Abstract
BACKGROUND
The aim was to determine the proportions and correlates of essential hypertension among children in a tertiary pediatric hypertension clinic.
METHODS
We evaluated 423 consecutive children and collected demographic and clinical history by retrospective chart review.
RESULTS
We identified 275 (65%) hypertensive children (blood pressure >95th percentile per the “Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents”) from 423 children referred to the clinic for history of elevated blood pressure. The remainder of the patients had normotension (11%), white coat hypertension (11%), prehypertension (10%), and pending diagnosis (3%). Among the 275 hypertensive children, 43% (n = 119; boys = 56%; median age = 12 years; range = 3–17 years) had essential hypertension and 57% (n = 156; boys = 66%; median age = 9 years; range = 0.08–19 years) had secondary hypertension. When compared with those with secondary hypertension, those with essential hypertension had a significantly older age at diagnosis (P = 0.0002), stronger family history of hypertension (94% vs. 68%; P < 0.0001), and lower prevalence of preterm birth (20% vs. 46%; P < 0.001). There was a bimodal distribution of age of diagnosis in those with secondary hypertension.
CONCLUSIONS
The phenotype of essential hypertension can present as early as 3 years of age and is the predominant form of hypertension in children after age of 6 years. Among children with hypertension, those with essential hypertension present at an older age, have a stronger family history of hypertension, and have lower prevalence of preterm birth.
Keywords: blood pressure, etiology, hypertension, pediatrics, primary hypertension, secondary hypertension.
Essential or primary hypertension is generally considered a disease of adulthood, with a prevalence of 30%,1 and although it is thought to be less common in children, it has its beginnings in childhood.2,3 Blood pressure (BP) tracks with age, and values in childhood have been found to be significant predictors of future BP rank.3 By the time the diagnosis of essential hypertension is made in adulthood, considerable damage may have already occurred to the arterial system, heart, and other organs as a result of long-standing high BP, the changes of which are already evident in childhood.4–7 Therefore, early recognition of such a prevalent and critical healthcare issue can be significant in reducing the associated morbidity and mortality.
Essential hypertension is the most frequent type of hypertension in adults (95%) and is diagnosed when there is sustained elevation of BP greater than 140/90mm Hg and when no etiology can be determined for the hypertension.8 However, the diagnosis of hypertension in children is more complicated than in adults and thus remains frequently underdiagnosed. A number of variables must be considered in children before one can label them “hypertensive.”9 The BP norms during childhood are influenced by the height, age, and sex of the child; hence a single threshold similar to adults (i.e., 140/90mm Hg) for labeling a child with hypertension does not exist.9,10 The BP measurements in children in the ambulatory clinic can be inaccurate; therefore it is imperative that the measurements are performed in accordance to a protocol9 and repeated several times11–13 before referral for further evaluation of hypertension. The BP cuff must correspond to the size of the child,9 which can vary widely. In addition, the child should be calm and rested before the BP measurements are taken, making the diagnosis even more difficult in early childhood and especially in crying infants.14 Therefore, it is not surprising to find that the BP is not routinely measured in children in various healthcare settings, even though the current recommendations are to monitor BP in all children after 3 years of age.9 Even in settings where BP is monitored in children, an abnormal BP for that child can go unrecognized.15
Since the first reported occurrence of childhood-onset essential hypertension in 1971,16 an increasing number of children are being diagnosed with it.17–26 The prevalence of hypertension (primary and secondary) in screened children is reported between 4.5%22,27 and 13%28 In the pediatric population, where secondary causes for hypertension are more prevalent than in adults, the prevalence of essential hypertension remains unknown. The secondary causes of hypertension in childhood are from myriad causes, including congenital defects of the kidney, heart, and other organs, and need to be evaluated in each child systematically.9
We evaluated children from a metropolitan pediatric hypertension clinic with a large multiethnic referral population and a screened population of more than 20,000 children from the regional school district. The aim of this study was to evaluate etiological factors identified leading to hypertension in a tertiary pediatric hypertension clinic and then determine the proportion of essential hypertension among these children.
METHODS
Institutional approval
The protocol (HSC-MS-07-0050) was approved by the institutional Committee for the Protection of Human Subjects or the Institutional Review Board at the University of Texas Health Science Center and Children’s Memorial Hermann Hospital, Texas Medical Center.
Patient population
We selected all children ages 0–19 years in our large, university-based, pediatric general and multispecialty ambulatory clinic populations with the diagnosis of hypertension (International Classification of Diseases, Ninth Revision (ICD 9) code 401.9). From the dataset, we excluded those patients that were not evaluated at the Pediatric Hypertension Clinic, a tertiary clinic established in 1998 at the University of Texas Medical School, Texas Medical Center. This clinic is a specialty clinic in pediatric nephrology, and any child identified with hypertension is referred to this clinic. We used an electronic medical record system to conduct a retrospective chart review of all consecutive children referred to our Pediatric Hypertension Clinic between 1 August 2005 and 31 August 2011. The patients with the diagnosis of hypertension (ICD 9 code 401.9) were identified through an electronic database of the entire pediatric multispecialty and primary care ambulatory population, which included children from all of the clinics, including general pediatric, renal, and cardiology clinics. A total of 423 consecutive patients with ICD code 401.9 were identified who were further evaluated at the tertiary Pediatric Hypertension Clinic for history of elevated BP measurements. One child with the diagnosis of hypertension (ICD code 401.9) at a general pediatric clinic and not evaluated at our Pediatric Hypertension Clinic was excluded.
Children evaluated in the Pediatric Hypertension Clinic consisted of a (i) referral study population and (ii) a recruited study population. Patients in the referral study population were referred to the clinic after detection of elevated BP from both ambulatory setting at primary care physicians and inpatient setting, including neonatal intensive care unit and the general pediatric floor. As a policy, most of the clinics in the university are encouraged to refer their patients with an elevated BP to this clinic. Patients in the recruited study population comprised a small proportion of patients in our clinic and were identified by systematic school-based screening for hypertension in students aged 11–18 years in Houston-area public schools. Parents were notified in advance by letter sent from each school regarding the screening program. Forms were provided for parents to sign and return if they did not wish their child to participate. At each screening, 3 seated BP measurements were made at least 1 minute apart using oscillometric monitors. Students found to have an average BP greater than the sex, age, and height percentile-specific 95th percentile per the Fourth Report9 underwent a second set of BP measurements 1–2 weeks later. Students found to have BP greater the 95th percentile at the second screening underwent a third set of BP measurements an additional 1–2 weeks later. Students with elevated BP on all 3 occasions were considered to be hypertensive. Families of hypertensive children were informed of the persistent BP elevation and invited for a clinic-based evaluation, either to our clinic or with their primary care physician. Patients in our clinic recruited by these 2 methods (i.e., school screening and referral as described above) have been shown to be similar upon our analysis and reported in a prior publication.29
BP protocol
BP was measured by a standard protocol on all children as follows in the clinic: Clinic hypertensive status was confirmed in all subjects at the first visit to the hypertension clinic by averaging the last 3 of 4 BP measurements performed by oscillometric method and confirmed by manual auscultation with a mercury sphygmomanometer by trained personnel using methodology recommended by the Fourth Report.9 Hypertension was diagnosed when 3 separate measurements of systolic and/or diastolic BP >95th percentile for postconceptual age, adjusted for height, age, and sex per the Fourth Report,9 were documented in the medical record. All children who were aged >5 years of age, except those admitted with a hypertensive emergency, underwent ambulatory BP monitoring (ABPM) using Spacelabs oscillometric monitors (Spacelabs, Redmond, WA). The children, along with their families, were instructed on avoidance of caffeinated beverages or supplements; any medications, herbals, or over-the-counter products; smoking, and alcohol for 24 hours before and during the ambulatory BP monitoring. While on ambulatory BP monitoring, the BP was automatically measured every 20 minutes for 24 hours. Subjects with 24-hour systolic BP or diastolic BP greater than the pediatric 95th percentile or BP load (percentage of BP values exceeding the 95th percentile for the 24-hour period) greater than 25% were considered to have ambulatory hypertension.30 Both BP and BP load were used to define the severity of ambulatory hypertension. Specifically, more severe ambulatory hypertension was defined as mean systolic or diastolic BP greater than the 95th percentile and BP load greater than 50%. Children with clinic hypertension but a 24-hour systolic BP and diastolic BP less than the pediatric 95th percentile and BP load less than 25% were considered to have white coat hypertension. Children with clinic hypertension and a 24-hour systolic BP and diastolic BP less than the pediatric 95th percentile but a BP load greater than 25% were considered to have ambulatory prehypertension. Children without clinic hypertension but a 24-hour systolic BP or diastolic BP greater than the pediatric 95th percentile and BP load greater than 25% were considered to have masked hypertension. Children with history of elevated BP before referral that had normal BP measurements in the hypertension clinic and a 24-hour systolic BP and diastolic BP less than the pediatric 95th percentile and BP load less than 25% were considered to have normotension.
Once hypertension was confirmed, all children underwent further evaluation for secondary hypertension per recommendations by the Fourth Working Group.9 Prematurity was defined as gestational age less than 37 weeks. Criteria for the diagnosis of essential hypertension were (i) clinic BP elevation greater the 95th percentile on 3 previous occasions, (ii) positive 24-hour ambulatory BP monitoring (except in those who with history of hypertensive emergency requiring admission or those who were aged <5 years), (iii) absence of secondary causes of hypertension, and (iv) no concurrent medication with the potential to raise BP (e.g., steroids, central stimulants). The diagnosis of secondary hypertension was made by extensive evaluation per recommendations by the Fourth Working Group,9 including a urinary evaluation, blood tests, renal ultrasound, and echocardiogram in all children; renal magnetic resonance imaging for evaluation for renal artery stenosis in all those with stage II hypertension or resistant hypertension; sleep study for those with obesity and/or symptoms, and so on.
Statistical analysis
Data from the medical records were abstracted and tabulated. Continuous variables were compared between groups using parametric (t tests, analysis of variance with post hoc Tukey) and nonparametric (Mann–Whitney, Kruskal–Wallis) tests depending on the distribution of the variable. χ2 tests were used to compare categorical variables across groups. Kernel density curves were drawn to graphically depict the distribution of age of diagnosis of hypertension. All analyses were performed in STATA version 10 (STATA Corp, College Station, TX). Statistical significance was assumed at a type I error rate of 0.05.
RESULTS
A total of 423 consecutive patients were identified who were evaluated at the tertiary Pediatric Hypertension Clinic for history of elevated BP measurements (Figure 1). Of the 423 study-eligible children (Figure 1), 65% were diagnosed with hypertension (boys = 62%, girls = 38%; mean age = 10 years, SD = 5.4 years), of whom 37% had a secondary cause for their hypertension (secondary hypertension) and 28% had no known cause identified (essential hypertension). There were those labeled with diagnosis pending (3%) who were lost to follow-up from our clinic with an incomplete evaluation and thus a pending diagnosis for hypertension at the time of the study. Certain demographic characteristics of the patients with hypertension (n = 275; 65%) are presented in Table 1.
Figure 1.
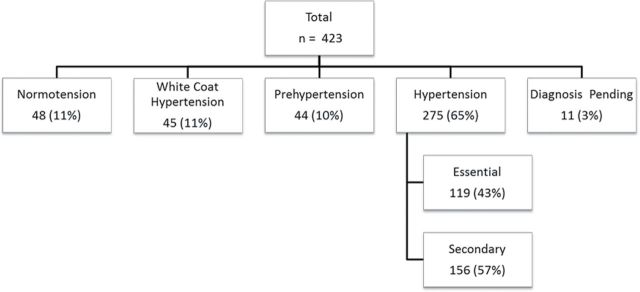
Children evaluated at the tertiary pediatric hypertension clinic for elevated blood pressure.
Table 1.
Clinical data on hypertensive children in a tertiary pediatric hypertension clinic
| Clinical data | All | Essential | Secondary | P value |
|---|---|---|---|---|
| No. (%) | 275 | 119 (43) | 156 (57) | |
| Age at diagnosis, y, median (range) | 11 (0.08–19) | 12 (3–17) | 9 (0.08–19) | 0.0002 |
| Male sex, no. (%) | 170 (62) | 67 (56) | 103 (66) | 0.10 |
| Ethnicity, no. (%) | ||||
| Black | 100 (36) | 51 (43) | 49 (31) | 0.12 |
| Non-Hispanic white | 70 (25) | 22 (18) | 48 (31) | |
| Hispanic | 85 (31) | 39 (33) | 46 (29) | |
| Asian | 7 (3) | 2 (2) | 5 (3) | |
| Others | 8 (3) | 4 (3) | 4 (3) | |
| Unknown | 5 (2) | 1 (1) | 4 (3) | |
| Family history of hypertension, no. (%)a | 209 (79) | 108 (94) | 101 (68) | <0.0001 |
| Prematurity <37 weeks, no. (%)b | 69 (35) | 17 (20) | 52 (46) | <0.001 |
aData unknown or missing for 12 patients.
bData unknown or missing for 75 patients.
Among those diagnosed with hypertension, we determined the proportion of children with essential hypertension to be 43% (boys = 56 %; mean age = 11.7±3.7 years) and the proportion with secondary hypertension to be 57% (boys = 66%; mean age 8.7±6.1 years). The differences for a number of parameters between those with essential vs. secondary hypertension were evaluated (Table 1). A higher proportion of black children had essential hypertension in comparison with non-Hispanic white children (43% vs. 18%; P = 0.01). Although essential hypertension was seen at a younger age in black children in comparison with the other race groups (Figure 2a), this difference did not reach statistical significance (P = 0.16).
Figure 2.
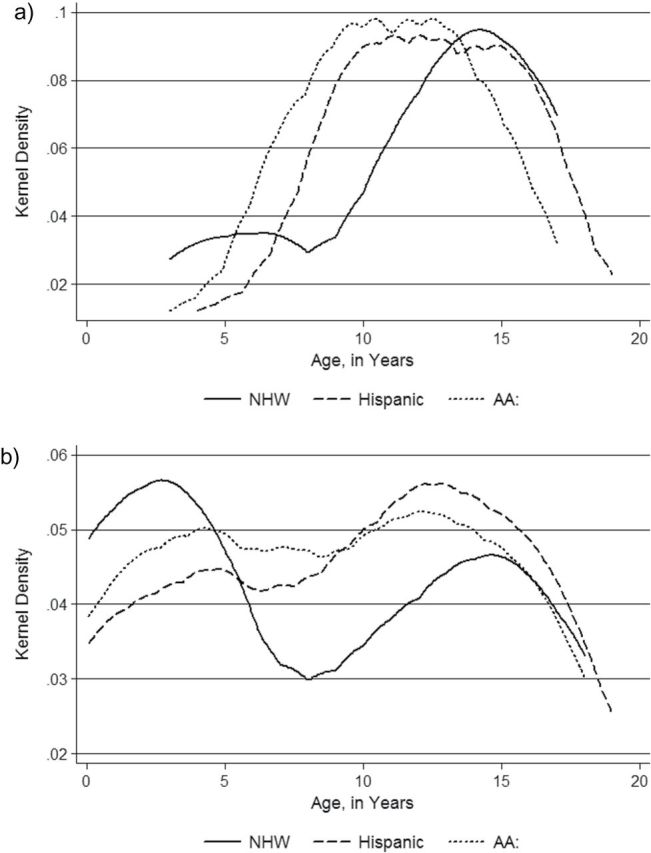
Race distribution curves. (a) Children with essential hypertension. (b) Children with secondary hypertension. NHW, non-Hispanic white.
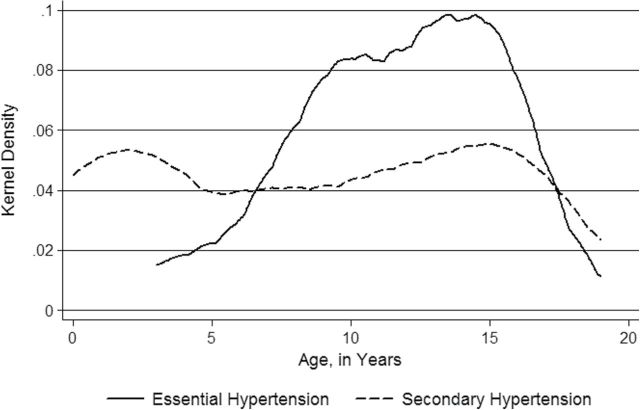
Based on the medical records, various causative factors for hypertension were identified for each child and tabulated based on the age of diagnosis (Tables 2 and 3). The most common cause in each age group was also tabulated (Table 4). The race distribution among the children with secondary hypertension was similar to that of children with essential hypertension (Figure 2b). The age distribution observed for essential hypertension (Figures 2b and 3) differed from the distribution for secondary hypertension (Figures 2b and 3), with a bimodal pattern of age of diagnosis seen in secondary hypertension. The etiologies for secondary hypertension in each subgroup were as follows: autoimmune (systemic lupus erythematosus, juvenile ankylosing spondylitis, antineutrophil cytoplasmic antibodies-associated vasculitis), cardiovascular (coarctation of aorta), endocrine (hypothyroidism, hyperthyroidism, adrenal neuroblastoma), gastrointestinal (gastroschisis), hematological (sickle cell disease), medication related (steroids, central stimulants, adrenocorticotropic hormone), neurological (atrioventricular malformation, severe intraventricular hemorrhage, hydrocephalus, brain tumor, neural tube defect, Arnold Chiari malformation), renal (hydronephrosis, nephrotic syndrome, glomerulonephritis, nephropathy, renal dysplasia, renal artery stenosis), respiratory (bronchopulmonary dysplasia, chronic lung disease), and sleep disordered breathing related (obstructive sleep apnea, mixed central with obstructive sleep apnea, narcolepsy). The following syndromes were seen in association with hypertension: Williams’s syndrome, Turner’s syndrome, and Leigh’s syndrome.
Table 2.
Causes of secondary hypertension in a tertiary pediatric hypertension clinic
| Causes | Total no. (%) | Age at diagnosis, y, median (range) | Male sex, no. (%) |
|---|---|---|---|
| Autoimmune | 3 (1) | 10.5 (9–17) | 2 (67) |
| Cardiac | 4 (3) | 4.5 (1–11) | 3 (75) |
| Endocrine | 9 (6) | 13 (6–17) | 2 (22) |
| Gastrointestinal | 2 (1) | 0.5 (0.17–0.75) | 2 (100) |
| Hematological | 1 (1) | 8 | 1 (100) |
| Medications | 21 (13) | 13 (0.08–18) | 16 (76) |
| Neurological | 19 (12) | 10 (0.25–18) | 14 (74) |
| Renal | 53 (34) | 10 (0.08–19) | 33 (62) |
| Respiratory | 32 (20) | 1 (0.01–17) | 20 (63) |
| Sleep disordered breathing | 12 (8) | 14 (4–17) | 10 (83) |
Table 3.
Causes of hypertension in a tertiary pediatric hypertension clinic by age
| Causes | Infancy, <1 y, no. (%) | Preschool, 1–5 y, no. (%) | Preteen, 6–12 y, no. (%) | Teen 13–19 y, no. (%) |
|---|---|---|---|---|
| No. of patients | 23 | 41 | 99 | 112 |
| Essential | 0 (0) | 8 (20) | 56 (57) | 55 (49) |
| Secondary | 23 (100) | 33 (80) | 43 (43) | 57 (51) |
| Autoimmune | 0 (0) | 0 (0) | 2 (5) | 1 (2) |
| Cardiac | 0 (0) | 3 (9) | 1 (2) | 0 (0) |
| Endocrine | 0 (0) | 0 (0) | 4 (9) | 5 (9) |
| Gastrointestinal | 2 (9) | 0 (0) | 0 (0) | 0 (0) |
| Hematological | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| Medications | 2 (9) | 3 (9) | 4 (9) | 12 (21) |
| Neurological | 2 (9) | 3 (9) | 7 (16) | 7 (12) |
| Renal | 3 (13) | 11 (34) | 17 (39) | 22 (39) |
| Respiratory | 14 (60) | 12 (36) | 4 (9) | 2 (3) |
| Sleep disordered breathing | 0 (0) | 1 (3) | 3 (12) | 8 (14) |
Table 4.
Most common causes of hypertension in a tertiary pediatric hypertension clinic by age
| Age groups | Most common etiology of hypertension |
|---|---|
| Infancy, <1 y | Respiratory (61%) |
| Renal (13%) | |
| Medication (9%) | |
| Preschool, 1–5 y | Respiratory (29%) |
| Renal (27%) | |
| Essential (19%) | |
| Preteen, 6–12 y | Essential (57%) |
| Renal (27%) | |
| Neurological (7%) | |
| Teen, 13–19 y | Essential (49%) |
| Renal (20%) | |
| Medication (11%) |
Figure 3.
Age of diagnosis distribution curve of children with essential hypertension in comparison with those with secondary hypertension.
DISCUSSION
Childhood-onset essential hypertension was diagnosed in 43% (56% in boys, 44% in girls; 25% in Non-Hispanic whites, 31% in Hispanics, 36% in blacks, 3% in Asians, and 5% in others) of children in our tertiary ambulatory clinic. Previous studies have reported the proportion of essential hypertension in a pediatric ambulatory care setting in the United States at 16% in the1980s,25 23% in the 1990s,26 and 48% in 2002.24 A more recent study in 2011 from Brazil reported that 15% of children in a pediatric ambulatory referral center had essential hypertension.31 It is important to note that nearly a quarter of the children (24%) referred to our clinic (those who were identified as having prehypertension (10%), white coat hypertension (11%), or diagnosis pending (3%)) have the potential to develop essential hypertension in the future.
In the general pediatric population, the prevalence of essential hypertension is unknown. However, various screening studies primarily in adolescents have identified a prevalence of any hypertension (essential or secondary) between 3.2% and 13.8%.22,32 The National Health and Nutrition Examination Survey 1999–2000 showed a prevalence of elevated BP near 8% among youth aged 12–19 years, but their definition of hypertension included the prehypertensive patients as well.33 A more recent multicenter study in 2006 found the prevalence of hypertension was 13.8%.32 We determined that secondary form of hypertension was diagnosed in 51% of the teenagers in the ambulatory hypertension clinic. This finding should caution us from labeling teenagers with essential hypertension without further evaluation for the etiology in the screening studies.
The phenotype of childhood-onset essential hypertension can present as early as 3 years of age, as demonstrated in our study of well-characterized, multiethnic, hypertensive children. After age 6 years, essential hypertension was the predominant etiology for hypertension in children in our patient population. Similar to other studies among children,24,31,34 we found that the majority were diagnosed with essential hypertension after the age of 13 years (66%). In our tertiary ambulatory referral center population, the mean age at presentation was 11.7±3.7 years, similar to that reported by other studies from the United States24 (13.3±3.9 years; earliest age at presentation reported as 1.1 years) and Brazil31 (13.07±5.67 years; earliest age at presentation reported as 2 years).
Similar to adults with essential hypertension, male sex was found to predominate in children with essential hypertension. We found 56% of male children with essential hypertension, which is higher than the 42.43% reported in the study from Brazil.31 Similar to adult-onset essential hypertension,8 essential hypertension was highest among black children. There was no significant difference between any of the other races and non-Hispanic whites or between the other races and blacks but this could be because of a small sample size and lack of statistical power.
Among the children with essential hypertension, we found 20% with preterm birth of less than 37 weeks of gestation. To our knowledge, this has never been reported previously in the literature. However, there has been a focus on birth size and fetal origins of hypertension,35–38 with higher BP in adulthood observed in relationship to smaller size at birth. This emphasizes the importance of determining prematurity among this population.
We found that children with essential hypertension, in comparison with those with secondary hypertension, presented at an older age, had a stronger family history of hypertension, and had a lower prevalence of preterm birth. Secondary hypertension is the predominant form of hypertension in infants and preschool children. Our patient population included children with neonatal hypertension who have perinatal risk factors (especially in preterm children) and secondary causes such as renal disease and so on for their hypertension, which require treatment.39 Although overall (Table 4) renal causes predominated as an etiology of secondary hypertension, we also had several younger children (aged <5 years) with predominantly respiratory causes of hypertension, a finding quite different from previous reports in children, where renal diseases predominated. With an increase in preterm birth, the etiology for hypertension among younger children in our study included those due to bronchopulmonary dysplasia that probably also included other perinatal risk factors for hypertension. Furthermore, we determined a bimodal pattern for diagnosis of secondary hypertension in children, which could be explained as follows: the first peak at a younger age due to congenital malformations and perinatal risk factors from prematurity; the second peak at an older age due to acquired diseases such as sleep disordered breathing and medications such as central stimulants for attention deficit disorder.
The limitations of this study included those of a retrospective, observational study design. Certain stratified analyses performed for this study were also limited in power by the small sample size in the stratified groups. Because our study involved retrospective chart review, we were not able to conduct a detailed study of the hypertensive patients and hence did not report their anthropometric, clinical, and BP characteristics or the contributors to the disease, the comorbid conditions, or the management of the disease. Although we had both referred and screened children in our hypertension clinic, our tertiary ambulatory clinic population ascertained from an electronic database is not representative of a general pediatric population, making prevalence estimates for such a population virtually impossible from our data. Unfortunately, unlike adults, in pediatrics the diagnosis code for essential hypertension is seldom used, and hence we do not have access to this data from national databases to determine the prevalence of essential hypertension in childhood. Furthermore, because our clinic is a referral clinic, the data may be subject to classification bias, although we evaluated the details from all investigations in each child to confirm the etiology of hypertension. Hypertension is a missed diagnosis in many instances; thus the children with hypertension from cardiac, renal, rheumatologic or other subspecialty clinic may be underrepresented in our patient population because they may have not have been labeled with hypertension and/or referred to the tertiary hypertension care clinic for their hypertension.
The phenotype of childhood-onset essential hypertension can present as early as 3 years of age, involving 43% of the ambulatory pediatric hypertension clinic patients, with preponderance in male sex and black ethnicity. After 6 years of age, essential hypertension is the predominant etiology for hypertension in children. Children with essential hypertension, in comparison with those with secondary hypertension, present at an older age, have a stronger family history of hypertension, and have lower prevalence of preterm birth.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by grant K23HL089391 “Determination of Genetics of Childhood-Onset Hypertension” (PI Monesha Gupta) from the National Heart, Lung, and Blood Institute . The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. Monesha Gupta receives funding from the National Institutes of Health. All others authors report no conflicts of interest to disclose.
REFERENCES
- 1. Gillespie CD, Hurvitz KA. Prevalence of hypertension and controlled hypertension–United States, 2007–2010. MMWR Surveill Summ 2013; 62:144–148. [PubMed] [Google Scholar]
- 2. Berenson GS, Wattigney WA, Bao W, Nicklas TA, Jiang X, Rush JA. Epidemiology of early primary hypertension and implications for prevention: the bogalusa heart study. J Hum Hypertens 1994; 8:303–311. [PubMed] [Google Scholar]
- 3. Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the bogalusa heart study. Am J Hypertens 1995; 8:657–665. [DOI] [PubMed] [Google Scholar]
- 4. Gruskin AB. The adolescent with essential hypertension. Am J Kidney Dis 1985; 6:86–90. [DOI] [PubMed] [Google Scholar]
- 5. Daniels SD, Meyer RA, Loggie JM. Determinants of cardiac involvement in children and adolescents with essential hypertension. Circulation 1990; 82:1243–1248. [DOI] [PubMed] [Google Scholar]
- 6. McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, Poffenbarger T, Sorof JM, Portman RJ. Left ventricular hypertrophy in hypertensive adolescents: Analysis of risk by 2004 national high blood pressure education program working group staging criteria. Hypertension 2007; 50:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daniels SR, Loggie JM, Khoury P, Kimball TR. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation 1998; 97:1907–1911. [DOI] [PubMed] [Google Scholar]
- 8. Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): Resetting the hypertension sails. Hypertension 2003; 41:1178–1179. [DOI] [PubMed] [Google Scholar]
- 9. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004; 114:555–576. [PubMed] [Google Scholar]
- 10. Shear CL, Burke GL, Freedman DS, Webber LS, Berenson GS. Designation of children with high blood pressure—considerations on percentile cut points and subsequent high blood pressure: the bogalusa heart study. Am J Epidemiol 1987; 125:73–84. [DOI] [PubMed] [Google Scholar]
- 11. Burke GL, Webber LS, Shear CL, Zinkgraf SA, Smoak CG, Berenson GS. Sources of error in measurement of children’s blood pressure in a large epidemiologic study: bogalusa heart study. J Chronic Dis 1987; 40:83–89. [DOI] [PubMed] [Google Scholar]
- 12. Podoll A, Grenier M, Croix B, Feig DI. Inaccuracy in pediatric outpatient blood pressure measurement. Pediatrics 2007; 119:e538––e543. [DOI] [PubMed] [Google Scholar]
- 13. Sinaiko AR, Gomez-Marin O, Prineas RJ. Prevalence of “significant” hypertension in junior high school-aged children: the children and adolescent blood pressure program. J Pediatr 1989; 114:664–669. [DOI] [PubMed] [Google Scholar]
- 14. Hart JT. Blood pressure in children: is screening for essential hypertension in children essential? BMJ. 2008; 336:1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bijlsma MW, Blufpand HN, Kaspers GJ, Bokenkamp A. Why pediatricians fail to diagnose hypertension: a multicenter survey. J Pediatr 2014; 164:173–177 e177. [DOI] [PubMed] [Google Scholar]
- 16. Gruskin AB, Linshaw M, Cote ML, Fleisher DS. Low-renin essential hypertension—another form of childhood hypertension. J Pediatr 1971; 78:765–771. [DOI] [PubMed] [Google Scholar]
- 17. Kilcoyne MM, Richter RW, Alsup PA. Adolescent hypertension. I. Detection and prevalence. Circulation 1974; 50:758–764. [DOI] [PubMed] [Google Scholar]
- 18. Dube SK, Kapoor S, Ratner H, Tunick FL. Blood pressure studies in black children. Am J Dis Child 1975; 129:1177–1180. [DOI] [PubMed] [Google Scholar]
- 19. Heyden S, Bartel AG, Hames CG, McDonough JR. Elevated blood pressure levels in adolescents, evans county, georgia. Seven-year follow-up of 30 patients and 30 controls. JAMA 1969; 209:1683–1689. [PubMed] [Google Scholar]
- 20. Londe S, Goldring D. Hypertension in children. Am Heart J 1972; 84:1–4. [DOI] [PubMed] [Google Scholar]
- 21. Loggie JM. Hypertension in children and adolescents. I. Causes and diagnostic studies. J Pediatr 1969; 74:331–335. [DOI] [PubMed] [Google Scholar]
- 22. McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr 2007; 150:640–644, 644 e641. [DOI] [PubMed] [Google Scholar]
- 23. Adrogue HE, Sinaiko AR. Prevalence of hypertension in junior high school-aged children: effect of new recommendations in the 1996 updated task force report. Am J Hypertens 2001; 14:412–414. [DOI] [PubMed] [Google Scholar]
- 24. Flynn JT, Alderman MH. Characteristics of children with primary hypertension seen at a referral center. Pediatr Nephrol 2005; 20:961–966. [DOI] [PubMed] [Google Scholar]
- 25. Feld LG, Springate JE. Hypertension in children. Curr Probl Pediatr 1988; 18:317–373. [DOI] [PubMed] [Google Scholar]
- 26. Arar MY, Hogg RJ, Arant BS, Jr, Seikaly MG. Etiology of sustained hypertension in children in the southwestern United States. Pediatr Nephrol 1994; 8:186–189. [DOI] [PubMed] [Google Scholar]
- 27. Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics 2004; 113:475–482. [DOI] [PubMed] [Google Scholar]
- 28. Sorof JM, Poffenbarger T, Franco K, Bernard L, Portman RJ. Isolated systolic hypertension, obesity, and hyperkinetic hemodynamic states in children. J Pediatr 2002; 140:660–666. [DOI] [PubMed] [Google Scholar]
- 29. Sorof JM, Turner J, Franco K, Portman RJ. Characteristics of hypertensive children identified by primary care referral compared with school-based screening. J Pediatr 2004; 144:485–489. [DOI] [PubMed] [Google Scholar]
- 30. Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S. Ambulatory blood pressure monitoring in children and adolescents: Recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young and the Council for High Blood Pressure Research. Hypertension 2008; 52:433–451. [DOI] [PubMed] [Google Scholar]
- 31. Gomes RS, Quirino IG, Pereira RM, Vitor BM, Leite AF, Oliveira EA, Simoes e Silva AC. Primary versus secondary hypertension in children followed up at an outpatient tertiary unit. Pediatr Nephrol 2011; 26:441–447. [DOI] [PubMed] [Google Scholar]
- 32. Jago R, Harrell JS, McMurray RG, Edelstein S, El Ghormli L, Bassin S. Prevalence of abnormal lipid and blood pressure values among an ethnically diverse population of eighth-grade adolescents and screening implications. Pediatrics 2006; 117:2065–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among U.S. adolescents, 1999–2000. Diabetes Care 2004; 27:2438–2443. [DOI] [PubMed] [Google Scholar]
- 34. Wyszynska T, Cichocka E, Wieteska-Klimczak A, Jobs K, Januszewicz P. A single pediatric center experience with 1025 children with hypertension. Acta Paediatr 1992; 81:244–246. [DOI] [PubMed] [Google Scholar]
- 35. Law CM, de Swiet M, Osmond C, Fayers PM, Barker DJ, Cruddas AM, Fall CH. Initiation of hypertension in utero and its amplification throughout life. BMJ 1993; 306:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Law CM, Egger P, Dada O, Delgado H, Kylberg E, Lavin P, Tang GH, von Hertzen H, Shiell AW, Barker DJ. Body size at birth and blood pressure among children in developing countries. Int J Epidemiol 2001; 30:52–57. [DOI] [PubMed] [Google Scholar]
- 37. Yliharsila H, Eriksson JG, Forsen T, Kajantie E, Osmond C, Barker DJ. Self-perpetuating effects of birth size on blood pressure levels in elderly people. Hypertension 2003; 41:446–450. [DOI] [PubMed] [Google Scholar]
- 38. Yiu V, Buka S, Zurakowski D, McCormick M, Brenner B, Jabs K. Relationship between birthweight and blood pressure in childhood. Am J Kidney Dis 1999; 33:253–260. [DOI] [PubMed] [Google Scholar]
- 39. Sahu R, Pannu H, Yu R, Shete S, Bricker JT, Gupta-Malhotra M. Systemic hypertension requiring treatment in the neonatal intensive care unit. J Pediatr 2013; 163:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]