Abstract
Background
We have reported that children with biopsy-proven minimal change disease (MCD) express CD80 (also known as B7.1) in their podocytes and excrete high levels of CD80 in their urine during active nephrotic syndrome. We also reported that polyIC, a Toll-like receptor 3 ligand, increases CD80 mRNA and protein expression in cultured human podocytes dose-dependently, with actin re-organization and a reduction in synaptopodin expression.
Methods
To determine the effect of polyIC in the kidney, we subjected mice to systemic injection of polyIC or phosphate buffered saline.
Results
Mice injected with polyIC developed significant proteinuria with increased urinary CD80 excretion. Glomeruli from mice injected with polyIC were normal by light microscopic examination, but showed increased CD80 production in podocytes by immunofluorescence staining. In isolated glomeruli from mice injected with polyIC, expressions of CD80 and interleukin 10 significantly increased with a mild non-significant increase in CTLA-4, and synaptopodin expression decreased significantly.
Conclusions
Our study demonstrates that systemically administered polyIC can induce transient proteinuria and urinary CD80 excretion with an increase in CD80 production in podocytes, increased glomerular CD80 and reduced synaptopodin expression. These findings may be relevant to the pathogenesis of proteinuria in MCD.
Keywords: animal models, CD80, minimal change disease, nephrotic syndrome, proteinuria
INTRODUCTION
Reiser et al. reported that CD80, a receptor normally expressed on antigen-presenting cells [1, 2], can be induced in podocytes where it causes actin reorganization and the development of proteinuria, and they hypothesized that this could be a mechanism for minimal change disease (MCD) [3]. Consistent with this hypothesis, we recently reported that subjects with MCD in relapse express CD80 in their podocytes and have increased urinary excretion of CD80 in their urine [4]. These changes were not observed in limited numbers of subjects with focal segmental glomerulosclerosis or other nephrotic diseases [5].
If proteinuria in MCD is due to podocyte CD80 expression, then identifying factors that can induce CD80 might provide an insight into the pathogenesis of this disease. To date, several models of CD80-associated proteinuria have been reported, including a rat model associated with interleukin (IL)-13 overexpression [6], and murine models associated with genetic knockout of α3 integrin, administration of the toxic compound aminonucleoside and by injection of lipopolysaccharide, which binds Toll-like receptor (TLR) 4 [3].
TLRs are particularly high in affinity as they are often activated by viral and bacterial infections, and viral infections are commonly associated with the induction or relapse of MCD. In this regard, we recently reported that TLR3 which recognizes viral dsRNA is expressed on cultured human podocytes and that polyIC, a TLR3 ligand, can induce CD80 expression with actin reorganization that can be blocked by corticosteroids [7]. In this article, we present the first description of a TLR3-mediated model of proteinuria with characteristics similar to that observed for MCD.
MATERIALS AND METHODS
Cell culture
Immortalized human podocytes were obtained from Dr Moin A. Saleem at the University of Bristol and were maintained as described previously [7]. Cells were kept in 1% fetal bovine serum (Invitrogen, Carlsbad, CA) for 18 h, and then were treated with 0.01–10 μg/mL of polyIC-LMW (PolyIC with a low molecular weight, average size of 0.2–1 kb, Invivogen, San Diego, CA) for 6 h. Cells were homogenized in RIPA Lysis Buffer (Santa Cruz Biotechnology, Santa Cruz, CA), or were subjected to mRNA extraction with QIAshredder (Qiagen, Valencia, CA). Western blotting for CD80 was performed as described previously [7].
Experimental animals
Wild-type male C57BL/6J mice, 6 weeks old (#000664, Jackson Laboratory, ME), were injected with 600 μg of polyIC-LMW with phosphate buffered saline (PBS) as vehicle control. Blood and kidney tissue were collected at 6 h, 24 h, 1 week and 2 weeks. Twenty-four-hour urine was collected at 24 h, 1 week and 2 weeks. Six-hour urine after the injections was collected at 6 h, and 18-h urine from 6 to 24 h after the injections was also collected. The harvested kidneys were directly frozen in liquid nitrogen for protein analysis or total RNA extraction, and then stored at –80°C. Urinary albumin-to-creatinine ratio was measured with Albuwell M (Exocell, Philadelphia, PA) and Liquid Creatinine Assay (Bioquant, San Diego, CA), respectively. CD80 null mice were obtained from Jackson Laboratory (#003611, B6.129S4-Cd80tm1Shr/J). Blood, kidney, spleen tissues and 24-h urine were collected from CD80 null mice. CD80 null mice were also injected with 600 μg of polyIC-LMW, then, 24-h urine was collected at 24 h. All experiments were conducted with adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Animal Care and Use Committee of the University of Colorado.
Renal histopathology
Kidney tissues were fixed in 10% formalin and embedded in paraffin for periodic acid-Schiff staining. For electron microscopy, kidney tissues were fixed in 2.5% glutaraldehyde overnight at 4°C and osmicated. They were then embedded in Epon 812 (Nisshin EM Co., Tokyo, Japan), and ultrathin sections were observed, transmission electron micrographs were taken using a Hitachi 7100 electron microscope.
Murine CD80 ELISA
Murine CD80 concentration in serum, urine and tissue lysates was determined with mouse B7-1/CD80 ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. For measurement of urinary mouse CD80 concentration, urine samples were concentrated by ultrafiltration using YM-10 Microcon centrifugal filter devices (Millipore, Bedford, MA). The amount of CD80 protein was normalized for creatinine concentration. Kidney and spleen samples were homogenized in RIPA Lysis Buffer (Santa Cruz Biotechnology), and then centrifuged. The amount of CD80 protein was determined by ELISA, and normalized for protein concentration (BCA Protein assay kit, Pierce, Rockford, IL).
Glomerular isolation
Cortical tissue of murine kidneys was minced in PBS and isolated by differential sieving [8].
RNA extraction, reverse transcription and quantitative RT-PCR
Total RNA was extracted using RNeasy mini kit (Qiagen). Each 1 μg of total RNA were reverse transcribed using iScript cDNA synthesis kit (Biorad, Hercules, CA). Quantitative real-time PCR was performed using i-Cycler (Bio-Rad) and iQ SYBR Green Supermix (Bio-rad). Primer pairs were designed using Beacon Designer software (Premier Biosoft International, Palo Alto, CA) and synthesized by IDT DNA (IDT DNA, Coralville, IA) (Table 1). Amplification were performed for one cycle at 95°C for 3 min, 45 cycles at 95°C for 15 s, and designated annealing temperature for 30 s; 72°C for 30 s. The results were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or β-actin expression.
Table 1.
Primer pairs used for quantitative RT-PCR
| Gene | Sequence (F, forward; R, reverse) | Annealing temperature (°C) |
|---|---|---|
| Mouse | ||
| CD80 | F: 5′-CGACTCGCAACCACACCATTAAG-3′ | 58 |
| R: 5′-TCCTGCCCCAAAGAGCACAAG-3′ | ||
| CTLA-4 | F: 5′-ACACATCTCAAGGCTTCTG-3′ | 53 |
| R: 5′-GGTCACCTGTATGGCTTC-3′ | ||
| IL-10 | F: 5′-GGTTGCCAAGCCTTATCG-3′ | 54 |
| R: 5′-TCTTCACCTGCTCCACTG-3′ | ||
| Synaptopodin | F: 5′-TATCAACCAGAACCCGTC-3′ | 51 |
| R: 5′-AATCAAGTGTGCCATTCG-3′ | ||
| β-actin | F: 5′-TCTTGGCTATGGAATCCTG-3′ | 60 |
| R: 5′-GTGTTGGCATAGAGGTCT-3′ | ||
| Angiopoietin-like 4 | F: 5′-GCACCAGAGCAAGTCTAA-3′ | 61 |
| R: 5′-CAGCAACGCTAGGACTCC-3′ | ||
| TLR1 | F: 5′-CTCATCTTCTACTGTATCATTGTT-3′ | 60 |
| R: 5′-TGGTAAGGTTTGCGTTTG -3′ | ||
| TLR2 | F: 5′-CCAGCAGAATCAATACAATAGA -3′ | 60 |
| R: 5′-CGAACCAGGAGGAAGATAA -3′ | ||
| TLR3 | F: 5′-TGGATTCTTCTGGTGTCTT-3′ | 60 |
| R: 5′-TCTTCTGAGTTGGTTGTGA-3′ | ||
| TLR4 | F: 5′-ATGTGATGTGACCATTGATG-3′ | 60 |
| R: 5′-ATGATTGATAAGGATTGCCATT-3′ | ||
| TLR5 | F: 5′-TTGGTAATATCTCCCTGTTCT-3′ | 60 |
| R: 5′-AGCCTCTCAGTGGTAGTA-3′ | ||
| TLR6 | F: 5′-GCATTCTTCTGGACTTAGTG-3′ | 60 |
| R: 5′-AATGGAGAACGGTGGTATT-3′ | ||
| TLR7 | F: 5′-GCTTTAGTGGACTCTCTGA-3′ | 60 |
| R: 5′-GTTAGATTCTCCTTCGTGATG-3′ | ||
| TLR8 | F: 5′-ACTCATCCATCCACATACAT-3′ | 60 |
| R: 5′-AACCAGGTAGAAGGAATCG-3′ | ||
| TLR9 | F: 5′-CTCTCCATACACTGAACTCT-3′ | 60 |
| R: 5′-CCTTCTTGCGGTAATTGAA-3′ | ||
| TLR11 | F: 5′-ACAACCAGTGTCACATCTA-3′ | 60 |
| R: 5′-GCTATAAGTCAGGCAATATCC-3′ | ||
| Human | ||
| CD80 | F: 5′-TTTGACCCTAAGCATCTGAAGC-3′ | 55 |
| R: 5′-ACCAGCCAGCACCAAGAG-3′ | ||
| GAPDH | F: 5′-CGCTGAGTACGTCGTGGAGT-3′ | 64 |
| R: 5′-AGAGGGGGCAGAGATGATG-3′ | ||
Statistical analysis
All data are presented as mean ± SEM. Data graphics and statistical analysis were performed using Prism 5 (GraphPad, San Diego, CA). Independent replicates for each data point (n) are identified in figure legends. Data were analyzed by one-way analysis of variance, Tukey post hoc test. When two groups were compared, the Mann–Whitney test was applied. A P-value of <0.05 was recognized as statistically significant.
RESULTS
In our previous report, we used polyIC, a TLR3 ligand, to induce CD80 expression and actin re-organization in cultured human podocytes. The expression of CD80 in human podocytes peaks at 6 h after stimulation with polyIC [7]. For our in vivo study, we selected polyIC-LMW which has a lower molecular weight (see the Materials and methods section). Western blotting and real-time PCR for CD80 revealed that polyIC-LMW significantly increased CD80 production and CD80 expression on cultured human podocytes (Figure 1a and b). This effect was dependent on the dose of polyIC-LMW.
FIGURE 1:
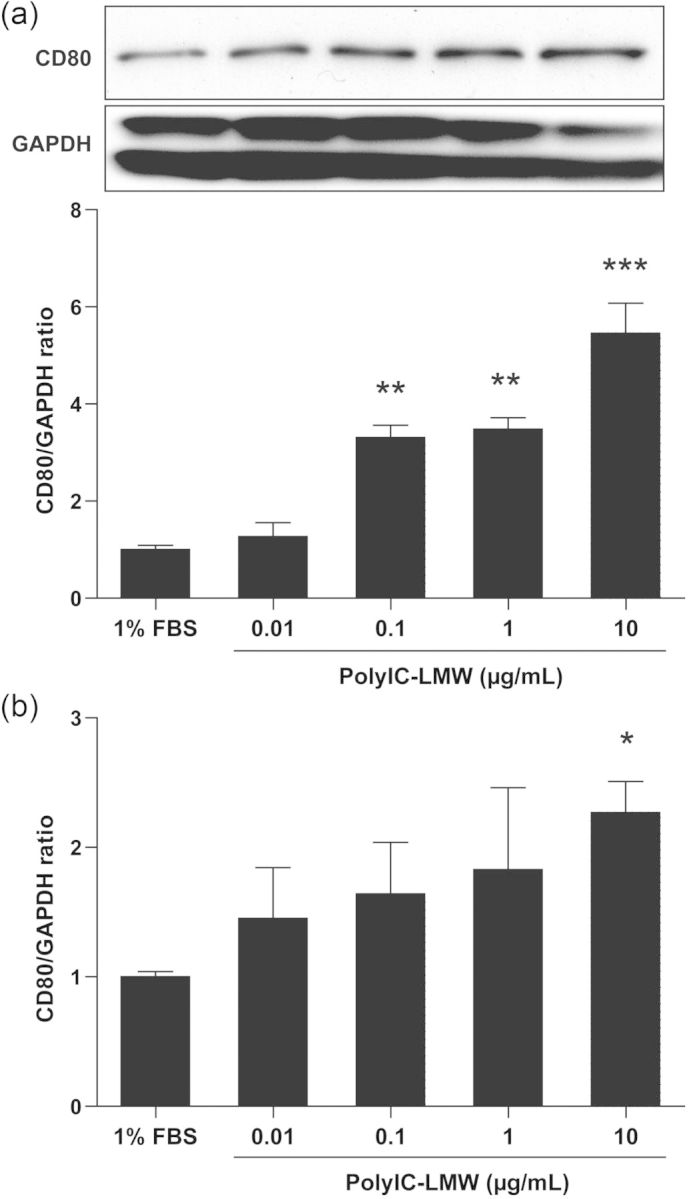
PolyIC-LMW increases CD80 on cultured human podocytes. Cultured human podocytes were stimulated with various concentrations of polyIC-LMW. FBS 1% was used as a control. (a) Total protein was extracted from the cells and processed for western blotting for human CD80. The results represent the means ± SEM (n= 3 wells) **P < 0.01; ***P < 0.001 versus 1% FBS. (b) Total RNA was extracted from the cells and processed for real-time PCR for human CD80. The results represent the means ± SEM (n= 3 wells). *P < 0.05 versus 1% FBS. GAPDH was used as an internal control.
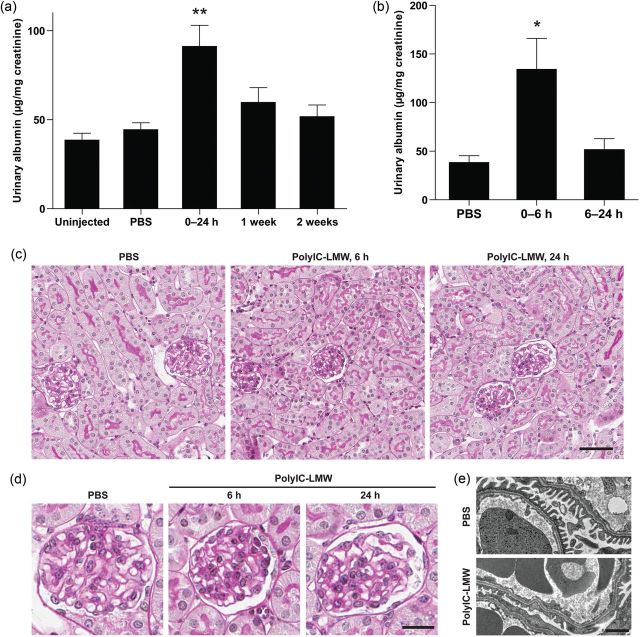
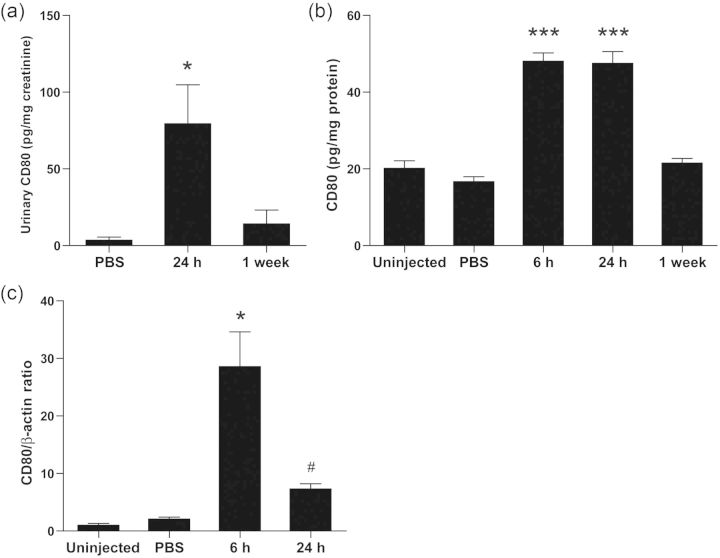
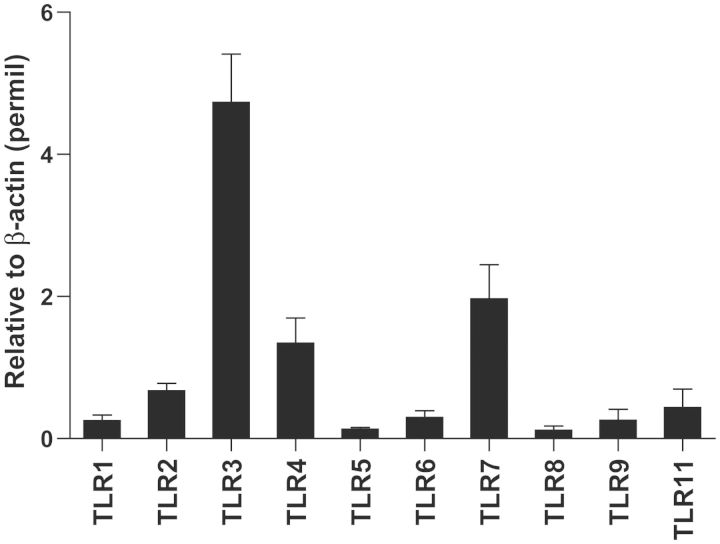
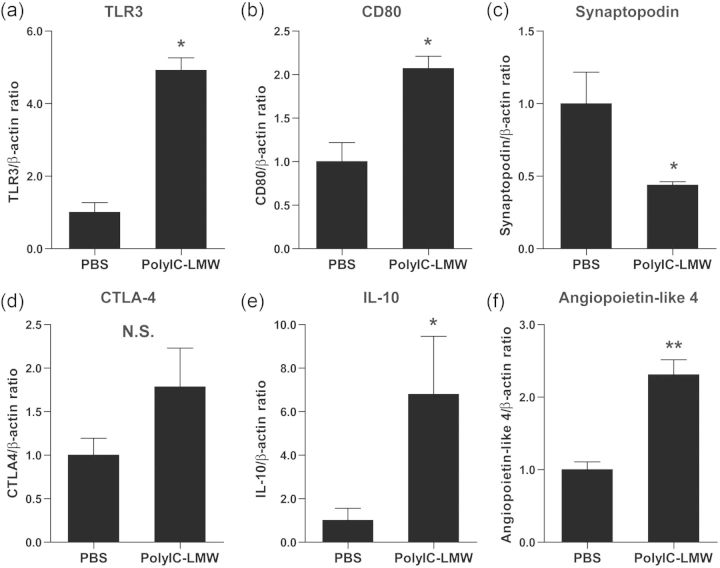
Intravenous injection of polyIC-LMW (PolyIC with a low molecular weight) resulted in significant albuminuria at 24 h compared with PBS-injected controls (Figure 2a), but was transient with a peak at 6 h (Figure 2b). Histological examination revealed normal appearing glomeruli by PAS staining in both polyIC-LMW- and PBS-injected mice at 6 and 24 h (PAS staining, Figure 2c and d). Immunofluorescence staining for macrophages (F4/80) and T-cells (CD3) did not show increased infiltration in the kidneys of polyIC-LMW injected mice at 6 and 24 h (data not shown). However, electron microscopy demonstrated partial foot process fusion in glomeruli of polyIC-LMW-injected mice (Figure 2e). Urinary CD80 levels were increased transiently by the intravenous injection of polyIC-LMW in association with transient albuminuria (Figure 3a). Whole kidney preparations also showed increased CD80 protein (Figure 3b) as well as CD80 mRNA (by quantitative real-time PCR, Figure 3c). Isolated glomeruli were also evaluated by quantitative real-time PCR for TLRs and CD80. In isolated murine glomeruli, TLR3 was predominantly expressed among TLRs (Figure 4). TLR3 was induced by polyIC along with CD80 (Figure 5a and b). Consistent with our in vitro study [7], synaptopodin expression was decreased (Figure 5c). Interestingly, both IL-10 and CTLA-4, which are known to down-regulate CD80 expression in antigen-presenting cells, were also induced (Figure 5d and e). We also examined the expression of angiopoietin-like 4, which has been implicated as a potential mediator of MCD [9]. Intravenous injection of polyIC-LMW significantly increased the expression of angiopoietin-like 4 in glomeruli (Figure 5f).
FIGURE 2:
Intravenous injection of polyIC-LMW induces transient albuminuria in mice. Mice were intravenously injected with polyIC-LMW or PBS. (a) Twenty-four-hour urine was collected at 24 h (0–24 h), 1 week and 2 weeks. Urinary albumin-to-creatinine ratio was measured. The results represent the means ± SEM (n = 5). **P < 0.01 versus uninjected, PBS and 2 weeks. (b) Six-hour urine (0–6 h) or 18-h (6–24 h) urine were collected at 6 or 24 h, respectively. Urinary albumin-to-creatinine ratio was measured. The results represent the means ± SEM (n = 5). *P < 0.05 versus PBS and 6–24 h. (c and d) Histological sections of the kidneys (at 6 and 24 h) of mice injected with polyIC-LMW or PBS were stained with PAS. Bar, 50 μm in (c) and 20 μm in (d). (e) Electron microscopy of the kidneys of mice injected with polyIC-LMW or PBS. Bar, 1 μm.
FIGURE 3:
PolyIC-LMW induces urinary CD80 excretion and CD80 production in kidneys. Mice were intravenously injected with polyIC-LMW or PBS. (a) Twenty-four-hour urine was collected at 24 h and 1 week. Urinary concentration of CD80 protein was determined by ELISA, and was corrected by creatinine concentration. The results represent means ± SEM (n = 5). *P < 0.05 versus PBS and 1 week. (b) The kidney samples at 6 and 24 h after the injection were homogenized and then centrifuged. The amount of CD80 in each supernatant was measured by ELISA. The results represent means ± SEM (n = 5). *P < 0.001 versus uninjected, PBS and 1 week. (c) Total RNA was extracted from the kidney samples at 6 and 24 h after the injection. The expression of CD80 was assessed by real-time PCR. The results represent the means ± SEM (n = 5). *P < 0.001 versus uninjected and PBS. #P < 0.01 versus 6 h. β-actin was used as an internal control.
FIGURE 4:
The expressions of TLRs in murine glomeruli. Isolated murine glomeruli from mice injected with PBS were subjected to real-time PCR for murine TLRs. The results represent the means ± SEM (n = 3). β-actin was used as an internal control.
FIGURE 5:
The effect of polyIC-LMW in glomeruli. Mice were intravenously injected with polyIC-LMW or PBS. Glomeruli were isolated at 6 h, and then subjected to real-time PCR for CD80 (a), TLR3 (b), synaptopodine (c), CTLA-4 (d), IL-10 (e) and angiopoietin-like 4 (f). The results represent means ± SEM (n = 5). **P < 0.01, *P < 0.05 versus PBS. N.S., not significant. β-actin was used as an internal control.
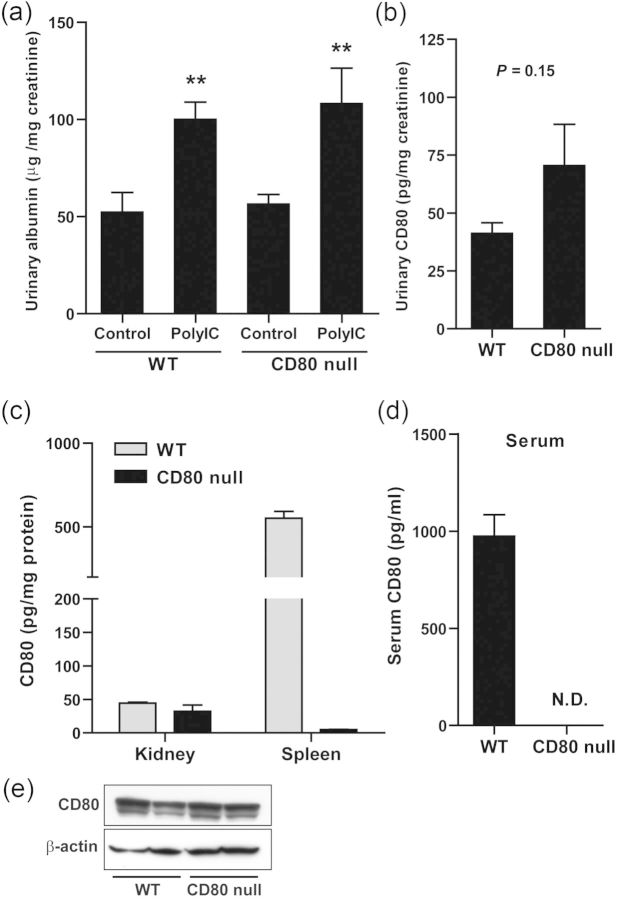
To investigate the involvement of CD80 in albuminuria induced by intravenous injection of polyIC-LMW, CD80 null mice were obtained from Jackson Laboratory. Intravenous injection of polyIC-LMW into CD80 null mice resulted in significant albuminuria at 24 h as the same as wild-type (WT) mice (Figure 6a). Urinary excretion of CD80 after the injection of polyIC-LMW was found in both WT mice and CD80 null mice (Figure 6b). To confirm the deletion of CD80 protein in CD80 null mice, ELISA and western blotting for murine CD80 were done. ELISA for CD80 revealed the absence of CD80 protein in serum and minimal CD80 protein in the spleen of CD80 null mice compared with WT mice (Figure 6c and d). However, ELISA for CD80 detected CD80 protein in the kidney of CD80 null mice as in the kidney of WT mice (Figure 6c). CD80 protein was also detected in the kidney of both WT mice and CD80 null mice by western blotting (Figure 6e). These results suggest that these CD80 null mice have a deletion of CD80 that affects the spleen and B-cells, but not the kidney.
FIGURE 6:
The examinations using CD80 null mice. (a and b) WT mice and CD80 null mice were intravenously injected with polyIC-LMW. (a) Twenty-four-hour urine was collected at 24 h (0–24 h). Control urine was collected 1 week prior to the injection of polyIC-LMW. Urinary albumin-to-creatinine ratio was measured. The results represent means ± SEM (n = 6). **P < 0.01 versus respective control. (b) Urinary concentration of CD80 protein was determined by ELISA, and was corrected by creatinine concentration. The results represent the means ± SEM (n = 5). (c) CD80 contents in the kidney and spleen of WT mice and CD80 null mice measured by ELISA. The results represent means ± SD (n = 3). (d) Serum CD80 concentration measured by ELISA. The results represent the means ± SD (n = 3). (e) Representative images of western blotting for CD80 using the kidney of WT mice and CD80 null mice.
DISCUSSION
In this study, we present a new model of proteinuria induced by the activation of the viral dsRNA receptor, TLR3. Specifically, the injection of a low molecular weight ligand for TLR3 (polyIC) resulted in transient proteinuria with focal foot process fusion and with CD80 expression in podocytes and urinary excretion of CD80, similar to that observed in MCD in humans. This model therefore provides a potential mechanism for the well-known phenomenon in which viral infections have been found to induce relapse in subjects with MCD.
Of note, we also administered polyIC to CD80 knockout mice obtained from Jackson Laboratories. Interestingly, these mice still developed proteinuria with urinary CD80 excretion. On further analysis, we were able to show that CD80 expression was absent or minimal in the spleen and circulation, but CD80 was still expressed in the kidney. Therefore, this particular CD80 knockout mouse is not truly a complete CD80 knockout, and hence could not be used to verify the importance of CD80 in mediating the proteinuria in this model.
One major difference between this model and MCD in humans is that MCD in humans is associated with nephrotic range proteinuria and frequently persists unless corticosteroid treatment is initiated. We recently proposed that this persistence may be due to the inability of the subject with MCD to down-regulate CD80 in podocytes. One such modulator is CTLA-4, a costimulatory receptor for CD80, which can be secreted by T reg cells as a soluble isoform and is known to down-regulate CD80 on antigen-presenting cells [10, 11]. We have previously shown that Treg are abnormal in MCD with impaired secretion of IL-10 and TGF-β [12]. In addition, urinary CTLA-4 levels of MCD patients in relapse are not different than those in MCD patients in remission [4]. We have also found evidence that podocytes can also express CTLA4. In contrast with MCD patients who have an impaired CTLA4 response, the rapid up-regulation of CTLA4 in glomeruli in our model might be able to account for the transient CD80 expression. IL-10 is also a candidate for driving this response, as it was also induced and is known to down-regulate CD80 expression in antigen-presenting cells. Thus, we would hypothesize that in order to induce a model with persistent nephrotic syndrome, one might have to knock down CTLA4 and/or IL-10 either in the podocyte or in the animal. In this regard, CTLA4 knockout mice die of an early age from anasarca, although it is not known if they develop proteinuria.
In conclusion, we present a new model of CD80-associated proteinuria accompanied by urinary excretion of CD80 that has some features suggestive of MCD. The observation that this pathway can be engaged by viral ligands provides a pathophysiologic mechanism linking viral infections with MCD.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
This work was in part funded by the start up funds from the University of Colorado for R.J.J. We thank Norihiko Suzuki for his excellent technical assistance for electron microscopy.
REFERENCES
- 1.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci USA. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbas AK, Sharpe AH. T-cell stimulation: an abundance of B7s. Nat Med. 1999;5:1345–1346. doi: 10.1038/70905. [DOI] [PubMed] [Google Scholar]
- 3.Reiser J, von Gersdorff G, Loos M, et al. Induction of B7–1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garin EH, Diaz LN, Mu W, et al. Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol. 2009;20:260–266. doi: 10.1681/ASN.2007080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garin EH, Mu W, Arthur JM, et al. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010;78:296–302. doi: 10.1038/ki.2010.143. [DOI] [PubMed] [Google Scholar]
- 6.Lai KW, Wei CL, Tan LK, et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol. 2007;18:1476–1485. doi: 10.1681/ASN.2006070710. [DOI] [PubMed] [Google Scholar]
- 7.Shimada M, Ishimoto T, Lee PY, et al. Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF-kappaB-dependent pathway. Nephrol Dial Transplant. 2012;27:81–89. doi: 10.1093/ndt/gfr271. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Bacon KB, Li L, et al. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J Exp Med. 1998;188:193–198. doi: 10.1084/jem.188.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clement LC, Avila-Casado C, Mace C, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–122. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers CA, Allison JP. Costimulatory regulation of T cell function. Curr Opin Cell Biol. 1999;11:203–210. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 11.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 12.Araya C, Diaz L, Wasserfall C, et al. T regulatory cell function in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol. 2009;24:1691–1698. doi: 10.1007/s00467-009-1214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]