Abstract
Background: We investigated the expression of CXCR4, CCR7, estrogen receptor (ER), progesterone receptor (PR) and HER2-neu in human metastatic breast cancers to determine whether these biological biomarkers were preferentially expressed in any organ-specific metastases.
Materials and methods: CXCR4, CCR7, ER, PR and HER2-neu expression levels were evaluated by immunohistochemical staining using paraffin-embedded tissue sections of metastatic breast cancers (n = 41) obtained by either diagnostic biopsy or surgical resection.
Results: The metastatic sites included the following: bone (n = 15), brain (n = 14), lung (n = 6), liver (n = 2), and omental metastases (n = 2). CXCR4 was expressed in 41% of cases, CCR7 expression was demonstrated in 10%, and HER2-neu overexpression was present in 27%. CXCR4 was more likely to be expressed in bone metastases than visceral metastases (67% versus 26%, P = 0.020). Visceral sites demonstrated a lower rate of CXCR4 positivity (33% and 23%, respectively, for lung and brain metastases). Similarly, CCR7 was more likely to be found in bone metastases than visceral sites (27% versus 0%, P = 0.037).
Conclusions: These results indicate that CXCR4 can contribute to the homing of breast cancer cells to the bone. This finding might have important clinical implications since patients with metastatic bone disease may achieve the highest benefit from a CXCR4-targeted therapy.
Keywords: bone metastases, CCR7, CXCR4, HER2-neu, metastatic breast cancer
introduction
The clinical behavior of primary breast cancer (PBC) is characterized by a long natural history and by clinical course heterogeneity among patients. Some of this heterogeneity is explained by differences in tumor growth rates, invasiveness, metastatic potential, and other mechanisms. The use of systemic adjuvant treatments reduces the risk of recurrence by 30%–50%. The failure of systemic treatments to completely prevent clinical evidence of recurrence is related to the persistence of cells or established colonies representing minimal residual disease. Once breast cancer progresses to the metastatic stage, current medical treatments have proven to be unable to eradicate the disease. Despite the fact that combination chemotherapy regimens elicit a 50%–70% objective remission rate in patients with metastatic breast carcinoma, complete response is uncommon and occurs in <20% [1]. Improvements in these response rates have been the subject of intense investigation and clearly new agents and/or strategies are being sought.
Several biological markers have been proposed as predictors of prognosis and target for treatments. Presently, only hormonal receptors and HER2-neu status have been demonstrated to be useful for these purposes and found widespread application [2]. Other markers have been proposed and are under investigation; these include the chemokine receptors [3–7].
The organ preference for metastatic disease is influenced by interactions between the circulating tumor cells (the seed) and the target host tissue (the soil). Chemokines are a superfamily of small, cytokine-like proteins that induce, through their interaction with G-protein-coupled receptors, cytoskeleton rearrangement, adhesion to endothelial cells, and directional migration [8]. These secreted proteins act in a coordinated fashion with cell-surface proteins to direct the specific homing of various subsets of hematopoietic cells to specific anatomical sites. It is well known that hematopoietic stem cells also home to bone during fetal life and during marrow transplantation. In this context, a CXC chemokine and its receptor, CXCR4 appear to be critical for these events. Similarly, tumor cells co-opt the same mechanisms to direct metastatic organ preference [9]. The CXCR4 ligand, CXCL12/SDF-1α, was preferentially expressed in organs like liver, bone marrow, lung, and lymph nodes whereas the CCR7 ligand, CCL21, was highly expressed in lymph nodes [9]. Therefore, the receptor/ligand pair CXCR4/CXCL12 was shown to interact in breast cancer metastases homing to bone, lung, and liver, and CCR7/CCL21 was demonstrated to play an important role in lymph node metastases.
HER2-neu is a well-known biomarker associated with increased metastatic potential in breast cancer [2]. However, there are limited studies regarding the predictive value of HER2-neu overexpression in breast cancer to direct metastatic organ preference [10]. We previously showed cross talk between CXCR4 and HER2-neu via HER2-neu transactivation by the SDF-1α/CXCR4 axis [11]. In fact, high CXCR4 expression along with CXCR4 coexpression with EGFR/HER2-neu was associated with bone marrow micrometastases in patients with breast cancer [12]. Furthermore, CXCR4 expression in primary tumor predicted liver metastases in patients with axillary node-positive breast cancer [13]. Therefore, we studied the differential expression of CXCR4, ER, PR, HER2-neu, and CCR7 in human primary and metastatic cancers to determine if these biological markers were preferentially expressed in any organ-specific metastasis. We also sought to determine the association between CXCR4 expression and hormone receptor and HER-2 status.
materials and methods
The Institutional Review Board of the University of Texas MD Anderson Cancer Center, approved this retrospective study and granted a waiver of informed consent. Sections of paraffin-embedded tissue samples were provided by the Institution's Breast Tumor Bank, retrospectively collected archival tissue material of metastatic breast cancers (n = 41) that had undergone surgical resection or diagnostic biopsy of the metastatic site along with their corresponding primary breast tumors. Patient samples obtained following completion of preoperative chemotherapy because of locally advanced disease were also included since we previously shown that chemotherapy does not adversely affect CXCR4 or CCR7 expression in inflammatory breast cancer patients by immunohistochemical analysis [14].
immunohistochemical analysis
The biomarkers including CCR7, CXCR4, ER, PR, and HER2-neu were assessed by immunohistochemical staining. The avidin–biotin complex method was used as reported before [15]. Tissue sections were deparaffinized and incubated by 0.3% H2O2 in phosphate-buffered saline (PBS) for CXCR4 and HER2-neu or by methanol for CCR7 to block endogenous peroxidase activity in the samples. Furthermore, a protein-blocking solution containing 5% normal horse serum and 1% normal goat serum in PBS was used to block nonspecific binding. Sections were incubated with the following antibodies, both for 18 h at 4°C: a primary antibody for CXCR4 (44.111, IgG2b, R&D Systems, Minneapolis, MN) at 1 : 150 dilution and for CCR7 (2H4, IgM, BD Biosciences, San Diego, CA) at 1 : 100 dilution. Sections were then incubated with a rat antimouse-IgG2b–horseradish peroxidase (HRP) (Serotec Inc., Raleigh, NC) for CXCR4 and with a goat-antimouse-IgM–HRP (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for CCR7. Color was developed with diaminobenzidine, and sections were counterstained with hematoxylin. Standard immunohistochemical staining of the diagnostic was carried out using the modified avidin–biotin complex method in a DAKO autostainer (DAKO, Carpinteria, CA) using primary antibodies against estrogen receptor (ER)-α (ER, clone: 6F11, Novocastra, 1 : 50) and progesterone receptor (PR) (PR Ab-9, clone: 1A6, NeoMarkers, Lab Vision Corporation, Fremont, CA, 1 : 30). HER2-neu antigen retrieval by microwave was required before incubation with the primary antibody (clone AB8, NeoMarkers, Lab Vision Corporation), at 1 : 300 dilution at room temperature.
interpretation of staining patterns
The intensity, staining percentage, and pattern of staining (nuclear and cytoplasmic) were assessed for CXCR4 and CCR7. The intensity was scored as low, moderate, and strong compared with background staining. The percentages of positive cells were estimated by the ratio of the positively stained invasive tumor cells to the total invasive tumor cells according to a scoring system, as reported before [15]. Briefly, moderate staining on >50% of cells or any strong cytoplasmic staining was considered positive for CXCR4 staining whereas strong cytoplasmic staining >50% was defined as positive for CCR7 staining. A predominantly nuclear staining for CCR7 or CXCR4 was considered positive when >80% of the tumor cells showed nuclear staining regardless of the presence of cytoplasmic staining. Furthermore, ER and PR were considered positive if nuclear staining of >5% of invasive tumor cells was observed. HER2-/neu was considered positive if >10% of tumor cells showed complete and strong membranous staining (HerceptTest 3+).
statistical analysis
The SPSS 10.1 software package (SPSS, Inc., Chicago, IL) and SAS 9.1 (SAS Institute, Inc., Cary, NC) were used for statistical analysis. The two-sided chi-square test or two-sided Fisher's exact test was used in categorical variables. Phi coefficient was used to assess the strength of the association between expression of a biomarker in primary tumors and the expression in the corresponding metastatic tumor. A P value of ≤0.05 was considered statistically significant. Missing cases were excluded from the analysis for each marker.
results
characteristics of patients and primary tumors
Patient and primary tumor characteristics are shown in Table 1. The median age was 44 years (27–79). Twenty-one patients (51%) were diagnosed with stage I (n = 6) or II (n = 15) disease, whereas 20 patients (49%) were diagnosed with stage III (n = 18) or IV (n = 2) disease. In the pathological assessment of the tumors, the majority of the tumors (80%) were poorly differentiated (60%) or intermediately differentiated (35%) invasive ductal carcinomas. Twelve patients (29%) with stage II/III disease received preoperative chemotherapy while 25 patients (61%) received adjuvant chemotherapy following surgery, and four patients (10%) with stage I ER-positive disease received only adjuvant hormonal therapy. Two patients with stage IV disease received chemotherapy after the biopsies of the metastatic bones. The median time from the diagnosis of cancer to the detection of first metastasis was 28 months (5–131).
Table 1.
Patient and primary tumor characteristics
| Characteristics | N |
| Median age (range, minimum–maximum) | 44 (27–79) |
| Stage of breast cancera | |
| I and II | 21 (51%) |
| III and IV | 20 (49%) |
| Histologic grade (n = 37) | |
| Well differentiated | 2 (5%) |
| Intermediately differentiated | 13 (35%) |
| Poorly differentiated | 22 (60%) |
| Tumor histology (n = 37) | |
| Invasive ductal carcinoma | 30 (81%) |
| Invasive lobular carcinoma | 2 (5%) |
| Invasive ductal and lobular mixed type | 4 (11%) |
| Invasive cancer | 1 (3%) |
| CCR7 (n = 29) | |
| + | 3 (10%) |
| − | 26 (90%) |
| CXCR4 (n = 29) | |
| + | 16 (55%) |
| − | 13 (45%) |
| HER2-neu (n = 39) | |
| + | 11 (27 %) |
| − | 28 (73 %) |
| CXCR4/HER2-neu (n = 29) | |
| + | 5 (17%) |
| − | 24 (83%) |
| ER (n = 36) | |
| + | 24 (67%) |
| − | 12 (33%) |
| PR (n = 33) | |
| + | 18 (55%) |
| − | 15 (45%) |
Sixth edition of American Joint Committee on Cancer Staging Manual.
bMissing cases were excluded from the analyses.
ER, estrogen receptor; PR, progesterone receptor.
According to the differential staining, 67% of the tumors were ER positive, whereas 55% of them were PR positive. Furthermore, more than half (55%) were shown to be CXCR4 positive, and 25% of the tumors were HER2 positive. However, 17% of the tumors were positive for both CXCR4 and HER2, while only 10% of the tumors were detected to be CCR7 positive.
expression of chemokine receptors and HER2-neu in metastatic breast tumors
The distribution of CXCR4, CCR7, and HER2-neu staining according to specific organs is shown in Table 2. CXCR4 was expressed in 41% of the metastatic tumors (16 of 39) (Figure 1A–D), whereas CCR7 expression was demonstrated in 10% of those same tumors (3 of 31) (Figure 1E). HER2-neu overexpression was present in 27% (9 of 34) (Figure 1F), whereas 13% (4 of 31) of the metastatic tumors expressed both HER2-neu and CXCR4. In this study, a predominantly nuclear staining was exclusively observed in three primary tumors either for CCR7 expression (n = 2) or CXCR4 expression (n = 1), whereas none of the metastatic tumors showed a predominantly nuclear staining pattern for these chemokine receptors. In our series of metastatic tumors, the predominant staining pattern of CXCR4 and CCR7 was cytoplasmic (Figure 1A–E).
Table 2.
Chemokine receptor expression in metastatic breast cancers
| Tumor characteristics | Bone (N = 15) | Visceral |
P | |||
| Brain (n = 14) | Lung (n = 6) | Liver/periton (n = 4) | Total (N = 24) | |||
| CXCR4metastatic tumor (n = 39) | 10/15 (67%) | 3/13 (23%) | 2/6 (33%) | 0/4 (0%) | 6/23 (26%) | 0.020 |
| HER2-neumetastatic tumor (n = 34) | 2/12 (17%) | 5/14 (36%) | 2/5 (40%) | 0/3 (0%) | 7/22 (32%) | 0.439 |
| CXCR4/HER2-neumetastatic tumor (n = 31) | 2/10 (20%) | 1/13 (8%) | 1/5 (20%) | 0/3 (0%) | 2/21 (10%) | 0.577 |
| CCR7metastatic tumor (n = 31) | 3/11 (27%) | 0/13 (0%) | 0/4 (0%) | 0/3 (0%) | 0/20 (0%) | 0.037 |
Figure 1.
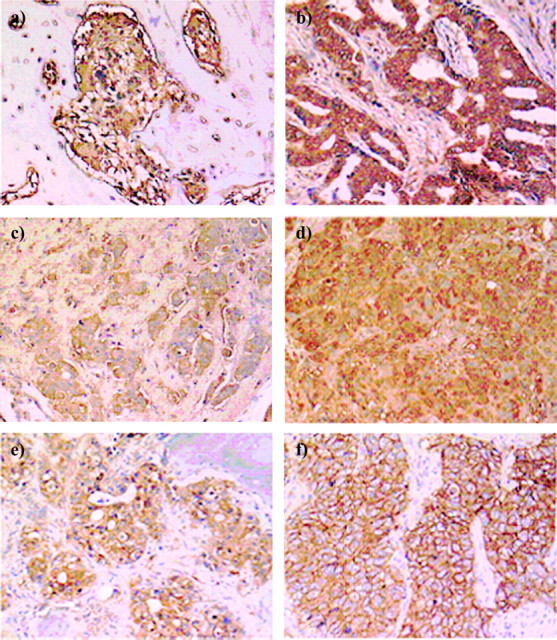
(A and B) Immunohistochemical staining for bone metastases with high cytoplasmic CXCR4 expression (×10). (C) Immunohistochemical staining for a brain metastasis with high cytoplasmic CXCR4 expression (×10). (D) Immunohistochemical staining for a lung metastasis with high cytoplasmic CXCR4 expression (×10). (E) Immunohistochemical staining for a bone metastasis with high cytoplasmic CCR7 expression (×10). (F) Immunohistochemical staining for a lung metastasis with HercepTest 3+ HER2/neu expression (×10).
Bone metastases were more likely to express CXCR4 compared with visceral metastases (bone, 67% versus visceral, 26%; P = 0.02). Similarly, CCR7 expression was exclusively found in bone metastases, whereas none of the visceral metastases were found to express CCR7 (bone, 27% versus visceral, 0%; P = 0.037). Unlike CXCR4 and CCR7 (Table 1), HER2-neu overexpression was more likely to be found in visceral (32%) than in bone metastases (17%). However, this association did not approach statistical significance (P = 0.439). No association was found between HER2/neu positivity in CXCR4-positive (5 of 16, 31%) or CXCR4-negative primary tumors (4 of 13, 31%) (P = 0.999). Similarly, no statistical difference was found between HER2-neu overexpression in CXCR4-positive (4 of 10, 40%) or CXCR4-negative (4 of 21, 19%) metastatic tumors (P = 0.381). All primary tumors of CXCR4-positive bone metastases (n = 8) expressed ER or PR, whereas this expression pattern was found in only one of six (17%) CXCR4-positive visceral metastases (P = 0.046).
correlations of chemokine receptor expression and HER2-neu in primary tumors with the corresponding metastatic tumors
Correlations of CXCR4 and CCR7 and HER2-neu expressions in primary tumors with matched metastatic tumors are shown in Table 3. It appears that CXCR4 is less likely to be positive in metastatic deposits of those with CXCR4-positive primary tumor (3 of 14, 21%) than for those with CXCR4-negative primary tumor (5 of 13, 38%). However, the association between the CXCR4 status in primary tumor and in metastatic tumor is not statistically significant (P = 0.42, Fisher’s exact test, Phi coefficient = −0.19). Those with positive expression of CCR7 in the primary tumor were more likely to have positive CCR7 expression in metastatic tumor (one of three, 33%) than those with negative CCR7 in the primary tumor (2 of 26, 8%), even though the association was not statistically significant (P = 0.29, Fisher’s exact test, Phi coefficient = 0.26). HER2-neu expression in metastatic tumor was highly associated with its expression in primary tumor (P = 0.0017, Phi coefficient = 0.58). In all, 64% (7 of 11) of the positive primary tumors also showed positive HER2-neu expression in the metastatic tumors, while only 9% (2 of 23) of the primary negative tumors showed positive HER2-neu expression in their corresponding metastatic tumors.
Table 3.
Concordances of chemokine receptors CXCR4 and CCR7, and HER2-neu expression in matched primary and metastatic tumors
| Variable | CXCR4metastatic tumor (−) | CXCR4metastatic tumor (+) | N |
| CXCR4primary tumor (−) | 8 | 5 | 13 |
| CXCR4primary tumor (+) | 11 | 3 | 14 |
| 19 | 8 | 27 | |
| CCR7metastatic tumor (−) | CCR7metastatic tumor (+) | ||
| CCR7primary tumor (−) | 24 | 2 | 26 |
| CCR7primary tumor (+) | 2 | 1 | 3 |
| 26 | 3 | 29 | |
| HER2-neumetastatic tumor (−) | HER2-neumetastatic tumor (+) | ||
| HER2-neuprimary tumor (−) | 21 | 2 | 23 |
| HER2-neuprimary tumor (+) | 4 | 7 | 11 |
| 25 | 9 | 34 |
discussion
In the present study, we investigated a series of matched primary and metastatic breast tumors, to demonstrate whether the expression of chemokine receptors CXCR4 and CCR7, novel biomarkers that were recently found to be associated with increased metastatic potential in preclinical models of breast cancer [9, 16–19], along with HER2-neu, were preferentially expressed in any organ-specific breast cancer metastases. Our findings demonstrated that CCR7 was exclusively detected in bone metastases whereas CXCR4 was found to be expressed in both bone and visceral metastases. Interestingly, however, the expression of CXCR4 was significantly more frequently detected in bone metastases compared with visceral metastases.
The CCR7 chemokine receptor ligand CCL21 was found to be highly expressed in lymph nodes [9]. CCR7 is usually demonstrated to be involved in the establishment of lymph node metastases in different cancer types including breast cancer, lung cancer, esophageal, and gastric carcinoma [15, 20–23]. Due to the lack of data in the published literature, it is surprising that CCR7 was exclusively detected in 27% of bone metastases in our series. Muller et al. [9] showed increased chemotaxis and chemoinvasion of MBA-231 breast cancer cells toward CCL21 gradients indicating that CCR7/CCL21 signaling may trigger the development of breast cancer metastases. Furthermore, in our previous report [14], patients with inflammatory breast cancer that expressed high levels of CCR7 were more likely to have poorer disease-free survival (CCF7−, 32% versus CCR7+, 18%; P = 0.502) and disease-specific survival (CCR7−, 36% versus CCR7+, 18%; P = 0.181) compared with those with inflammatory breast cancer that expressed low levels of CCR7. Therefore, these findings indicate that CCR7 expression in PBC may indicate a more aggressive tumor phenotype. However, it warrants further investigation how CCR7 was preferentially expressed in bone similar to the chemokine receptor CXCR4.
The observation that the majority of bone metastases of patients with breast cancer in the current study expressed high levels of CXCR4 indicates that the CXCL12/CXCR4 axis may play an important role in the pathogenesis of bone metastases in breast cancer in concordance with numerous previous studies [24–30]. CXCR4 appears to be associated with an increase rate of bone metastases in breast and prostate cancer (PC) [24–30]. Osteoblasts and marrow endothelial cells were shown to express SDF-1 protein, and PC cells were observed migrating across bone marrow endothelial cell monolayers in response to SDF-1 [28]. Furthermore, in in vitro adhesion assays, pretreatment of PC cells with SDF-1 significantly increased their adhesion to osteosarcomas and endothelial cells and such increased adhesion was inhibited by the CXCR4 antibody [28]. In vivo, direct intratibial injections of PC cells followed by neutralizing CXCR4 antibody or a specific peptide that blocks CXCR4 inhibited the intraosseous growth of tumor cells compared with controls [29]. Chinni et al. [30] reported that bone stromal cells and bone tissue-conditioned media induced the migration of PC cells through CXCL12/CXCR4-signaling pathways that could be blocked by a CXCR4 antibody. Furthermore, exogenous CXCL12 induced MMP-9 gene expression and chemoinvasion of PC cells that could be abrogated by specific pharmacological inhibition of phosphoinositide 3-kinase and mitogen-activated protein kinase pathways.
Similarly, Muller et al. [9] reported that CXCR4/CXCL12 signaling induced chemotaxis and migration of breast cancer cells. Kang et al. [26] interestingly demonstrated that CXCR4 has been found as one of the limited number of genes that are enriched in a subpopulation of the breast cancer cell line MDA231 with enhanced metastatic potential to bone, and overexpression of CXCR4 alone significantly increased the number of bone metastasis in vivo. Furthermore, high CXCR4 expression in the primary tumor has been found to be associated with bone marrow and/or bone metastases in breast cancer or neuroblastoma [12, 31]. All these data indicate a close link between CXCR4/CXCL12 signaling and the development of bone metastases. Moreover, all the primary tumors of the bone metastases in our series were ER and/or PR positive. CXCL12 was also shown to be a proliferative and chemotactic factor particularly for ER-positive breast cancer suggesting that these patients may benefit from an antiestrogen-based therapy [32].
In our series, CXCR4 was also found to be highly expressed in visceral metastases including lung and brain. As reported previously, the CXCR4 ligand, CXCL12, has been expressed in lung, liver, and brain [33]. Expression of CXCR4 has been demonstrated to be associated with lung metastases in breast cancer, malignant melanoma, and primary brain tumors [9, 19, 34–37]. Furthermore, lung metastasis of breast cancer cells in immunodeficient mice could be inhibited by a neutralizing antibody against CXCR4 [9], or the growth of the lung metastases could be inhibited by using small interfering RNA duplexes of CXCR4 [34, 36] or by specific peptide CXCR4 antagonists including TN14003 or AMD3100 [19, 34]. In concordance with our findings, all these data indicate that CXCR4 plays a potentially critical role in promoting lung metastases or brain tumors. A recent phase I/II study of CTCE-9908, a novel anticancer agent that inhibits CXCR4, in patients with advanced solid cancer including patients with breast cancer indicated that CTCE-9908 as a single anticancer agent has been well tolerated and has shown preliminary signs of efficacy [38]. However, further detailed studies are warranted to explore its efficacy in certain patient populations with CXCR4-positive metastatic cancers as targeted therapy and/or in combination with chemotherapy regimen.
In the current study, our findings showed HER2-neu overexpression in visceral and bone metastases favoring visceral organ metastases including brain and lung in concordance with previous data [39, 40]. In the recent study of Palmieri et al. [39], 36.2% of 124 archival brain metastases from patients with breast cancer overexpressed HER2-neu. In vivo, HER2-neu-transfected MBA-231-BR cells, which selectively metastasize to brain, yielded larger tumors in the brain compared with low-HER2-neu-expressing 231-BR clones indicating that HER2-neu overexpression may increase the outgrowth of brain metastases. Along with visceral organ metastases, HER2-neu overexpression was detected in 10% of bone metastases in the present study comparable to the study reported by Lorincz et al. [41]. Interestingly, all the bone metastases with HER2-neu overexpression also coexpressed CXCR4.
Our previous study demonstrated HER2-neu transactivation through CXCL12/SDF-1α signaling of breast cancer cell lines [22], and CXCR4 expression alone and/or with HER2-neu overexpression was associated with bone marrow metastases in patients with breast cancer [12]. Furthermore, few patients with high cytokeratin positivity in bone marrow exclusively expressed high levels of CXCR4 with EGFR/HER2-neu. Therefore, it is intriguing whether breast cancer cells coexpressing HER2-neu with high cytoplasmic CXCR4, as opposed to nuclear could reveal enhanced metastatic behavior. However, due to the small percentage of CXCR4/HER2-neu positivity in metastatic samples (4 of 31, 13%) in our series, it is difficult to draw conclusions about this aspect of our study. A recent report has further suggested that expression of CXCR4 could be regulated by HER2-neu by increasing the expression of CXCR4, which is required for HER2-mediated invasion in vitro and lung metastases in vivo [42]. CXCR4 expression was also associated with HER2-neu overexpression in concordance with some reports [43]. However, this remains a controversial issue since we and others could not confirm a correlation between HER2-neu and CXCR4 overexpression in PBC [44, 45]. These discrepancies might be due to the differences in scoring of CXCR4 and HER2-neu. Furthermore, even though HER2-neu overexpression was concordant in the majority of matched primary and metastatic samples as established before in many studies [40, 41, 46, 47], the concordance of positive expression of chemokine receptors, CXCR4 and CCR7, in matched primary and metastatic samples was strikingly poor and warrants further investigation in future.
In conclusion, our data indicate that chemokine receptors are differentially expressed in metastatic sites and particularly CXCR4/SDF-1 axis can contribute to the homing of breast cancer cells to the bone. Our results might have important clinical implications since patients with metastatic bone disease or life-threatening visceral metastases may achieve the highest benefit from CXCR4-targeted therapy.
funding
Nellie B. Connally Breast Cancer Research Fund.
Acknowledgments
Part of this study was presented at the American Association of Cancer Research, 2004.
References
- 1.Robain M, Pierga JY, Jouve M, et al. Predictive factors of response to first-line chemotherapy in 1426 women with metastatic breast cancer. Eur J Cancer. 2000;36:2301–2312. doi: 10.1016/s0959-8049(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 2.Meric FM, Hung MC, Hortobagyi GN, Hunt KK. HER2/neu in the management of invasive breast cancer. J Am Coll Surg. 2002;194:488–501. doi: 10.1016/s1072-7515(02)01121-3. [DOI] [PubMed] [Google Scholar]
- 3.Bogenrieder T. Axis of evil: molecular mechanisms of cancer metastases. Oncogene. 2003;22:6524–6539. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 4.Zlotnik A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004;14:181–185. doi: 10.1016/j.semcancer.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 6.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–2029. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 7.Ruffini PA, Morandi P, Cabioglu N, et al. Manipulating the chemokine-chemokine receptor network to treat cancer. Cancer. 2007;109:2392–2404. doi: 10.1002/cncr.22706. [DOI] [PubMed] [Google Scholar]
- 8.Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 9.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 10.Yi B, Williams PJ, Niewolna M, et al. Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res. 2002;62:917–923. [PubMed] [Google Scholar]
- 11.Cabioglu N, Summy J, Miller C, et al. CXCL12-/Stromal cell-derived factor-1 alpha transactivates HER2-neu in breast cancer cells by a novel pathway involving Src-kinase activation. Cancer Res. 2005;65:6493–6497. doi: 10.1158/0008-5472.CAN-04-1303. [DOI] [PubMed] [Google Scholar]
- 12.Cabioglu N, Sahin AA, Doucet M, et al. Chemokine receptor CXCR4 expression in breast cancer as a potential predictive marker of isolated tumor cells in bone marrow. Clin Exp Metastasis. 2005;22:39–46. doi: 10.1007/s10585-005-3222-y. [DOI] [PubMed] [Google Scholar]
- 13.Andre F, Cabioglu N, Assi H, et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol. 2006;17:945–951. doi: 10.1093/annonc/mdl053. [DOI] [PubMed] [Google Scholar]
- 14.Cabioglu N, Gong Y, Islam R, et al. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann Oncol. 2007;18:1021–1029. doi: 10.1093/annonc/mdm060. [DOI] [PubMed] [Google Scholar]
- 15.Cabioglu N, Yazici MS, Arun B, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 16.Helbig G, Christopherson KW, II, Bhat-Nakshatri P, et al. NF-kappa B promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 17.Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2:327–338. [PubMed] [Google Scholar]
- 18.Fernandis AZ, Prasad A, Band H, et al. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23:157–167. doi: 10.1038/sj.onc.1206910. [DOI] [PubMed] [Google Scholar]
- 19.Liang Z, Wu T, Lou H, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–4308. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 20.Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 2003;5:144–150. doi: 10.1186/bcr627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takanami I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. Int J Cancer. 2003;105:186–189. doi: 10.1002/ijc.11063. [DOI] [PubMed] [Google Scholar]
- 22.Ding Y, Shimada Y, Maeda M, et al. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res. 2003;9:3406–3412. [PubMed] [Google Scholar]
- 23.Mashino K, Sadanaga N, Yamaguchi H, et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–2941. [PubMed] [Google Scholar]
- 24.Sloan EK, Anderson RL. Genes involved in breast cancer metastases to bone [review] Cell Mol Life Sci. 2002;59:1491–1502. doi: 10.1007/s00018-002-8524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–180. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- 26.Kang H, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Loberg R, Taichman RS. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastases. Cancer Metastasis Rev. 2006;25:573–587. doi: 10.1007/s10555-006-9019-x. [DOI] [PubMed] [Google Scholar]
- 28.Taichman RS, Cooper C, Keller ET. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 29.Sun YX, Schneider A, Jung Y, et al. Skeletal localization and neutralization of the SDF-1/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 30.Chinni SR, Sivalogan S, Dong Z, et al. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate. 2005;9999:1–17. doi: 10.1002/pros.20318. [DOI] [PubMed] [Google Scholar]
- 31.Russell HV, Hicks J, Okcu MF, Nuchtern JG. CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J Pediatr Surg. 2004;39:1506–1511. doi: 10.1016/j.jpedsurg.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Hall JM, Korach KS. Stromal cell-derived factor 1, a novel target for estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2005;17:792–803. doi: 10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- 33.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 34.Smith MCP, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 35.Murakami T, Maki W, Cardones AR, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- 36.Liang Z, Yoon Y, Votaw J, et al. Silencing of CXCR4 blocks breast cancer metastases. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotte SJ, Hirte HW, Moretto P, et al. Final results of a Phase I/II study of CTCE-9908, a novel anticancer agent that inhibits CXCR4, in patients with advanced solid cancers. 20th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics Abstract Book, Geneva, Switzerland, October 21–24, 2008. Eur J Cancer Suppl 2008; 6: 192. [Google Scholar]
- 39.Palmieri D, Bronder JL, Herring JM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 40.Tapia C, Savic S, Wagner U, et al. HER2 gene status in primary breast cancers and matched distant metastases. Breast Cancer Res. 2007;9:R31. doi: 10.1186/bcr1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorincz T, Toth J, Badalian G, et al. HER-2/neu genotype of breast cancer may change in bone metastasis. Pathol Oncol Res. 2006;12:149–152. doi: 10.1007/BF02893361. [DOI] [PubMed] [Google Scholar]
- 42.Li YM, Pan Y, Wei Y, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Salvucci O, Bouchard A, Baccarelli A, et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006;97:275–283. doi: 10.1007/s10549-005-9121-8. [DOI] [PubMed] [Google Scholar]
- 44.Holm NT, Brynes K, Li BDL, et al. Elevated levels of chemokine receptor CXCR4 in HER-2 negative breast cancer specimens predict recurrence. J Surg Res. 2007;141:53–59. doi: 10.1016/j.jss.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Kim R, Arihiro K, Emi M, et al. Potential role of HER-2 in primary breast tumor with bone metastasis. Oncol Rep. 2006;15:1477–1484. [PubMed] [Google Scholar]
- 46.Gong Y, Booser DJ, Sneige N. Comparison of HER-2 status determined by fluorescence in situ hybridization in primary and metastatic breast carcinoma. Cancer. 2005;103:1763–1769. doi: 10.1002/cncr.20987. [DOI] [PubMed] [Google Scholar]
- 47.Gancberg D, Di Leo A, Cardoso F, et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol. 2002;13:1036–1043. doi: 10.1093/annonc/mdf252. [DOI] [PubMed] [Google Scholar]


