Herbaspirillum species are capable of causing systemic infections in immunocompromised patients. It may present as a nosocomial common-source cluster or sporadic hospital or community-onset infection and is most often misidentified as Burkholderia cepacia.
Keywords: Herbaspirillum, cancer, outbreak, immunocompromised, misdiagnosis
Abstract
Background. Herbaspirillum species are gram-negative Betaproteobacteria that inhabit the rhizosphere. We investigated a potential cluster of hospital-based Herbaspirillum species infections.
Methods. Cases were defined as Herbaspirillum species isolated from a patient in our comprehensive cancer center between 1 January 2006 and 15 October 2013. Case finding was performed by reviewing isolates initially identified as Burkholderia cepacia susceptible to all antibiotics tested, and 16S ribosomal DNA sequencing of available isolates to confirm their identity. Pulsed-field gel electrophoresis (PFGE) was performed to test genetic relatedness. Facility observations, infection prevention assessments, and environmental sampling were performed to investigate potential sources of Herbaspirillum species.
Results. Eight cases of Herbaspirillum species were identified. Isolates from the first 5 clustered cases were initially misidentified as B. cepacia, and available isolates from 4 of these cases were indistinguishable. The 3 subsequent cases were identified by prospective surveillance and had different PFGE patterns. All but 1 case-patient had bloodstream infections, and 6 presented with sepsis. Underlying diagnoses included solid tumors (3), leukemia (3), lymphoma (1), and aplastic anemia (1). Herbaspirillum species infections were hospital-onset in 5 patients and community-onset in 3. All symptomatic patients were treated with intravenous antibiotics, and their infections resolved. No environmental source or common mechanism of acquisition was identified.
Conclusions. This is the first report of a hospital-based cluster of Herbaspirillum species infections. Herbaspirillum species are capable of causing bacteremia and sepsis in immunocompromised patients. Herbaspirillum species can be misidentified as Burkholderia cepacia by commercially available microbial identification systems.
Herbaspirillum species are gram-negative, flagellate members of the Betaproteobacteria class of bacteria, which includes Burkholderia, Ralstonia, and other plant-associated bacteria. Most species of Herbaspirillum are nitrogen-fixing bacteria that are commonly found in the rhizosphere and colonize plants endophytically. They are found in the roots and stems of maize, rice, beans, bananas, sugar cane, pineapple, and other plants [1, 2] and they have been recovered from groundwater [3] and drinking water distribution systems [4] as well. In the published literature, Herbaspirillum species have been recovered from respiratory secretions of patients with cystic fibrosis (CF) [5, 6] and described in case reports of 4 patients with bacteremia, 2 of whom had an underlying malignancy [6–9]. Its close phylogenetic and phenotypic resemblance to Burkholderia cepacia complex led to initial misidentification in 2 of the 4 reported cases [6, 9]. To our knowledge, no outbreaks or clusters of cases have been previously reported in the literature.
Between June and August 2011, we identified an initial cluster of 5 patients with bloodstream infection caused by a gram-negative bacillus, initially identified as B. cepacia complex. The isolates were susceptible to all antibiotics tested, whereas over the years, previous B. cepacia isolates recovered in our institution were resistant to at least 1 and up to 4 of the antibiotics tested. These isolates were submitted to the Cystic Fibrosis Foundation Burkholderia cepacia Research Laboratory and Repository at the University of Michigan and were subsequently reidentified as Herbaspirillum species.
We conducted a joint investigation with the Texas Department of State Health Services (TDSHS) and the Centers for Disease Control and Prevention (CDC) to determine the nature and extent of the cluster of Herbaspirillum species infections. This report describes the clinical and epidemiologic findings from our investigation and provides a review of the literature.
MATERIALS AND METHODS
Setting
The University of Texas MD Anderson Cancer Center is a National Cancer Institute–designated comprehensive cancer center in Houston, Texas, with approximately 620 beds, 25 000 admissions in 2011, and >1 million patient visits per year.
Case Definition
We defined a case as a Herbaspirillum species clinical isolate confirmed by genetic sequencing in a patient who received care at MD Anderson between 1 January 2006 and 15 October 2013. The University of Texas MD Anderson Cancer Center Institutional Review Board approved this study and a waiver for informed consent was granted.
Case Finding
We performed a retrospective laboratory review of B. cepacia clinical isolates recovered at our institution from 1 January 2006 to 24 October 2011, to identify Herbaspirillum species cases that may have been misidentified previously, and we reviewed microbiology records prospectively until 15 October 2013 to identify additional cases. Antibiotic susceptibility was determined using Etest and interpreted according to Clinical and Laboratory Standards Institute (CLSI) 2011 standards. All pan-sensitive B. cepacia clinical isolates were submitted to the Cystic Fibrosis Foundation Burkholderia cepacia Research Laboratory and Repository at the University of Michigan for 16S ribosomal DNA (16s rDNA) sequencing as described previously [6, 10]. Any isolate identified as Herbaspirillum species was sent to TDSHS, where pulsed-field gel electrophoresis (PFGE) was performed according to methods previously described [11].
Case Description
We reviewed the medical records of all Herbaspirillum species cases and reviewed their demographic and clinical characteristics. We also assessed for any common exposures among identified cases.
Definitions
Herbaspirillum species case-patients were considered to have sepsis if they had any 2 of the following: heart rate >90 beats per minute; respiratory rate >20 breaths per minute; temperature >38°C or <36°C; or white blood cell count >12 000 cells/µL or <4000 cells/µL or >10% bands. For this investigation, catheter-related bloodstream infection (CRBSI) was defined as per the guidelines of the Infectious Diseases Society of America [12]. Herbaspirillum species infections were considered community-onset when cultures were drawn as an outpatient or prior to hospital day 3, or hospital-onset when the infection was diagnosed after 3 days of hospitalization and within 3 days of discharge.
Facility Observations and Infection Prevention Assessments
We conducted an in-depth site inspection between October and November 2011, which included an inspection and review of the schematics of the hospital water system to determine the water supply for rooms occupied by case-patients. We also observed the handling, transport, and processing of blood cultures to exclude laboratory contamination.
We observed and interviewed healthcare personnel within selected areas of the hospital where case-patients had received care. The following infection prevention practices were assessed: hand hygiene and personal protective equipment use, preparation of injectable medications (in patient care areas and the central pharmacy), infusion technique and maintenance of central lines, environmental cleaning and disinfection, and the reprocessing of bronchoscopes (1 of the case-patients had a preceding bronchoscopy).
Environmental and Product Sampling
To investigate potential sources of Herbaspirillum species, we tested various sources of water and surface samples in case-patient-specific and common areas of the hospital. Prefilled saline syringes commonly used to flush intravenous medications were also tested.
For each small surface sample (eg, faucet aerator or showerhead hose in rooms of case-patients), we removed the aerator or showerhead and swabbed the inside surface with a sterile swab. The swab was then sealed, refrigerated, and transported to the CDC, where the swab was processed by adding 3 mL of phosphate-buffered saline and vortexed. Various concentrations of the resulting extract were placed on MacConkey II (MAC) and tryptic soy agar (TSA) plates (Becton Dickinson Diagnostics, Sparks, Maryland).
For each large surface sample (eg, ice machine, water fountain in the hospital lobby, pharmacy hood, and pneumatic tube canister), we wiped a surface up to 2 square feet with a sterile 3M Sponge-Stick (3M, St Paul, Minnesota). Sponges were then placed in sterile bags, sealed, refrigerated, and transported to the CDC. The sponges were then processed by adding 100 mL of phosphate-buffered saline with 0.2% tween 80, and homogenized in a stomacher at 200 rpm for 1 minute. The resulting eluent was centrifuged, and the pellet was resuspended and plated on TSA and MAC plates.
For each water sample (from sinks, showerhead, water fountain, and facility water tanks), we collected 1 L of water in a sterile container with sodium thiosulfate tablets. Upon receipt by the CDC laboratory, 10 mL of each water sample was filtered through a sterile 0.45-µm membrane filter; the filters were then placed on a TSA plate, and 0.1 mL of each water sample was also spread on a control TSA plate. The remaining volume was filtered and placed on a MAC plate. All plates were incubated for 48 hours at 30°C. All environmental sampling and processing was performed according to standardized protocols developed by the Clinical and Environmental Microbiology Branch of the Division of Healthcare Quality Promotion at the CDC.
For each saline syringe sample, the exterior of each syringe was sanitized with 70% ethanol and the saline was pooled based on matching manufacturer and lot number. Membrane filtration was then performed as described in US Pharmacopeia Convention Chapter <71>, Sterility Tests [13].
RESULTS
Case Finding
Retrospective Review of B. cepacia Isolates
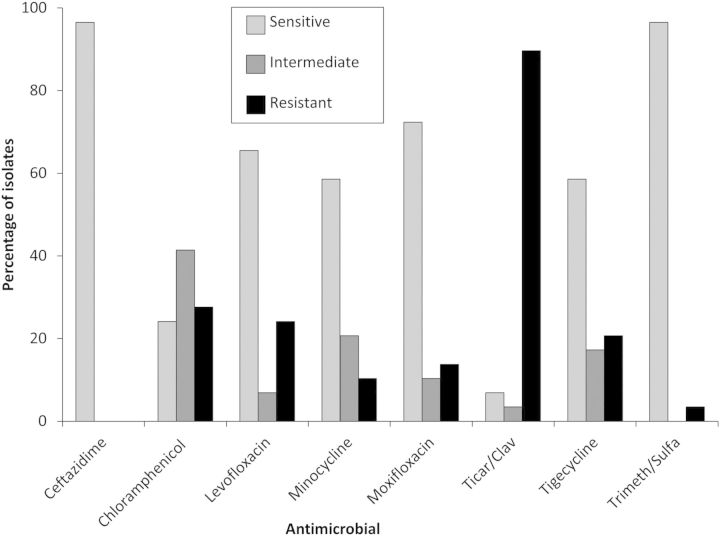
There were 46 clinical isolates of B. cepacia identified between 1 January 2006 and 24 October 2011. Nine were from blood cultures, 24 were from sputum, and 13 were from other sources. All B. cepacia isolates were resistant to at least 1 antibiotic tested, most frequently ticarcillin-clavulanate (Figure 1).
Figure 1.
Antimicrobial susceptibility of the isolate of Burkholderia cepacia cases, 2006–2011. Abbreviations: Ticar/Clav, ticarcillin–clavulanate; Trimeth/Sulfa, trimethoprim–sulfamethoxazole.
Herbaspirillum Species Cases
Eight patients (A–H), including the original 5 (A–E) and 3 additional cases identified prospectively (F–H), had 21 positive Herbaspirillum species cultures between 5 July 2011 (first isolate) and 3 August 2012 (last isolate) (Table 1). Eighteen cultures were obtained from blood, and 1 each was obtained from sputum, central venous catheter (CVC) tip, and an extracted infusaport. Isolates from 4 patients (A, C, D, and E) were indistinguishable by PFGE analysis, whereas 3 patients (F–H) had different PFGE patterns (Figure 2). The isolate from 1 patient (patient B) was not available for typing. Interestingly, the 4 cases with indistinguishable PFGE pattern isolates (A, C, D, and E) were clustered temporally within a period of 10 days.
Table 1.
Cases of Herbaspirillum Infection
| Patient | Age/Sex | Cancer/HSCT | Immunosuppression | Reason for Admission | Clinical Presentation | Type of CVC | CVC Removed | Days From Admission to Positive Culture | Date of First Positive Culture | Days From Index Case | Source (No. Positive) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 48/F | Ovarian adenocarcinoma | Chemotherapy (−30 d) | Pseudomonas fluorescens BSI | Sepsis—CRBSI | Infusaport | Yes | 7 | 5 July 2011 | 0a | Blood (3), Line tip (1) |
| B | 67/F | Leukemia | Chemotherapy (−4 d) | Chemo, MRSA pneumonia | Sepsis—BSI | PICC in right arm | No | 11 | 19 July 2011 | 14 | Blood (1) |
| C | 58/M | Leukemia/HSCT | High-dose steroids (30 d) | GI GVHD—GI bleed | BSI | PICC in right subclavian; apheresis CVC in left subclavian | Yes | 36 | 26 July 2011 | 21 | Blood (4) |
| D | 55/F | Leukemia/HSCT | High-dose steroids (8 d) | GI GVHD | BSI | PICC in right arm | Yes | 15 | 25 July 2011 | 20 | Blood (1) |
| E | 2/M | Ependymoma | High-dose steroids (14 d) | Herbaspirillum | Sepsis—BSI | PICC (basilic) | Yes | −2 | 2 Aug 2011 | 28 | Blood (6) |
| F | 66/F | Lung | Radiation therapy | Herbaspirillum | Sepsis—pneumonia | None | NA | 0 | 20 Sept 2011 | 77 | Sputum (1) |
| G | 18/M | Lymphoma | Chemotherapy (−4 d) | Chemotherapy | Sepsis—BSI | PICC (basilic) | Yes | 5 | 10 Mar 2012 | 249 | Blood (2) |
| H | 51/F | Aplastic anemia/HSCT | Tacrolimus | Herbaspirillum | Sepsis—CRBSI | PICC (basilic) | Yes | −1 | 3 Aug 2012 | 395 | Blood (1), Line tip (1) |
Abbreviations: BSI, bloodstream infections; Chemo, chemotherapy; CRBSI, catheter-related bloodstream infection; CVC, central venous catheter; GI, gastrointestinal; GVHD, graft-vs-host disease; HSCT, hematopoietic stem cell transplant; MRSA, methicillin-resistant Staphylococcus aureus; NA, not applicable; PICC, peripherally inserted central catheter.
a Index case.
Figure 2.
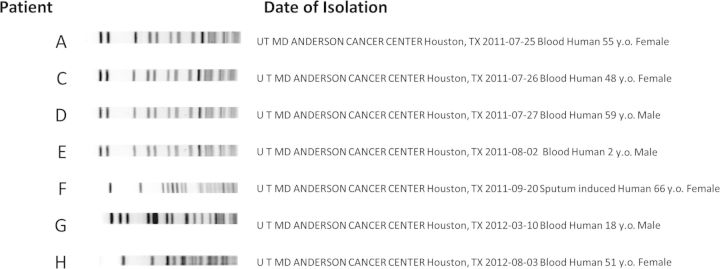
Pulsed-field gel electrophoresis patterns of Herbaspirillum species isolates obtained from 7 of 8 patients reported.
All Herbaspirillum species isolates were susceptible to ceftazidime, levofloxacin, minocycline, moxifloxacin, ticarcillin/clavulanate, tigecycline, and trimethoprim/sulfamethoxazole. All were susceptible to chloramphenicol except 2 patients’ isolates, which were intermediate (patients F and G; Table 1).
Case Description
The clinical presentation was bacteremia in 7 patients and polymicrobial pneumonia in 1 patient. Sepsis was present in 6 of 8 patients, including 2 with CRBSI (1 peripherally inserted central catheter and 1 infusaport), 3 with bacteremia, and 1 with pneumonia. Five of 8 patients were female (Table 1), and the median age was 53 years (range, 2–67 years). Three patients were hospitalized for community-onset Herbaspirillum species infections (E, F, and H), and the remaining 5 patients with hospital-onset infections (A–D, G) were originally hospitalized for either chemotherapy administration (2 patients), complications of graft-vs-host disease (GVHD) of the gastrointestinal tract (2 patients), or Pseudomonas fluorescens bacteremia (1 patient). Possible risk factors for poor outcomes from infection in 6 patients included cytotoxic chemotherapy 4–6 days before the first positive cultures (3 patients); high doses of corticosteroids (>1 mg/kg/day of prednisone or equivalent) 8–30 days prior to their first positive culture (3 patients); receipt of tacrolimus for GVHD prophylaxis (1 patient); and a history of emphysema, lung irradiation, and recurrent pneumonia in the only patient who had pneumonia secondary to Herbaspirillum species. All patients had CVCs at the time of the infection except patient F, who had pneumonia without bacteremia. Interestingly, no patients were neutropenic immediately before or during infection onset, with absolute neutrophil counts between 2110 and 23 160 cells/µL.
All patients were treated with intravenous antibiotics, either cefepime (5 patients), ceftazidime (1 patient), moxifloxacin (1 patient), or meropenem (1 patient) initially, followed by ceftriaxone or a fluoroquinolone. The CVCs were removed from all patients except patient B, who improved promptly with intravenous antibiotics and had negative repeat blood cultures. All patients responded to therapy, and only 1 patient (patient A) had a recurrence, which resolved once the implanted port was removed.
No common exposures were identified among the initial 5 cases (A–E). Cases A–E (including community-onset case E) were all hospitalized from 19 to 28 July 2011, but they were located on different wards on different floors and did not share common healthcare providers. Cases A–E also had different types of central lines (Table 1). Only 1 of the initial cases had used the outpatient infusion services, and another had been directly transferred to our hospital from an out-of-state hospital. The only common intravenous medications among all 5 cases were individually packaged sterile saline flushes and single-use vials of morphine. There was no evidence to suggest reuse or sharing of these sterile medications. There were no common procedures, and the only common diagnostic test was a nonportable chest radiograph. Patients F–H were identified by prospective surveillance and had positive cultures several months apart (identified on 20 September 2011, 10 March 2012, and 3 August 2012, respectively), and their Herbaspirillum species isolates had different patterns by PFGE (Figure 2).
Facility Observations and Infection Prevention Assessment
Our inspection of the schematics of the hospital water system revealed that case-patients received care in rooms supplied by many subsegments of the water supply system; therefore, only the main water supply system was common to all case-patients. We did not observe opportunities for contamination during the storage, transport, and handling of microbiology specimens.
Over the course of 11 days of observation, all available opportunities for observing hand hygiene, personal protective equipment use, medication preparation, central line care, and infusions resulted in no clear systematic breaches in infection control practices.
Environmental and Product Sampling
We collected 40 environmental samples, including 19 water samples, 12 large surface samples, and 9 small surface samples. No Herbaspirillum species or B. cepacia were recovered. Non-lactose-fermenting gram-negative rods were isolated from 13 samples: ice machine water (2), ice machine surface (3), patient sink water (1), patient shower water (2), patient shower surface (1), water from the main water tank (2), outdoor waterfall water (1), and lobby fountain water (1). Specific organisms that were identified in 7 of these samples included P. fluorescens, Pseudomonas putida, Pseudomonas aeruginosa, Cupriavidus pauculus, and Aeromonas salmonicida. Finally, we tested 6 different lots of saline flushes and all were sterile.
DISCUSSION
We report a hospital-based cluster of Herbaspirillum species infections in patients with cancer as well as subsequent sporadic cases of hospital- and community-onset infections. The first 5 cases at our institution were initially misidentified as a cluster of B. cepacia infections, and 4 of them, when tested by PFGE, were indistinguishable from each other, strongly suggesting a common source from their common hospitalization period that was not identified. Continued epidemiologic surveillance revealed 3 additional cases of Herbaspirillum species that presented 1, 7, and 11 months, respectively, after the initial cluster. These 3 isolates were unrelated to the initial cluster by PFGE, which is more consistent with sporadic infections. Two of these latter cases had community-onset infections, as did 3 of 4 cases reported in the literature [7–9]. Because Herbaspirillum species are water- and soil-based organisms, we suspected an environmental source, as in 3 previous case reports [7–9]. However, our joint public health investigation and environmental sampling did not reveal a source of Herbaspirillum species. Although other opportunistic soil and waterborne bacteria were recovered, these organisms were not found in greater than expected counts for treated municipal water. This may suggest that unique and transient circumstances introduced Herbaspirillum species into either the water supply, or another reservoir such as a contaminated medical product or a contaminated food product. We did not identify any pervasive infection control lapses that could explain acquisition of this organism. Thus, the source and mechanism of transmission of this cluster remain unknown.
No clusters of Herbaspirillum species infections have been reported in the literature, and limited case descriptions suggest different mechanisms of acquisition. Whereas all but 1 case in this study presented as bacteremia or CRBSI, only 4 separate cases of Herbaspirillum species bacteremia have been reported in the literature [6–9]; only 2 of these cases had central lines [6, 9], and no infusion-related acquisition was identified. Although the frequency of bacteremia in our study makes infusion-related acquisition plausible, no likely source was identified during our investigation. Herbaspirillum species may also be acquired via the respiratory tract in patients with preexisting lung pathology, as reported in CF patients [6], potentially explaining acquisition in our case-patient with pneumonia who had previously undergone lung radiation therapy and had a history of recurrent pneumonia. Herbaspirillum species may also enter through skin lacerations, as described in a cirrhotic patient who developed cellulitis and bacteremia from injuries sustained while jumping into a canal in Florida [8]. A leukemia patient in China developed Herbaspirillum species bacteremia after ingesting juice from sugar cane [7], one of several plants known to be colonized by this organism [4], suggesting that the ingestion of uncooked, Herbaspirillum species–contaminated food by vulnerable cancer patients could potentially explain the sporadic appearance of new cases, and the acquisition of infection inside and outside the hospital environment. Neutropenic patients in our institution may not have been affected because of heightened dietary precautions that include the avoidance of uncooked vegetables and/or the use of antimicrobial prophylaxis.
Herbaspirillum species can be incorrectly identified as B. cepacia by commercially available rapid microbial identification systems [5, 6]. Respiratory isolates from patients with CF [6] and 2 of the reported bacteremia cases [5, 9], as well as our clinical isolates, were initially misidentified as B. cepacia. We did not find additional Herbaspirillum species cases in our retrospective review of B. cepacia isolates based on susceptibility patterns. Antimicrobial susceptibility patterns can potentially serve as a surrogate marker for suspecting Herbaspirillum species instead of B. cepacia and suggest the need for further examination of the isolate(s). 16S rDNA sequencing can reliably confirm either isolates. Previous outbreaks and sporadic infections may have been incorrectly attributed to B. cepacia in other institutions. Furthermore, all cases but one were documented in the United States and most of these isolates were identified at the same referral laboratory at the University of Michigan. These findings suggest that there might be an underestimation of the prevalence of this pathogen due to the inability to identify it correctly at local laboratories. Increased awareness and improved diagnostic methods are therefore needed to accurately identify cases at other institutions. Interestingly, without any specific interventions, our prospective surveillance failed to identify further cases after August 2012.
This investigation has several limitations. First, more than a month had elapsed from isolation of Herbaspirillum species from case E to the start of the investigation. If there had been a common exposure that could have transmitted Herbaspirillum species, it may no longer have been present. Second, very little is known about the pathogenesis of Herbaspirillum species infections or the reservoirs and modes of exposure that lead to human infection. Therefore, generating and testing plausible hypotheses for Herbaspirillum species acquisition was challenging, especially in light of the small number of confirmed cases. Finally, because B. cepacia isolates were not saved before this cluster was identified, we were unable to reexamine the likelihood of these isolates being Herbaspirillum species.
In summary, Herbaspirillum species are opportunistic pathogens capable of colonizing the airways of patients with CF and causing bacteremia and sepsis in CF and immunocompromised patients, particularly those who have cancer or have undergone hematopoietic stem cell transplant. Even though most patients in this study were septic at presentation, their infections resolved with appropriate antibiotic therapy in all cases and removal of their CVCs in all but 1 case. Herbaspirillum species infections may be hospital-onset, either in a cluster or sporadic, as well as community-onset. Although a source and mechanism of acquisition was not identified in this study, the organism may enter the body via intravenous infusions, the respiratory tract [5, 6], environmental contamination of lacerated skin [8], or ingestion of uncooked vegetable products [7]. In addition, research is needed to determine whether infections caused by Herbaspirillum species and other genera of plant-associated bacteria, such as Burkholderia, Enterobacter, Ochrobactrum, Pseudomonas, Ralstonia, and Stenotrophomonas, could be acquired by immunocompromised hosts via consumption or exposure to plants in their environment [14]. Herbaspirillum species may be widely misidentified as B. cepacia by current testing methods; therefore, improved identification methods and increased awareness are necessary to document the true prevalence of this pathogen in human hosts and identify preventive measures.
Notes
Acknowledgments. The authors thank Markeda Wade (Department of Scientific Publications, The University of Texas MD Anderson Cancer Center) for her editorial support. We also thank the Texas Department of State Health Services Laboratory, Austin, for their technical support.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This research was supported in part by the National Institutes of Health through the MD Anderson Cancer Center (support grant CA016672). J. J. L. receives support from the Cystic Fibrosis Foundation.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Baldani JI, Pot B, Kirchhof G, et al. Emended description of Herbaspirillum; inclusion of [Pseudomonas] rubrisubalbicans, a milk plant pathogen, as Herbaspirillum rubrisubalbicans comb. nov.; and classification of a group of clinical isolates (EF group 1) as Herbaspirillum species 3. Int J Syst Bacteriol. 1996;46:802–10. doi: 10.1099/00207713-46-3-802. [DOI] [PubMed] [Google Scholar]
- 2.Schmid M, Baldani J, Hartmann A. The genus Herbaspirillum. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The prokaryotes. New York: Springer; 2006. pp. 141–50. [Google Scholar]
- 3.Ghosh S, Sar P. Identification and characterization of metabolic properties of bacterial populations recovered from arsenic contaminated ground water of north east India (Assam) Water Res. 2013;47:6992–7005. doi: 10.1016/j.watres.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Jia RB, Li L. Bacterial community of iron tubercles from a drinking water distribution system and its occurrence in stagnant tap water. Environ Sci Process Impacts. 2013;15:1332–40. doi: 10.1039/c3em00171g. [DOI] [PubMed] [Google Scholar]
- 5.Coenye T, Goris J, Spilker T, Vandamme P, LiPuma JJ. Characterization of unusual bacteria isolated from respiratory secretions of cystic fibrosis patients and description of Inquilinus limosus gen. nov., sp. nov. J Clin Microbiol. 2002;40:2062–9. doi: 10.1128/JCM.40.6.2062-2069.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spilker T, Uluer AZ, Marty FM, et al. Recovery of Herbaspirillum species from persons with cystic fibrosis. J Clin Microbiol. 2008;46:2774–7. doi: 10.1128/JCM.00460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Su Z, Liu Y, et al. Herbaspirillum species: a potential pathogenic bacteria isolated from acute lymphoblastic leukemia patient. Curr Microbiol. 2011;62:331–3. doi: 10.1007/s00284-010-9703-5. [DOI] [PubMed] [Google Scholar]
- 8.Tan M, Oehler R. Lower extremity cellulitis and bacteremia with Herbaspirillum seropedicae associated with aquatic exposure in a patient with cirrhosis. Infect Dis Clin Pract. 2005;13:277–9. [Google Scholar]
- 9.Ziga ED, Druley T, Burnham CA. Herbaspirillum species bacteremia in a pediatric oncology patient. J Clin Microbiol. 2010;48:4320–1. doi: 10.1128/JCM.01479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LiPuma JJ, Dulaney BJ, McMenamin JD, et al. Development of rRNA-based PCR assays for identification of Burkholderia cepacia complex isolates recovered from cystic fibrosis patients. J Clin Microbiol. 1999;37:3167–70. doi: 10.1128/jcm.37.10.3167-3170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutty PK, Moody B, Gullion JS, et al. Multistate outbreak of Burkholderia cenocepacia colonization and infection associated with the use of intrinsically contaminated alcohol-free mouthwash. Chest. 2007;132:1825–31. doi: 10.1378/chest.07-1545. [DOI] [PubMed] [Google Scholar]
- 12.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Pharmacopeial Convention, Inc. Rockville, MD: US Pharmacopeial Convention, Inc; 2012. US Pharmacopeia 36—National Formulary 31; pp. 71–970. Chapter <71> sterility tests. [Google Scholar]
- 14.Berg G, Eberl L, Hartmann A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol. 2005;7:1673–85. doi: 10.1111/j.1462-2920.2005.00891.x. [DOI] [PubMed] [Google Scholar]