Abstract
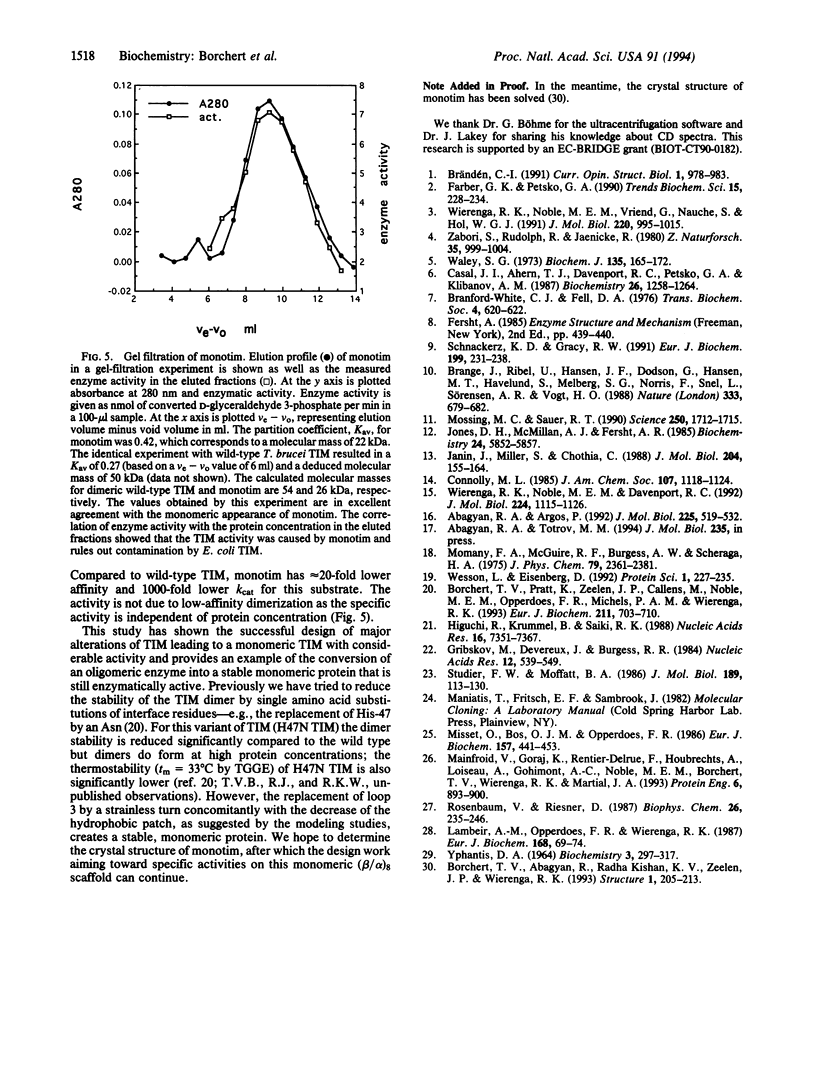
Protein engineering on trypanosomal triosephosphate isomerase (TIM) converted this oligomeric enzyme into a stable, monomeric protein that is enzymatically active. Wild-type TIM consists of two identical subunits that form a very tight dimer involving interactions of 32 residues of each subunit. By replacing 15 residues of the major interface loop by another 8-residue fragment, a variant was constructed that is a stable and monomeric protein with TIM activity. The length, sequence, and conformation of the designed fragment were suggested by extensive modeling.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abagyan R., Argos P. Optimal protocol and trajectory visualization for conformational searches of peptides and proteins. J Mol Biol. 1992 May 20;225(2):519–532. doi: 10.1016/0022-2836(92)90936-e. [DOI] [PubMed] [Google Scholar]
- Borchert T. V., Abagyan R., Kishan K. V., Zeelen J. P., Wierenga R. K. The crystal structure of an engineered monomeric triosephosphate isomerase, monoTIM: the correct modelling of an eight-residue loop. Structure. 1993 Nov 15;1(3):205–213. doi: 10.1016/0969-2126(93)90021-8. [DOI] [PubMed] [Google Scholar]
- Borchert T. V., Pratt K., Zeelen J. P., Callens M., Noble M. E., Opperdoes F. R., Michels P. A., Wierenga R. K. Overexpression of trypanosomal triosephosphate isomerase in Escherichia coli and characterisation of a dimer-interface mutant. Eur J Biochem. 1993 Feb 1;211(3):703–710. doi: 10.1111/j.1432-1033.1993.tb17599.x. [DOI] [PubMed] [Google Scholar]
- Branford White C. J., Fell D. A. Evidence that the monomers of dimeric triose phosphate isomerase are active. Biochem Soc Trans. 1976;4(4):620–622. doi: 10.1042/bst0040620. [DOI] [PubMed] [Google Scholar]
- Brange J., Ribel U., Hansen J. F., Dodson G., Hansen M. T., Havelund S., Melberg S. G., Norris F., Norris K., Snel L. Monomeric insulins obtained by protein engineering and their medical implications. Nature. 1988 Jun 16;333(6174):679–682. doi: 10.1038/333679a0. [DOI] [PubMed] [Google Scholar]
- Casal J. I., Ahern T. J., Davenport R. C., Petsko G. A., Klibanov A. M. Subunit interface of triosephosphate isomerase: site-directed mutagenesis and characterization of the altered enzyme. Biochemistry. 1987 Mar 10;26(5):1258–1264. doi: 10.1021/bi00379a009. [DOI] [PubMed] [Google Scholar]
- Farber G. K., Petsko G. A. The evolution of alpha/beta barrel enzymes. Trends Biochem Sci. 1990 Jun;15(6):228–234. doi: 10.1016/0968-0004(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Miller S., Chothia C. Surface, subunit interfaces and interior of oligomeric proteins. J Mol Biol. 1988 Nov 5;204(1):155–164. doi: 10.1016/0022-2836(88)90606-7. [DOI] [PubMed] [Google Scholar]
- Jones D. H., McMillan A. J., Fersht A. R., Winter G. Reversible dissociation of dimeric tyrosyl-tRNA synthetase by mutagenesis at the subunit interface. Biochemistry. 1985 Oct 8;24(21):5852–5857. doi: 10.1021/bi00342a024. [DOI] [PubMed] [Google Scholar]
- Lambeir A. M., Opperdoes F. R., Wierenga R. K. Kinetic properties of triose-phosphate isomerase from Trypanosoma brucei brucei. A comparison with the rabbit muscle and yeast enzymes. Eur J Biochem. 1987 Oct 1;168(1):69–74. doi: 10.1111/j.1432-1033.1987.tb13388.x. [DOI] [PubMed] [Google Scholar]
- Mainfroid V., Goraj K., Rentier-Delrue F., Houbrechts A., Loiseau A., Gohimont A. C., Noble M. E., Borchert T. V., Wierenga R. K., Martial J. A. Replacing the (beta alpha)-unit 8 of E.coli TIM with its chicken homologue leads to a stable and active hybrid enzyme. Protein Eng. 1993 Nov;6(8):893–900. doi: 10.1093/protein/6.8.893. [DOI] [PubMed] [Google Scholar]
- Misset O., Bos O. J., Opperdoes F. R. Glycolytic enzymes of Trypanosoma brucei. Simultaneous purification, intraglycosomal concentrations and physical properties. Eur J Biochem. 1986 Jun 2;157(2):441–453. doi: 10.1111/j.1432-1033.1986.tb09687.x. [DOI] [PubMed] [Google Scholar]
- Mossing M. C., Sauer R. T. Stable, monomeric variants of lambda Cro obtained by insertion of a designed beta-hairpin sequence. Science. 1990 Dec 21;250(4988):1712–1715. doi: 10.1126/science.2148648. [DOI] [PubMed] [Google Scholar]
- Rosenbaum V., Riesner D. Temperature-gradient gel electrophoresis. Thermodynamic analysis of nucleic acids and proteins in purified form and in cellular extracts. Biophys Chem. 1987 May 9;26(2-3):235–246. doi: 10.1016/0301-4622(87)80026-1. [DOI] [PubMed] [Google Scholar]
- Schnackerz K. D., Gracy R. W. Probing the catalytic sites of triosephosphate isomerase by 31P-NMR with reversibly and irreversibly binding substrate analogues. Eur J Biochem. 1991 Jul 1;199(1):231–238. doi: 10.1111/j.1432-1033.1991.tb16114.x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Waley S. G. Refolding of triose phosphate isomerase. Biochem J. 1973 Sep;135(1):165–172. doi: 10.1042/bj1350165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson L., Eisenberg D. Atomic solvation parameters applied to molecular dynamics of proteins in solution. Protein Sci. 1992 Feb;1(2):227–235. doi: 10.1002/pro.5560010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga R. K., Noble M. E., Davenport R. C. Comparison of the refined crystal structures of liganded and unliganded chicken, yeast and trypanosomal triosephosphate isomerase. J Mol Biol. 1992 Apr 20;224(4):1115–1126. doi: 10.1016/0022-2836(92)90473-w. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Noble M. E., Vriend G., Nauche S., Hol W. G. Refined 1.83 A structure of trypanosomal triosephosphate isomerase crystallized in the presence of 2.4 M-ammonium sulphate. A comparison with the structure of the trypanosomal triosephosphate isomerase-glycerol-3-phosphate complex. J Mol Biol. 1991 Aug 20;220(4):995–1015. doi: 10.1016/0022-2836(91)90368-g. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Zabori S., Rudolph R., Jaenicke R. Folding and association of triose phosphate isomerase from rabbit muscle. Z Naturforsch C. 1980 Nov-Dec;35(11-12):999–1004. doi: 10.1515/znc-1980-11-1224. [DOI] [PubMed] [Google Scholar]