Sensitive viral diagnostic methods have identified increasing prevalence of asymptomatic viral infection. This study determined the epidemiologic characteristics and etiology of asymptomatic upper respiratory tract infection in the first year of life and the association with acute otitis media complication.
Keywords: acute otitis media, common cold, asymptomatic infection, respiratory viruses, rhinovirus
Abstract
Background. Sensitive diagnostic assays have increased the detection of viruses in asymptomatic individuals. The clinical significance of asymptomatic respiratory viral infection in infants is unknown.
Methods. High-throughput, quantitative polymerase chain reaction assays were used to detect 13 common respiratory viruses from nasopharyngeal specimens collected during 2028 visits from 362 infants followed from near birth up to 12 months of age. Specimens were collected at monthly interval (months 1–6 and month 9) and during upper respiratory tract infection (URTI) episodes. Subjects were followed closely for acute otitis media (AOM) development.
Results. Viruses were detected in 76% of 394 URTI specimens and 27% of asymptomatic monthly specimens. Rhinovirus was detected most often; multiple viruses were detected in 29% of the specimens. Generalized mixed-model analyses associated symptoms with increasing age and female sex; detection of respiratory syncytial virus (RSV), influenza, rhinovirus, metapneumovirus, and adenovirus was highly associated with symptoms. Increasing age was also associated with multiple virus detection. Overall, 403 asymptomatic viral infections in 237 infants were identified. Viral load was significantly higher in URTI specimens than asymptomatic specimens but did not differentiate cases of URTI with and without AOM complication. The rate of AOM complicating URTI was 27%; no AOM occurred following asymptomatic viral infections. AOM development was associated with increasing age and infection with RSV, rhinovirus, enterovirus, adenovirus, and bocavirus.
Conclusions. Compared to symptomatic infection, asymptomatic viral infection in infants is associated with young age, male sex, low viral load, specific viruses, and single virus detection. Asymptomatic viral infection did not result in AOM.
(See the Editorial Commentary by Barenkamp on pages 10–11.)
Viral upper respiratory tract infection (URTI) affects all ages but is particularly prevalent among children. Viral URTI in infants and young children is often complicated by secondary bacterial infections including acute otitis media (AOM) and acute bacterial sinusitis [1–3]. AOM is one of the most common diseases seen in pediatric practice and is the most common reason children receive antibiotics or undergo surgery. The incidence and causative viruses of URTI and AOM complication have been more extensively studied in children 1–5 years of age [1, 2] than in infants aged <1 year. Early AOM onset results in recurrent and chronic otitis media later in life [4, 5]. Increased understanding of viral URTI and its association with AOM in the first year of life is essential to advance prevention efforts for this highly prevalent disease.
Advances in molecular testing such as use of polymerase chain reaction (PCR) assays have increased the detection of viruses in asymptomatic individuals. In case-control studies, respiratory viruses were detected in 58%–90% of symptomatic cases and 28%–52% of asymptomatic controls [6–10]. Positive PCR results in asymptomatic children may indicate acute asymptomatic viral infection, recent symptomatic infection with residual shedding, or viral persistence from past infection. The characteristics of asymptomatic respiratory viral infection in the first year of life and its association with AOM complication have not been described.
The aim of this study was to identify viruses causing asymptomatic and symptomatic URTI in infants and to establish the associations with viral load, viral coinfections, URTI symptoms, and AOM development. We tested the following hypotheses: (1) asymptomatic viral URTI in infants leads to AOM; and (2) high viral load in nasopharyngeal (NP) specimens is associated with URTI symptoms and AOM complication.
METHODS
Study Design and Subjects
The study was part of a prospective, longitudinal study of infants in the first year of life to evaluate the prevalence and risks for URTI and AOM development. The study was approved by the University of Texas Medical Branch (UTMB) Institutional Review Board; written informed consent was obtained for all subjects. Between October 2008 and April 2013, 367 subjects were enrolled. Healthy subjects living in Galveston County were recruited from the newborn nursery or primary care pediatric clinics at UTMB. Preterm infants and those with major medical problems or anatomical/physiological defects of the ear or nasopharynx were excluded. Prior to enrollment, subjects were prescreened for polymorphisms of tumor necrosis factor α (TNF−308) and interleukin 6 (IL-6−174), which we previously found to be associated with AOM susceptibility [11, 12].
Subjects were enrolled from near birth (<1 month) and completed the study at age 6 months, if AOM developed prior to 6 months, or followed to 12 months for AOM. Upon enrollment, data were collected on family history, daycare attendance, cigarette smoke exposure, and breastfeeding vs formula feeding. Nasopharyngeal swabs were collected at 1, 2, 3, 4, 5, and 6 months, and at 9 months if the subject remained in the study.
During follow-up, parents were instructed to report to the study team when the subject developed cold (URTI) symptoms (defined below). Time and travel were compensated. Symptomatic subjects were evaluated as soon as possible after URTI onset and followed for AOM complication for 28 days. At the initial URTI visit, otoscopic examination and tympanometry were performed and NP specimens were collected for viral studies. A follow-up visit was provided 3–5 days later. Weekly telephone calls were made for follow-up of possible AOM symptoms. Parents were encouraged to bring the subject in for examination any time they suspected the infant might have an ear infection. AOM diagnosis was made based on presentations of acute symptoms (fever, irritability, otalgia), signs of tympanic membrane inflammation (intense redness, bulging, opaque tympanic membrane), and presence of middle ear fluid as documented by pneumatic otoscopy and/or tympanometry. Study personnel who made AOM diagnosis were trained and experienced otoscopists ( T. C., J. A. P., or D. P. M.).
In addition, study personnel called the parents twice monthly to identify any URTI, AOM, or lower respiratory tract infection (LRTI) missed since the last contact. A comprehensive chart review was performed at the end of follow-up to capture URTI, AOM, or LRTI episodes that may have been diagnosed by other providers. The UTMB electronic medical record, used at all UTMB sites, provided optimal capture of AOM and LRTI episodes, as UTMB is the only primary care and emergency provider for infants and children in Galveston.
Clinical Definitions
URTI Episode
Presence of the following symptoms: nasal congestion, rhinorrhea, cough, and/or sore throat, with or without constitutional symptoms such as fever, decreased appetite, and restless sleep.
Monthly Visit With Symptom History
Specimens collected during monthly visit without URTI symptoms but history of URTI documented 7 days before or after specimen collection.
Asymptomatic Viral Infection
Presence of virus(es) in monthly visit specimen without history of symptoms within 7 days of specimen collection, excluding cases with the same virus detected within the previous 30 days.
AOM Complicating URTI
AOM that occurred within 28 days after the onset of URTI (unless a new-onset URTI occurred during this period; in that case, AOM was considered to have complicated the most recent URTI).
AOM Associated With Asymptomatic Viral Infection
AOM diagnosed within 28 days after asymptomatic viral infection in the absence of URTI symptoms within this time period.
Specimens
Included in this report were respiratory specimens collected by 15 October 2013 that were available for testing by high-throughput, quantitative, multiplex PCR (HT-qPCR) assay developed for 13 respiratory viruses [13]. NP swab specimens were collected by trained personnel during the monthly visit by introducing a flocked swab (FLOQSwabs, COPAN, Murrieta, California) into the nose until resistance was met. The swab was rotated gently 180° and then placed in a 1-mL tube of ESwab transport medium (COPAN Diagnostics, Murrieta, California) and transported to the lab on ice within 1 hour of collection. An aliquot was sent for bacterial culture; the remaining aliquots were kept frozen at −80°C until testing. During a URTI episode, additional nasopharyngeal secretion (NPS) samples were also collected using vacuum suction into a mucus trap as previously described [13].
Molecular Virologic Studies
HT-qPCR assay was used in this study to detect 13 respiratory viruses including adenovirus; bocavirus; coronaviruses 229E, NL63, and OC43; enterovirus; human metapneumovirus; influenza A and B, parainfluenza viruses 1 and 3; respiratory syncytial virus (RSV); and rhinovirus. The assays were developed at the Assay Development Service Division, Galveston National Laboratory, UTMB as previously described; appropriate positive and negative controls were included [13]. The limit of detection (LOD) with 99% confidence was approximately 15 copies for all viruses except enterovirus and RSV, which both had an LOD of 100 copies. Viral load was calculated as viral genome copies per milliliter of the original specimen based on the original dilution of the specimens and subsequent assay dilutions.
Statistical Analysis
Because each subject in the study contributed >1 specimen, we used a generalized mixed model with logit link to analyze outcomes of symptomatic episodes; “subject” was included as a random intercept term. Other outcomes of viral detection, multiple viral detection, and AOM were also modeled and tested using the same modeling framework. Viral load was modeled using a mixed model with a log-transformed response. Grouping of patients experiencing symptomatic and asymptomatic viral detection were tested using Fisher exact test. All calculations were done in R (version 3.0.2); all mixed models were tested in the lme4 library of R.
RESULTS
Subjects and Specimens
A total of 362 subjects (aged 0–12 months) yielded 2153 NP swabs or NPS specimens that were tested for viruses by HT-qPCR. Characteristics of the subjects and specimens are summarized in Table 1. The average duration of follow-up was 9.4 months (median, 12 months). Two hundred forty-one (66%) subjects contributed ≥6 specimens; 70 (19%) subjects contributed 3–5, and 51 (14%) contributed 1–2 specimens. In 125 of 394 (32%) URTI episodes, NP swab or NPS specimens were collected twice (average of 4 days apart), because the subjects developed AOM or continued to have symptoms. To prevent overrepresentation, the viral detection in these cases were combined. Single NP swab specimens were collected during 1634 monthly visits with no apparent symptoms; a retrospective chart review showed that URTI symptoms were present within 7 days before or after specimen collection in 147 of these cases that were subsequently reclassified as “monthly visit with symptom history.” The results reported here represent 2028 URTI and monthly visit events; of these, 1487 were collected when subjects were asymptomatic (Table 2 and Supplementary Table 1A and 1B).
Table 1.
Characteristics of the Subjects and Numbers of Specimens Tested for Viruses
| Characteristic | No. | % |
|---|---|---|
| No. of subjects | 362 | |
| Male | 195 | 54 |
| Race | ||
| White | 275 | 76 |
| Black | 82 | 23 |
| Asian | 5 | 1 |
| Ethnicity | ||
| Hispanic/Latino | 180 | 50 |
| Breastfed, any duration | 237 | 65 |
| Cigarette smoke exposure | 71 | 17 |
| Daycare attendance | 3 | 1 |
| TNF-308 polymorphism (358 tested) | 104 | 29 |
| IL-6-174 polymorphism (359 tested) | 165 | 46 |
| Subjects followed for <6 mo | 41 | 11 |
| Subjects followed 6 to <12 mo | 118 | 33 |
| Subjects followed for 12 mo | 203 | 56 |
| Total duration of follow-up, patient-months | 3408 | |
| Total duration of follow-up, first 6 mo of life | 2056 | 60 |
| Total duration of follow-up, 6–12 mo | 1352 | 40 |
| Total URTI episodes with virus results | 394 | |
| URTI episodes with 2 specimens tested for viruses | 125 | |
| Total URTI specimens | 519 | |
| Total specimens collected during monthly visits | 1634 | |
| Month 1 | 300 | 18 |
| Month 2 | 260 | 16 |
| Month 3 | 228 | 14 |
| Month 4 | 230 | 14 |
| Month 5 | 225 | 14 |
| Month 6 | 245 | 15 |
| Month 9 | 146 | 9 |
| Monthly specimens, symptomatic | 147 | 9 |
| Monthly specimens, asymptomatic | 1487 | 91 |
| Total number of specimens tested for viruses | 2153 | |
| Total URTI and monthly visit eventsa | 2028 |
Abbreviations: IL-6, interleukin 6; TNF, tumor necrosis factor; URTI, upper respiratory tract infection.
a Virus results were combined for 125 episodes for which 2 specimens were collected.
Table 2.
Virus Detection Rates by Type of Specimens
| Virus Type | All |
URTI |
Monthly Visits |
|||||
|---|---|---|---|---|---|---|---|---|
| Samples |
Samples |
With Symptom History |
Asymptomatic |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Total | 2028 | 394 | 19 | 147 | 7 | 1487 | 73 | |
| Virus-positive samples, % | 795 | 39 | 299 | 76 | 75 | 51 | 421 | 28 |
| Total viruses detected | 1079 | 455 | 95 | 529 | ||||
| Single-virus samples | 567 | 180 | 59 | 328 | ||||
| Rhinovirus | 244 | 43a | 99 | 55 | 22 | 37 | 123 | 38 |
| Coronavirusesb | 120 | 21 | 15 | 8 | 13 | 22 | 92 | 28 |
| Parainfluenza 1, 3 | 67 | 12 | 15 | 8 | 5 | 8 | 47 | 14 |
| RSV | 35 | 6 | 20 | 11 | 6 | 10 | 9 | 3 |
| Metapneumovirus | 31 | 5 | 13 | 7 | 3 | 5 | 15 | 5 |
| Adenovirus | 23 | 4 | 7 | 4 | 2 | 3 | 14 | 4 |
| Enterovirus | 20 | 4 | 5 | 3 | 4 | 7 | 11 | 3 |
| Bocavirus | 20 | 4 | 3 | 2 | 2 | 3 | 15 | 5 |
| Influenza A/B | 7 | 1 | 3 | 2 | 2 | 3 | 2 | 1 |
| Multiple-virus samples | 228c | 119 | 16 | 93 | ||||
| 2 viruses | 181d | 23e | 90 | 30 | 12 | 16 | 79 | 19 |
| ≥3 or more viruses | 47f | 6 | 29 | 10 | 4 | 5 | 14 | 3 |
Abbreviations: RSV, respiratory syncytial virus; URTI, upper respiratory tract infection.
a Percentage of single virus.
b Coronaviruses 229E, OC43, and NL63.
c Five hundred twelve viruses.
d Three hundred sixty-two viruses (Supplementary Table 1A).
e Percentage of virus-positive samples (total single virus = 71%; multiple viruses = 23% + 6% = 29%).
f One hundred fifty viruses (Supplementary Table 1B).
Detection of Viruses and Presence of Symptoms
A total of 1079 viruses were detected in 795 (39%) URTI or monthly visit specimens. Virus types detected and their prevalence are shown in Figure 1. Virus detection rates by specimen type are shown in Table 2. Virus detection occurred more often during URTI, compared to monthly visits with symptom history (P < .0001) and asymptomatic visits (P < .00001). Multiple virus detection occurred more often in URTI samples than samples collected during asymptomatic visits (P < .0001). Both positive virus detection and multiple virus detection increased with age (P < .0001 and P < .004, respectively).
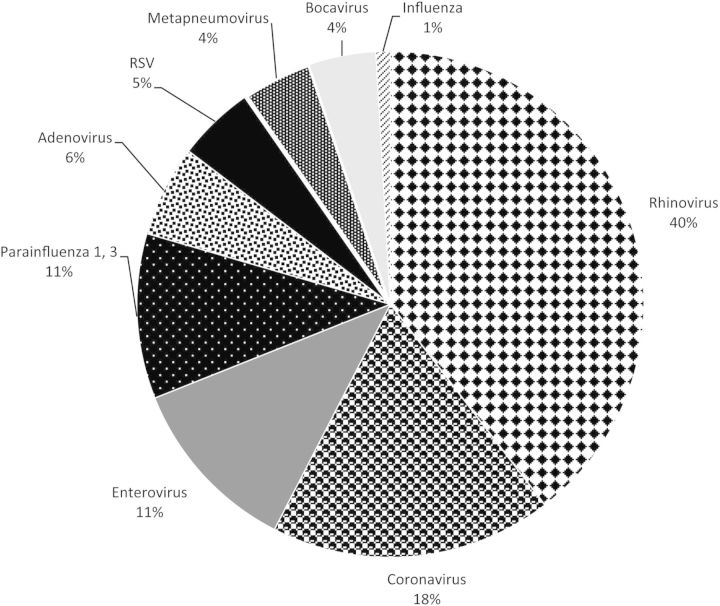
Figure 1.
Proportion of respiratory viruses in 795 respiratory samples (1079 viruses) detected singly or in combination with other viruses. Rhinovirus was the most common and accounted for 40% of all detected viruses. In 567 (71%) specimens, a single virus was detected; 2 viruses were detected in 181 (23%) specimens; ≥3 viruses were identified in 47 (6%) specimens. Abbreviation: RSV, respiratory syncytial virus.
Overall, the presence of symptoms was associated with detection of virus (P < .0001) and with increasing age (P < .0001). Using a mixed model with random intercept (including age effect), significant positive association with symptoms was detected for rhinovirus, RSV, adenovirus, metapneumovirus (P ≤ .0001 for all), influenza (P = .007), parainfluenza (P = .007), and coronavirus (P = .014). For bocavirus and enterovirus, positive detection was not significantly associated with presence of symptoms. Table 3 shows the estimated odds ratios for the presence of symptoms when specific viruses were detected. Detection of RSV, influenza, rhinovirus, metapneumovirus, and adenovirus were highly associated with symptoms, with odds ratios >4.
Table 3.
Estimated Odds Ratios for the Presence of Symptoms When Specific Viruses Were Detecteda
| Virus | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| RSV | 11.67 | 6.1–27.2 |
| Influenza A/B | 7.28 | 1.4–50.2 |
| Rhinovirus | 5.08 | 3.7–6.8 |
| Metapneumovirus | 4.38 | 2.3–8.5 |
| Adenovirus | 4.29 | 2.4–8.4 |
| Parainfluenza 1, 3 | 1.95 | 1.2–3.1 |
| Bocavirus | 1.86 | .9–3.8 |
| Coronavirus (229E, OC43, NL63) | 1.60 | 1.1–2.4 |
| Enterovirus | 1.54 | 1.0b–2.5 |
Abbreviation: RSV, respiratory syncytial virus.
a Mixed model.
b 0.995.
Repeated Viral Detection in Asymptomatic Patients
Of 1487 asymptomatic monthly specimens, 421 specimens from 242 infants were positive for 529 viruses; 328 specimens contained 1 virus, 79 had 2, and 14 had ≥ 3. Because there can be a prolonged presence of viral nucleic acids in the respiratory tract, we used available longitudinal data from each patient to establish the repeated presence of the same viruses within 30 days. Of these, 18 specimens contained 27 viruses that were detected 30 days previously and were categorized as “prolonged presence” (Supplementary Table 2). These results were not included in subsequent analyses. The remaining 403 specimens with positive viruses collected during asymptomatic monthly visits were considered new asymptomatic viral infections.
Asymptomatic Respiratory Viral Infections
Characteristics of 403 asymptomatic viral infections in 237 infants are shown in Table 4. Percentage of positive specimens by month for symptomatic and asymptomatic infections is shown in Figure 2. There is a higher rate of asymptomatic virus detection during the Galveston non-RSV season (April to August, P = .04); this high rate is more pronounced in late spring and early summer (April to June, P = .000087).
Table 4.
Characteristics of 403 Asymptomatic Viral Infections in 237 Infants
| Characteristic | No. |
|---|---|
| Total specimens collected from monthly asymptomatic visits | 1487 |
| Total specimens positive for viruses | 421 |
| Repeated detection of the same virus within previous 30 d | |
| By virus type | 27 |
| By number of specimens | 18 |
| Asymptomatic viral infections | 403 (27%) |
| No. of subjects with asymptomatic viral infections | 237 |
| Mean age | 4 mo |
| Median age | 4 mo |
| Infections with 1 virus | 316 |
| Rhinovirus | 119 |
| Coronavirus NL63 | 65 |
| Parainfluenza 1, 3 | 43 |
| Coronavirus OC43 | 19 |
| Human metapneumovirus | 15 |
| Adenovirus | 14 |
| Enterovirus | 11 |
| Bocavirus | 11 |
| Coronavirus 229E | 10 |
| RSV | 7 |
| Influenza A | 2 |
| Influenza B | 0 |
| Infections with 2 viruses | 76 |
| Infections with 3 viruses | 10 |
| Infections with 4 viruses | 1 |
| Total viruses | 502 |
| AOM within 28 d of asymptomatic viral infections | 9 |
| AOM attributed to asymptomatic viral infections | 0 |
Abbreviations: AOM, acute otitis media; RSV, respiratory syncytial virus.
Figure 2.
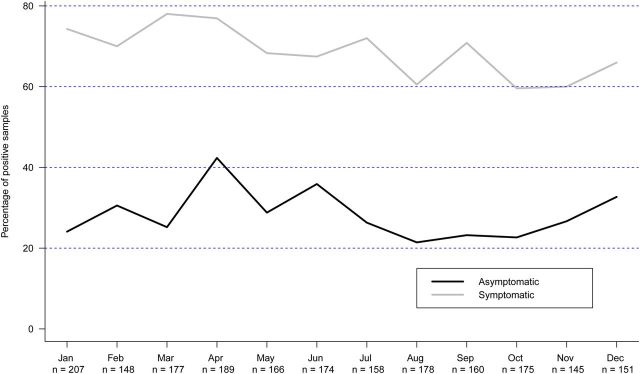
Percentage of virus-positive samples (asymptomatic vs symptomatic infections) by month (combined data 2009–2013). Symptomatic infection includes upper respiratory tract infection episodes and monthly visits with symptom history.
Of 362 infants, 49 never had virus detected from their specimens, 76 had only symptomatic infections, 101 had only asymptomatic infections, and 136 had both symptomatic and asymptomatic infections. Characteristics of infants in these 4 groups are shown in Supplementary Table 3A; types of viruses detected in each group are shown in Supplementary Table 3B. Categorical data analyses of demographic, genetic, and environmental factors of infants with asymptomatic vs symptomatic infections showed association between asymptomatic infections and male sex (P = .004). Factors not associated with symptoms included race, ethnicity, TNF−308 and IL-6−174 polymorphisms, breastfeeding vs formula feeding, cigarette smoke exposure, and daycare attendance.
AOM Occurrences
A total of 111 AOM episodes were documented (Table 5); 95% of AOM episodes were diagnosed by the study physician; the remaining were identified through chart review. AOM complicated 27% of URTI episodes; AOM occurred in 4% of monthly visits with a symptom history. Of 1487 asymptomatic monthly visits, there were 9 AOM cases within 28 days of specimen collection. In those cases, the subjects developed URTI 8–22 days after asymptomatic specimen collection; therefore, AOM episodes were attributed to URTI and not from prior asymptomatic viral detection. Mixed-model analysis showed significant association between AOM development and increasing age (P < .0001) and number of viruses detected (P = .009). RSV and rhinovirus, both as single and part of multiple virus detections, were associated with AOM occurrence (P < .001 in all). Enterovirus (P < .0001), adenovirus (P < .002), and bocavirus (P < .04) were also associated with AOM development marginally; coronavirus, influenza, metapneumovirus, and parainfluenza viruses were not found to be significantly associated with AOM development in this model.
Table 5.
Rate of Acute Otitis Media Following Upper Respiratory Tract Infection and Symptomatic and Asymptomatic Viral Detection
| Type of Viral Infection | Total Cases | AOM | % | Average Days After URTI | Median Days | By Virus | AOM | % | Average Days After URTI | Median Days |
|---|---|---|---|---|---|---|---|---|---|---|
| After URTI | After URTI | Detection | After URTI | After URTI | ||||||
| URTI episode | 394 | 105 | 27 | 6 | 4 | Positive | 86 | 29 | 5 | 4 |
| Negative | 19 | 20 | 6 | 5 | ||||||
| Monthly visits, symptom history | 147 | 6 | 4 | 10 | 7 | Positive | 2 | 3 | 7 | 7 |
| Negative | 4 | 5 | 12 | 9 | ||||||
| Monthly visits, asymptomatic | 1487 | 0 | 0 | Positive | 0 | 0 | ||||
| Negative | 0 | 0 |
Abbreviations: AOM, acute otitis media; URTI, upper respiratory tract infection.
Viral Load
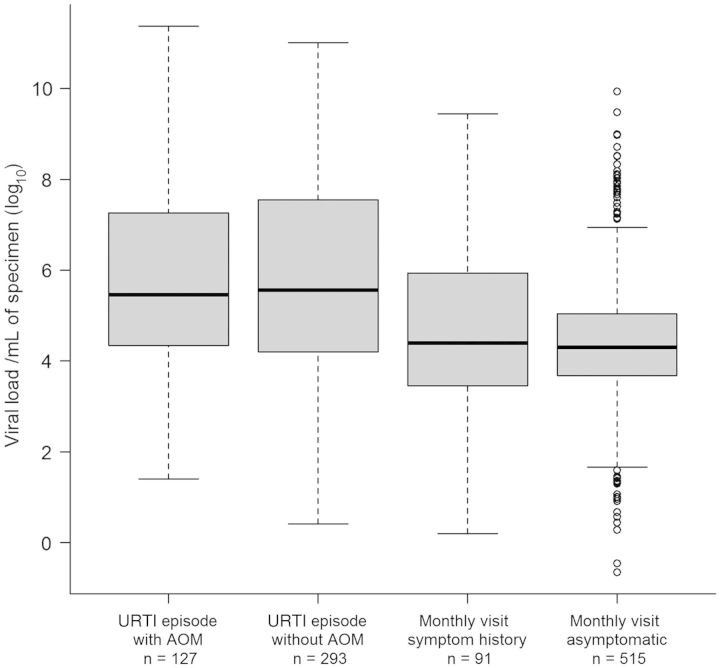
Viral load data were available for 1026 (95%) of the virus-positive specimens; 53 detections could not be accurately quantified due to unknown specimen dilution. Viral load data are shown in Figure 3. Overall, viral loads ranged between 2.26 × 10−1 and 2.35 × 1011 copies/mL of specimen. Median viral load in URTI specimens with AOM complication, URTI without AOM, monthly visits with symptom history, and asymptomatic monthly visit specimens was 2.85 × 105, 3.63 × 105, 2.5 × 104, and 2.00 × 104 copies/mL, respectively. Viral loads were highest in URTI groups (with or without AOM), compared with the other groups (P < .00001). However, there was no significant difference in viral load in the URTI group, with and without AOM (P = .59). Viral load in monthly visits with symptom history specimens was not different from asymptomatic specimens (P = .55). For each specific virus, viral loads were also higher in URTI specimens, compared with other specimens (P < .0001 for all viruses; P = .018 for bocavirus), except for influenza virus (no significant difference).
Figure 3.
Viral load by specimen type; the median is represented by the heavy horizontal line. The interquartile range is shown by the boxes. The number of specimens in each category is shown at the bottom of each column. Abbreviations: AOM, acute otitis media; URTI, upper respiratory tract infection.
DISCUSSION
In this prospective, longitudinal study of infants in the first year of life, >2000 specimens collected from both symptomatic and asymptomatic infants were tested for the presence of 13 common viruses. Rhinovirus was the most common virus detected overall. Asymptomatic viral infection was associated with young age, male sex, low viral load, bocavirus, enterovirus, and single virus detection. Importantly, asymptomatic viral infection did not lead to AOM development.
To our knowledge, this is the largest series describing asymptomatic respiratory viral infections in infants (n = 403). Because viruses are intracellular microorganisms, detection of viruses in clinical specimens suggest replication of viruses in the host cells; thus, the term “infection” is appropriate regardless of symptoms. We defined “asymptomatic infection” by a 7-day symptom-free period before and after specimen collection; this history was carefully obtained from both parents and medical record review. This study is unique in that we utilized longitudinal data from the same infant to distinguish a new infection from repeated detection due to prolonged presence of virus nucleic acid in the respiratory tract. We used a 30-day time frame to separate the 2 situations for uniformity with previous reports [14, 15]. We are aware that survival of nucleic acid in the respiratory tract may vary depending on the virus species identified [15, 16]. Therefore, some of the defined asymptomatic viral infections in this study could still be from prolonged presence (>30 days) of viral nucleic acids in the respiratory tract. To ascertain “new” viral infection in a successive series of positive detections of the same virus over time, sequencing of the detected viruses will be required but is beyond the scope of this report.
We found that URTI symptoms in infants associated with numerous host and virus factors and many of these factors were interrelated. Interestingly, URTI symptoms in the first year of life associated with increasing age (eg, infants 6–12 months of age expressed more symptoms than younger infants). Consistent with our finding, studies of older children [8, 17, 18] found that the ages of 6–12 months were a risk factor for symptoms. Collectively, these data indicate that 6- to 12-month-old infants are more likely to express symptoms than younger infants or older children. It is well known that young infants express less specific symptoms when they are ill and they are less susceptible to infections due to preexisting maternal antibodies, which wane with age. Older infants had more frequent single and multiple virus detection. Increased exposure to infections in older infants likely increases infection by multiple viruses. This may also contribute to symptom expression in older infants; association of multiple viral infections and symptoms/severity has previously been documented [8, 19, 20].
In this report focusing on URTI (but not LRTI), we found rhinovirus to be the most commonly detected virus in both symptomatic and asymptomatic infants. Miller et al [21] recently reported similar findings in 175 URTI episodes in infants (<12 months) over 4 consecutive years. Our study also showed positive correlations between rhinovirus, symptoms, and AOM development. Other viruses highly associated with symptoms included RSV, influenza, and metapneumovirus; the seasonality of these 3 viruses may explain the higher rate of symptomatic infection during winter respiratory season and higher rate of asymptomatic infection from April to August. A recent case-control study performed in Sweden in children aged <5 years during non–influenza season also showed high proportion of RSV and metapneumovirus in symptomatic cases [22]. Prolonged presence of bocavirus may partially explain a lack of association between symptoms and this virus detection [23].
AOM occurs mostly as a bacterial complication of viral URTI. Our finding that asymptomatic viral infection did not lead to AOM highlights the fact that a necessary step in the pathogenesis of AOM is an inflammation of the nasopharynx and the Eustachian tubes severe enough to cause URTI symptoms and associated Eustachian tube dysfunction. We clearly showed a strong correlation between expression of URTI symptoms and high viral load for all viruses except for influenza, which only accounted for a small number. Others have also shown the correlation between viral load and symptom severity of specific viruses [23–26]. It is interesting that the development of AOM was not associated with a higher viral load during URTI. In previous studies of an older cohort, we have also shown a lack of association between AOM complication and high viral load in metapneumovirus and bocavirus URTI [27, 28]. It is possible that viral load has lesser importance on the development of AOM when considered along with other known AOM risk factors such as demographic and genetic risks, feeding status, daycare attendance, nasopharyngeal bacterial colonization, pneumococcal antibody status, and type of URTI virus [2, 12, 29, 30].
In summary, this large study of asymptomatic respiratory viral infections in the first year of life showed rhinovirus to be the most commonly detected virus. Asymptomatic viral infection was more common in young infants and did not lead to AOM development.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study subjects and their parents; and Alejandro Diego, Stella Kalu, Johanna Nokso-Kiovisto, Linda Ede, Esther Valdivia, Lilia Rodriquez, Carrie Maxwell, and Ying Xiong for their assistance with the study subjects and specimen processing.
Financial support. This work was supported by the National Institutes of Health (research grants R01DC005841, R01DC005841-10S1, and UL1TR000071).
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Alper CM, Winther B, Mandel EM, Hendley JO, Doyle WJ. Rate of concurrent otitis media in upper respiratory tract infections with specific viruses. Arch Otolaryngol Head Neck Surg. 2009;135:17–21. doi: 10.1001/archotol.135.1.17. [DOI] [PubMed] [Google Scholar]
- 2.Chonmaitree T, Revai K, Grady JJ, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–23. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marom T, Alvarez-Fernandez PE, Jennings K, Patel JA, McCormick DP, Chonmaitree T. Acute bacterial sinusitis complicating viral upper respiratory tract infections in young children. Pediatr Infect Dis J. 2014;33:803–8. doi: 10.1097/INF.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kvaerner KJ, Nafstad P, Hagen JA, Mair IW, Jaakkola JJ. Recurrent acute otitis media: the significance of age at onset. Acta Otolaryngol. 1997;117:578–84. doi: 10.3109/00016489709113441. [DOI] [PubMed] [Google Scholar]
- 5.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 6.Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631–6. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singleton RJ, Bulkow LR, Miernyk K, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82:1282–90. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CS, Wolfs TF, van der Ent CK. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154:396–400. doi: 10.1016/j.jpeds.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Gageldonk-Lafeber AB, Heijnen ML, Bartelds AI, Peters MF, van der Plas SM, Wilbrink B. A case-control study of acute respiratory tract infection in general practice patients in the Netherlands. Clin Infect Dis. 2005;41:490–7. doi: 10.1086/431982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Advani S, Sengupta A, Forman M, Valsamakis A, Milstone AM. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J. 2012;31:1221–6. doi: 10.1097/INF.0b013e318265a804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel JA, Nair S, Revai K, et al. Association of proinflammatory cytokine gene polymorphisms with susceptibility to otitis media. Pediatrics. 2006;118:2273–9. doi: 10.1542/peds.2006-0764. [DOI] [PubMed] [Google Scholar]
- 12.Revai K, Patel JA, Grady JJ, Nair S, Matalon R, Chonmaitree T. Association between cytokine gene polymorphisms and risk for upper respiratory tract infection and acute otitis media. Clin Infect Dis. 2009;49:257–61. doi: 10.1086/599833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeffelholz MJ, Pong DL, Pyles RB, et al. Comparison of the Filmarray(R) respiratory panel and prodesse(R) real-time PCR assays for detection of respiratory pathogens. J Clin Microbiol. 2011;49:4083–8. doi: 10.1128/JCM.05010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalu SU, Loeffelholz M, Beck E, et al. Persistence of adenovirus nucleic acids in nasopharyngeal secretions: a diagnostic conundrum. Pediatr Infect Dis J. 2010;29:746–50. doi: 10.1097/INF.0b013e3181d743c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nokso-Koivisto J, Pitkaranta A, Blomqvist S, et al. Viral etiology of frequently recurring respiratory tract infections in children. Clin Infect Dis. 2002;35:540–6. doi: 10.1086/341773. [DOI] [PubMed] [Google Scholar]
- 16.Martin ET, Fairchok MP, Kuypers J, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis. 2010;201:1625–32. doi: 10.1086/652405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues F, Foster D, Nicoli E, et al. Relationships between rhinitis symptoms, respiratory viral infections and nasopharyngeal colonization with Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in children attending daycare. Pediatr Infect Dis J. 2013;32:227–32. doi: 10.1097/INF.0b013e31827687fc. [DOI] [PubMed] [Google Scholar]
- 18.Butler CC, Hood K, Kinnersley P, Robling M, Prout H, Houston H. Predicting the clinical course of suspected acute viral upper respiratory tract infection in children. Fam Pract. 2005;22:92–5. doi: 10.1093/fampra/cmh713. [DOI] [PubMed] [Google Scholar]
- 19.Rotzén-Östlund M, Eriksson M, Tiveljung Lindell A, Allander T, Zweygberg Wirgart B, Grillner L. Children with multiple viral respiratory infections are older than those with single viruses. Acta Paediatr. 2014;103:100–4. doi: 10.1111/apa.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jartti T, Lee WM, Pappas T, Evans M, Lemanske RF, Jr, Gern JE. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J. 2008;32:314–20. doi: 10.1183/09031936.00161907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller EK, Gebretsadik T, Carroll KN, et al. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. Pediatr Infect Dis J. 2013;32:950–5. doi: 10.1097/INF.0b013e31829b7e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhedin S, Lindstrand A, Rotzén-Östlund M, et al. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014;133:e538–45. doi: 10.1542/peds.2013-3042. [DOI] [PubMed] [Google Scholar]
- 23.Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis. 2008;62:382–8. doi: 10.1016/j.diagmicrobio.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houben ML, Coenjaerts FE, Rossen JW, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82:1266–71. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franz A, Adams O, Willems R, et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010;48:239–45. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuller JA, Njenga MK, Bigogo G, et al. Association of the CT values of real-time PCR of viral upper respiratory tract infection with clinical severity, Kenya. J Med Virol. 2013;85:924–32. doi: 10.1002/jmv.23455. [DOI] [PubMed] [Google Scholar]
- 27.Nokso-Koivisto J, Pyles RB, Miller AL, Patel JA, Loeffelholz M, Chonmaitree T. Viral load and acute otitis media development after human metapneumovirus upper respiratory tract infection. Pediatr Infect Dis J. 2012;31:763–6. doi: 10.1097/INF.0b013e3182539d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nokso-Koivisto J, Pyles RB, Miller A, Jennings K, Loeffelholz MJ, Chonmaitree T. Role of human bocavirus in upper respiratory tract infections and acute otitis media. J Pediatr Infect Dis Soc. 2014;3:98–103. doi: 10.1093/jpids/pit061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Revai K, Mamidi D, Chonmaitree T. Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin Infect Dis. 2008;46:e34–7. doi: 10.1086/525856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffman HJ, Daly KA, Bainbridge KE, et al. Panel 1: Epidemiology, natural history, and risk factors. Report of the 10th Research Conference on Recent Advances in Otitis Media. Otolaryngol Head Neck Surg. 2013;148(4 suppl):E1–25. doi: 10.1177/0194599812460984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.