shown at original magnification of ×200.
Mucosal Cellular Immune Activation
We next assessed the activation status of total and vector-specific CD4+ T lymphocytes in gated cell populations from colorectal mucosa by multiparameter flow cytometry (Figure 5A and 5B). No increased Ki67 activation or CCR5 expression was observed on total or vector-specific CD4+ T lymphocytes in colorectal mucosa following Ad26 vaccination (Figure 5A and B; top panels show total colorectal CD4+ T lymphocytes that express Ki67 or CCR5, bottom panels show Ad26 vector-specific colorectal CD4+ T lymphocytes that express Ki67 or CCR5). These data demonstrate that Ad26-EnvA vaccination of both Ad26– and Ad26+ subjects did not result in increased numbers or activation status of total or vector-specific CD4+ T cells in colorectal mucosa at these time points.
Figure 5.

Ki67 and CCR5+ CD4+ T-cell mucosal activation. A, Percentage of Ki67 expression by total and adenovirus serotype 26 (Ad26)-specific mucosal CD4+ T lymphocytes. B, Percentage of CCR5+ expression by total and Ad26-specific mucosal CD4+ T lymphocytes. A and B, Group 1 vs group 2: P = not significant (weeks 2 and 24) for total and Ad26-specific responses by Ki-67 and CCR5. In the placebo group, filled circles = placebo Ad26− subjects and open circles = placebo Ad26+ subjects at baseline.
DISCUSSION
In this study, we demonstrate that a single intramuscular immunization with the Ad26-EnvA vectored HIV-1 vaccine resulted in both peripheral and mucosal immune responses in the majority of healthy human subjects. The magnitude of peripheral cellular and humoral immune responses in this study was similar to those observed in the first-in-human Ad26-EnvA dose escalation study [6]. Specifically, the T-cell and binding antibody responses in the peripheral blood in the present study were comparable to responses observed following the first dose of 5 × 1010 viral particles of the prior Ad26-EnvA study [6].
Although the T-cell responses by ELISpot assays appeared slightly lower in the baseline Ad26+ subjects in 2 of the 3 time points evaluated, the ICS and ELISA responses were comparable between subjects who were baseline Ad26+ and Ad26–, both in peripheral blood and in colorectal mucosa. In addition, systemic and mucosal responses persisted for at least 6 months in the majority of subjects after a single intramuscular vaccine dose. Although there were a small number of subjects studied, these data suggest that the immunogenicity of this vaccine in Ad26– and Ad26+ subjects appeared similar. These data show that systemically primed immune responses can result in clearly detectable and persistent, for at least 6 months, cellular and humoral immune responses in colorectal mucosa.
The Step Study, which assessed an adenovirus serotype 5–based HIV-1 vaccine, and some nonhuman primate data have raised concerns that a vaccine that induced activated vector-specific or total CD4+ T lymphocytes at the mucosal portal of entry may lead to enhanced HIV-1 acquisition [3, 22, 23, 25–29]. In the current study, we found that Ad26-EnvA immunization elicited Env-specific mucosal humoral and cellular immune responses without detectable vector-specific or total CD4+ T-lymphocyte activation in colorectal mucosa in humans. However, these observations are limited by the few time points assessed and anatomic sites sampled.
Taken together, our data demonstrate that intramuscular immunization with an Ad26-vectored HIV-1 Env vaccine elicits mucosal humoral and cellular Env-specific immune responses without increasing activated total or vector-specific CD4+ T cells in colorectal mucosa. Direct evaluation of mucosal immunogenicity and inflammation will likely be important for future HIV-1 vaccine candidates, as well as for candidate vaccines for other pathogens.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the safety monitoring committee (Drs Peter Wright [Chair], Michael Keefer, and Paul Goepfert); the National Institutes of Health (NIH) Vaccine Research Center for the EnvA vaccine antigen, EnvA protein, and peptides; the Investigational Drug Service at Brigham and Women's Hospital (Alka Patel and Kinara Yang); the clinical research staff at Brigham and Women's Hospital (Rozalia Kocjan, Patrick Falahee, Jon Gothing, Daniel Worrall, Nicolas Issa, Francisco Marty, Robert Tucker, Kathleen Krause, Teress Votto); gastrointestinal surgery staff at Brigham and Women's Hospital (Joel Goldberg); pathology staff at Brigham and Women's Hospital (Gerald S. Pinkus, Alyson Smeedy Campbell, Alana Pradham, and Meghan Smith); research staff at Beth Israel Deaconess Medical Center (Kara Brandariz, Annalena LaPorte, Rebecca Dilan, Giannina Santos, Elizabeth Christian, Alexis Burbank, Avinash Oza, Justin Dalrymple, Elise Zablowsky, Shirin Bajimaya, David Dominguez, and Lauren E. Grandpre); Crucell staff (Laura Digilio, Sandra Kik, Jerry Sadoff, Hanneke Schuitemaker, Jenny Hendriks, Japp Goudsmit); and National Institute of Allergy and Infectious Diseases/NIH staff (Mike Pensiero, Alan Fix, Elizabeth Adams, MaryAnne Luzar).
Financial support. The project was supported in part by the NIH (grant numbers AI060354, AI066305, AI069412, AI078526, AI096040, RR025758); and Ragon Institute of MGH, MIT, and Harvard.
Potential conflicts of interest. M. G. P. and M. W. are employees of Crucell Holland BV. Patents for the Ad26 vector are held in part by Crucell and Beth Israel Deaconess Medical Center. No licensing agreements, royalties, or income are associated with these patents. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammer SM, Sobieszczyk ME, Janes H, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369:2083–92. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perreau M, Welles HC, Harari A, et al. DNA/NYVAC vaccine regimen induces HIV-specific CD4 and CD8 T-cell responses in intestinal mucosa. J Virology. 2011;85:9854–62. doi: 10.1128/JVI.00788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden LR, Walsh SR, Seaman MS, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001) J Infect Dis. 2013;207:240–7. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch DH, Liu J, Peter L, et al. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001) J Infect Dis. 2013;207:248–56. doi: 10.1093/infdis/jis671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch DH, Kik SV, Weverling GJ, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–9. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mast TC, Kierstead L, Gupta SB, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–7. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Rhee EG, Masek-Hammerman K, Teigler JE, Abbink P, Barouch DH. Adenovirus serotype 26 utilizes CD46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. J Virol. 2012;86:10862–5. doi: 10.1128/JVI.00928-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Perreau M, Welles HC, Pellaton C, et al. The number of Toll-like receptor 9-agonist motifs in the adenovirus genome correlates with induction of dendritic cell maturation by adenovirus immune complexes. J Virol. 2012;86:6279–85. doi: 10.1128/JVI.00123-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penaloza-MacMaster P, Provine NM, Ra J, et al. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J Virol. 2013;87:1373–84. doi: 10.1128/JVI.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan WG, Jin HT, West EE, et al. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J Virol. 2013;87:1359–72. doi: 10.1128/JVI.02055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letvin NL, Rao SS, Montefiori DC, et al. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. J Virol. 2011;85:11007–15. doi: 10.1128/JVI.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Ewald BA, Lynch DM, et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol. 2008;82:4844–52. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, O'Brien KL, Lynch DM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprangers MC, Lakhai W, Koudstaal W, et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41:5046–52. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masek-Hammerman K, Li H, Liu J, et al. Mucosal trafficking of vector-specific CD4+ T lymphocytes following vaccination of rhesus monkeys with adenovirus serotype 5. J Virol. 2010;84:9810–6. doi: 10.1128/JVI.01157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien KL, Liu J, King SL, et al. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat Med. 2009;15:873–5. doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutnick NA, Carnathan DG, Dubey SA, et al. Baseline Ad5 serostatus does not predict Ad5 HIV vaccine-induced expansion of adenovirus-specific CD4+ T cells. Nat Med. 2009;15:876–8. doi: 10.1038/nm.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frahm N, DeCamp AC, Friedrich DP, et al. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest. 2012;122:359–67. doi: 10.1172/JCI60202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fauci AS, Marovich MA, Dieffenbach CW, Hunter E, Buchbinder SP. Immunology. Immune activation with HIV vaccines. Science. 2014;344:49–51. doi: 10.1126/science.1250672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray GE, Allen M, Moodie Z, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11:507–15. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duerr A, Huang Y, Buchbinder S, et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J Infect Dis. 2012;206:258–66. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bukh I, Calcedo R, Roy S, et al. Increased mucosal CD4+ T cell activation following vaccination with an adenoviral vector in rhesus macaques. J Virol. 2014;88:8468–78. doi: 10.1128/JVI.03850-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


