Background: PDCD4 is regulated by multiple mechanisms and is involved in tumor promotion.
Results: PDCD4 mRNA is bound by the RNA-binding proteins HuR and TIA1, resulting in modulation of PDCD4 mRNA and protein levels.
Conclusion: This study describes a dynamic regulation of PDCD4 mRNA via the 3′UTR.
Significance: This work reveals an additional regulatory mechanism that modulates levels of the tumor suppressor PDCD4.
Keywords: Imaging, Post-transcriptional Regulation, RNA-binding Protein, RNA Processing, RNA-Protein Interaction, HuR, PDCD4, TIA1
Abstract
Post-transcriptional processing of mRNA transcripts plays a critical role in establishing the gene expression profile of a cell. Such processing events are mediated by a host of factors, including RNA-binding proteins and microRNAs. A number of critical cellular pathways are subject to regulation at multiple levels that allow fine-tuning of key biological responses. Programmed cell death 4 (PDCD4) is a tumor suppressor and an important modulator of mRNA translation that is regulated by a number of mechanisms, most notably as a target of the oncomiR, miR-21. Here, we provide evidence for post-transcriptional regulation of PDCD4 by the RNA-binding proteins, HuR and TIA1. Complementary approaches reveal binding of both HuR and TIA1 to the PDCD4 transcript. Consistent with a model where RNA-binding proteins modulate the PDCD4 transcript, knockdown of HuR and/or TIA1 results in a significant decrease in steady-state PDCD4 mRNA and protein levels. However, fractionation experiments suggest that the mode of regulation of the PDCD4 transcript likely differs in the cytoplasm and the nucleus as the pool of PDCD4 mRNA present in the cytoplasm is more stable than the nuclear pool of PDCD4 transcript. We observe a competitive mode of binding between HuR and TIA1 on the PDCD4 transcript in the cytoplasm, suggesting that these two factors dynamically interact with one another as well as the PDCD4 transcript to maintain tight control of PDCD4 levels. Overall, this study reveals an additional set of regulatory interactions that modulate the expression of PDCD4, a key pro-apoptotic factor, and also reveals new insights into how HuR and TIA1 functions are integrated to achieve such regulation.
Introduction
Post-transcriptional processing events are critical to ensure the proper gene expression profile of a cell. These events include the capping, splicing, 3′ end processing, export from the nucleus, translation, and eventual decay of an mRNA transcript in the cytoplasm (1). A myriad of RNA-binding proteins and noncoding RNAs, such as microRNAs (miRNAs),3 mediate these post-transcriptional events and therefore are key factors in modulating gene expression (2, 3). Highlighting the importance of these events, there are a growing number of examples in which the dysregulation of RNA-binding proteins or miRNAs and, in turn, the post-transcriptional processing events they mediate are associated with various types of cancer (4–6). The 3′-untranslated region (UTR) of an mRNA transcript is the site of binding and regulation for many post-transcriptional factors. The AU-rich element-binding proteins represent one class of RNA-binding proteins that mediate their post-transcriptional function by binding to AU- or U-rich RNA elements (AREs) located primarily within the 3′UTRs of target mRNAs. This protein family, which includes the mRNA stability factor human antigen R (HuR) and the translational repressor T-cell intracellular antigen 1 (TIA1), plays a role in the post-transcriptional regulation of a number of biologically important mRNAs (7–9).
HuR is the ubiquitously expressed member of the embryonic lethal abnormal vision (ELAV) family of RNA-binding proteins (10). Two N-terminal RNA recognition motifs (RRMs) within HuR mediate recognition of AREs located primarily within the 3′UTR of target mRNA transcripts (11, 12). A third RRM in the C terminus of HuR has affinity for polyadenosine RNA and is thought to bind to the poly(A) tail of target mRNAs (13, 14). The majority of HuR localizes to the nucleus at steady state but can shuttle out of the nucleus when the cell experiences stress such as oxidative stress (15) or transcriptional inhibition (16). For the majority of HuR target transcripts, association with HuR in the cytoplasm leads to increased stability of the HuR-bound target mRNA, ultimately resulting in an increase in the steady-state protein level (17). In addition to this conventional role of HuR in stabilizing mRNAs, recent work has revealed that HuR can positively regulate the translation of target transcripts without affecting their stability (18, 19) or even repress the expression of target transcripts via cooperative interactions with the miRNA machinery (20). Together, these studies reveal a complex role for HuR in the post-transcriptional processing of target mRNAs. Further adding to this complexity is the observation that HuR interacts with other AU- or U-rich element RNA-binding proteins, both cooperatively (21) and competitively (22), to fine-tune the expression of target transcripts.
TIA1 is an RRM-containing RNA-binding protein that modulates the splicing and translation of target mRNAs (23, 24). TIA1 contains three tandem RRMs at the N terminus of the protein followed by a glutamine-rich C-terminal domain (25). RRM2 of TIA1 has specificity for U-rich RNA and mediates high affinity binding to intronic and 3′UTR regions of target mRNAs (26, 27). TIA1 is present in both the nucleus and the cytoplasm and can shuttle between the two compartments (28, 29). Similar to HuR, TIA1 localization shifts toward the cytoplasm upon transcriptional inhibition (29). During oxidative stress, TIA1 binding to the 3′UTR of target transcripts promotes the formation and sorting of translationally incompetent preinitiation complexes into stress granules, resulting in translational repression (30).
Recent transcriptome-wide analyses of both HuR and TIA1 binding reveal a large number of candidate mRNA targets that could be bound and regulated by these RNA-binding proteins (12, 27, 31–33). These studies suggest HuR binding to ∼10% of the transcriptome, specifically within the 3′UTRs and introns of these candidate target transcripts. Further analyses of these sequencing studies reveal a robust preference of HuR for U-rich sequences, which challenges the previously held belief that HuR is an exclusively AU-rich RNA-binding protein (12, 31–33). Secondary structural predictions of the candidate HuR-binding sites suggest a preference for single-stranded RNA for binding to HuR, specifically within the context of a loop (12, 34). A recent TIA1 iCLIP study reveals a high degree of TIA1 binding to U-rich regions found within the 3′UTRs of target transcripts (27). The observation that HuR and TIA1 both bind preferentially to U-rich 3′UTR regions suggests that these two proteins may coordinately regulate a number of target mRNAs.
These transcriptome-wide studies validate many well characterized HuR targets, including the cell cycle regulators cyclin A, B1, D1, and E1 (35–37), p53 (19, 38), and estrogen receptor α (ERα) (38) transcripts. Recently, a number of apoptotic factors whose transcripts are bound by HuR have been identified, including prothymosin α (18), cytochrome c (21), Bcl-2, and Mcl-1 (39). Interestingly, TIA1 also regulates a number of apoptotic mRNAs, including the tumor necrosis factor α mRNA (TNF-α) (24, 40). These studies suggest a potential role for HuR and TIA1 in coordinating the apoptotic program (39).
Among the biologically important targets of HuR and TIA1 are several putative cancer-relevant targets. One such transcript identified in transcriptome-wide analyses is the novel tumor suppressor, programmed cell death 4 (PDCD4). The PDCD4 protein binds to and inhibits the activity of the eukaryotic translation initiation factor 4A (eIF4A) (41), which is an RNA helicase that is responsible for unwinding the secondary structure in the 5′UTRs of translating mRNAs (42). Reduction of PDCD4 protein levels, which is observed in a number of cancer types (43), results in an increase in protein production and increased tumor promotion (41, 44). The finding that PDCD4 is a key regulator of global translational levels suggests that PDCD4 is likely regulated by multiple factors. The oncomiR, miR-21, which is overexpressed in almost all cancer types examined (45), is a well defined regulator of PDCD4 that functions by binding to the 3′UTR of the PDCD4 transcript and reducing PDCD4 protein levels (46, 47). Furthermore, PDCD4 expression is regulated at the transcriptional (48, 49) and post-translational levels (43, 50, 51). The recent transcriptome-wide sequencing studies on HuR and TIA1 described above suggest that the PDCD4 transcript may be a target of these proteins; therefore, an additional mode of regulation could modulate PDCD4 protein levels.
In this study, we extensively characterized the PDCD4 transcript as a novel target of HuR and TIA1 in a breast cancer cell line, MCF-7 (52). In addition to RNA-immunoprecipitation (RNA-IP) with HuR and TIA1, we employed RNA-imaging probes deliverable to live cells (53) and proximity ligation assay (PLA) (54, 55) to examine the interplay between HuR and TIA1 on the PDCD4 transcripts with single-interaction sensitivity on a per cell basis. Contrary to previous studies that describe a cooperative relationship between HuR and TIA1 in binding to RNA (21), we observe a competitive interaction between HuR and TIA1 on the PDCD4 transcript in the cytoplasm. Analysis of the nuclear and cytoplasmic pools of PDCD4 mRNA reveals a significantly more stable population of PDCD4 mRNA in the cytoplasm compared with the nucleus. Knockdown of HuR and/or TIA1 results in a steady-state decrease of PDCD4 mRNA and protein levels, supporting a role for these factors in positively regulating PDCD4 mRNA levels. Together, these data present a novel and dynamic mode of regulation of PDCD4 mRNA by multiple factors that contribute to fine-tuning the level of PDCD4 protein in breast cancer cells.
EXPERIMENTAL PROCEDURES
Cell Culture
MCF-7 cells (ATCC HTB-22; ER positive (ER+) breast cancer cell line (52)) were obtained from ATCC and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and antibiotics. DNA plasmids and siRNA (Invitrogen) were transfected into cultured cells using Lipofectamine2000 (Invitrogen) or Neon Electroporation System (Invitrogen) according to manufacturer's protocol. Cells were plated on number 1.5 glass coverslips (Ted Pella) 1 day prior to transfection for imaging.
Plasmids and Chemicals
A FLAG fusion construct for HuR was generated using PCR primers that include the FLAG sequence, creating an N-terminal FLAG-tagged protein. The PCR product was then subcloned into the pcDNA3.1 vector (Invitrogen). The HuR RNA-binding mutant (HuR(BM); N21A, Y109A, R147A) was generated by site-directed mutagenesis using the QuikChange kit (Stratagene). Primers used throughout the study are shown in Table 1. MCF-7 cells were transfected with 0.8 μg of HuR-GFP or 0.8 μg of TIA1-GFP plasmid (gifts from Dr. Myriam Gorospe, NIA, National Institutes of Health). mRNA-targeted probes were delivered 36 h after plasmid transfection. A set of three pre-designed Stealth siRNAs (assay ID numbers s4608, s4609, and s4610; Invitrogen) or 200 nm On-TARGET SMARTpool HuR siRNA (Thermo Scientific Dharmacon) was employed for knockdown of HuR. A set of three pre-designed Stealth siRNAs (assay ID numbers s14131, s14132, and s14133; Invitrogen) was employed for knockdown of TIA1. For control, 200 nm On-TARGETplus nontargeting siRNA number 1 (Thermo Scientific Dharmacon) was used. mRNA-targeted probes were delivered 48 h after siRNA transfection.
TABLE 1.
Primer sequences used in this study
| Gene name | Forward | Reverse |
|---|---|---|
| 18 S rRNA | GAGACTCTGGCATGCTAACTAG | GGACATCTAAGGGCATCACAG |
| RPLP0 | GGGCGACCTGGAAGTCCAACT | CCCATCAGCACCACAGCCTTC |
| GAPDH | AAGGTCGGAGTCAACGGATTTGG | GATGACAAGCTTCCCGTTCTC |
| ΑCTB (β-Actin) | GGACTTCGAGCAAGAGATGG | AGCACTGTGTTGGCGTACAG |
| c-Myc | TTCGGGTAGTGGAAAACCAGCAGC | CCGAGTCGTAGTCGAGGTCATAGTTCC |
| PDCD4 | GACTACCAAAGAAAGGTGGTGCAGGAGG | CATAAACACAGTTCTCCTGGTCATCATCATAGTTAGGAT |
| ERα | TGAACCGTCCGCAGCTCAAGATC | GTCTGACCGTAGACCTGCGCGTTG |
| PTMA | CACCCAACCCAAACCATGAGAATTTGC | CATGGTCACACCACAAGTAAAGTCAGAAGTAG |
| CYCS | GTTCAACTTTTCACAAAGATGGTGAGTGCC | CCTTTAAGAGGCAAATGAACATGAACACAAAACAC |
| HuR N21A | GACTGCAGGGGTGACATCGGGAGAACGGCTTTGATCGTCAACTACCTCCCTCAGAACATG | CATGTTCTGAGGGAGGTAGTTGACGATCAAAGCCGTTCTCCCGATGTCACCCCTGCAGTC |
| HuR Y109A | GTGATCAAAGACGCCAACTTGGCCATCAGCGGGCTCCCGCGG | CCGCGGGAGCCCGCTGATGGCCAAGTTGGCGTCTTTGATCAC |
| R147A | GTCCTCGTGGATCAGACTACAGGTTTGTCCGCAGGGGTTGCGTTTATCCGGTTTGACAAA | TTTGTCAAACCGGATAAACGCAACCCCTGCGGACAAACCTGTAGTCTGATCCACGAGGAC |
| 3′RACE camp | GACGAGCTCGGATCCTGCAGTTTTTTTTTTTTTTTTTTVN | |
| Outer 3′RACE primer | GACGAGCTCGGATCCTGCAGTTTTTT | |
| GAPDH 3′RACE primer | CAACAGGGTGGTGGACCTCATG | |
| PDCD4 3′RACE primer | GAGGTCGTCTTAAACCAGAGAGCTACTGA |
Immunoblotting
MCF-7 cells were harvested and washed in 1× PBS and then lysed on ice in RIPA-2 buffer (150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mm Tris, pH 8.0) containing protease inhibitors (PLAC: 3 μg/ml of pepstatin, leupeptin, aprotinin, chymostatin, and 0.5 mm PMSF). Immunoblotting was performed using standard methods (56). Briefly, 30 μg of total protein lysate per sample was resolved by SDS-PAGE and transferred onto a nitrocellulose membrane. For immunoblotting, a 1:1,000 dilution of HuR or TIA1 antibody (Santa Cruz Biotechnology; clones 3A2 and c-20, respectively), a 1:2,000 dilution of HSP90 (Santa Cruz Biotechnology; clone F-8) or poly(ADP-ribose) polymerase antibody (Pharmingen; clone 4C10-5), a 1:4,000 dilution of PDCD4 antibody (Rockland; rabbit polyclonal), or a 1:5,000 dilution of α-tubulin antibody (Sigma; clone DM1A) was used followed by 1:3,000 dilutions of HRP-conjugated goat anti-mouse IgG, HRP-conjugated goat anti-rabbit IgG, or HRP-conjugated mouse anti-goat IgG secondary antibodies (Jackson ImmunoResearch).
RNA Isolation
Total RNA was isolated from MCF-7 cells using TRIzol reagent (Invitrogen) in accordance with the manufacturer's instructions. Reverse transcriptase reactions with Moloney murine leukemia virus RT (Invitrogen) used 1 μg of RNA for a final concentration of 50 ng/μl cDNA per sample that was used for quantitative RT-PCR.
Isolation and enrichment of miRNAs from MCF-7 cells was performed using an miRNeasy mini kit (Qiagen). For reverse transcription, 10 ng of isolated RNA was used with the TaqMan miRNA RT kit (Invitrogen; miR-21 and RNU48 assays) according to the manufacturer's instructions.
Quantitative RT-PCR
For qRT-PCR analyses, 1 μg of total RNA was transcribed to cDNA as described above. Relative mRNA levels were measured by quantitative PCR analysis of triplicate samples of 5 ng of cDNA with QuantiTect SYBR Green Master Mix using an Applied Biosystems real time machine (ABI). Results were analyzed using the ΔΔCT method (57) and normalized to the 18 S rRNA or RPLP0 transcript. Statistical significance was determined using one-way ANOVA. A list of primers used for these analyses is shown in Table 1.
miR-21 and RNU48 levels were detected using TaqMan primers for each transcript and the TaqMan Universal PCR Master Mix, no UNG (Invitrogen). Results were analyzed using the ΔΔCT method (57) and normalized to the RNU48 transcript. Statistical significance was determined using one-way ANOVA.
RNA Immunoprecipitation
RNA-IPs to assay endogenous HuR/PDCD4 mRNA interactions were performed using standard methods (58). Briefly, MCF-7 cells were grown to confluency in 100-mm dishes and rinsed twice with ice-cold PBS. Lysates were prepared with an equal pellet volume of polysome lysis buffer (PLB: 10 mm HEPES, pH 7.0, 100 mm KCl, 5 mm MgCl2, 0.5% Nonidet P-40, 1 mm DTT, RNase OUT (Invitrogen), and 1 cOmplete protease inhibitor tablet (Roche Applied Science)) and stored at −80 °C. Protein A-Sepharose beads (Santa Cruz Biotechnology) were incubated at 4 °C overnight with either mouse IgG or HuR antibody (Santa Cruz Biotechnology). Beads coated in antibody were resuspended in NT2 buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm MgCl2, 0.05% Nonidet P-40) supplemented with RNase OUT (Invitrogen) and 1 mm DTT. Thawed and clarified cell lysates were added, and the bead/antibody/cell lysate mixture was incubated at 4 °C for 2 h while tumbling end-over-end. After incubation, beads were spun down and washed five times with cold NT2 buffer. The bound RNA was isolated with TRIzol (Invitrogen) and purified according to the manufacturer's instructions.
To detect FLAG-HuR/PDCD4 mRNA interactions, MCF-7 cells were grown to near confluency in 100-mm plates and transfected with pcDNA3, FLAG-HuR, or -HuR(BM) plasmids. After 48 h, cell lysates were prepared and frozen as described above. FLAG-M2 magnetic beads (Sigma) were resuspended in supplemented NT2 buffer described above. Thawed and clarified cell lysates were added to beads and incubated at 4 °C for 2 h while tumbling end-over-end. After incubation, beads were magnetized and washed five times with cold NT2 buffer. FLAG-HuR·RNA complexes were eluted with excess FLAG peptide (Sigma), and bound RNA was isolated with TRIzol (Invitrogen) and purified according to the manufacturer's instructions.
Immunofluorescence
Prior to visualization by indirect immunofluorescence, MCF-7 cells were fixed with 1–2% formaldehyde (EM Science) for 10 min, permeabilized with 0.1% Triton X-100 for 5 min, and incubated with Hoechst or DAPI (Invitrogen) to mark the position of the nucleus. To localize endogenous HuR, FLAG-HuR, or HuR-GFP by immunofluorescence, cells were probed with mouse monoclonal anti-HuR (1:1,000; 3A2, Santa Cruz Biotechnology), mouse monoclonal anti-FLAG (1:1,000; M2, Sigma), or goat polyclonal anti-GFP (1:500; ab5450, Abcam) antibody followed by staining with Texas Red or fluorescein-conjugated secondary antibodies (Jackson ImmunoResearch). For immunostaining endogenous TIA1 or TIA1-GFP, cells were probed with polyclonal rabbit anti-TIA1 (1:500; AV40981, Sigma) or anti-GFP (1:500; ab5450, Abcam) antibody followed by staining with Cy3 or fluorescein-conjugated secondary antibody (Jackson ImmunoResearch). Images were obtained using an Olympus IX81 microscope with a 0.3 numerical aperture (NA) 100 × Zeiss Plan-Neofluar objective or an Axiovert 200 M microscope (Zeiss) with a 1.4 NA 63 × Plan-Apochromat objective unless otherwise stated. Images were captured using a Hamamatsu digital camera with SlideBook software (version 1.63) or Volocity acquisition software (PerkinElmer Life Sciences) and globally processed for brightness and contrast using Adobe Photoshop.
3′-Rapid Amplification of cDNA Ends (RACE)
To determine the precise length of the PDCD4 3′UTR in human cell lines, we isolated RNA from HeLa (ATCC CCL-2), MDA-MB-231 (ATCC HTB-26), and MCF-7 (ATCC HTB-22) cells using TRIzol reagent (Invitrogen) in accordance with the manufacturer's instructions. Reverse transcriptase reactions were carried out as follows: 1 μg of RNA was combined with 1 μl of dNTPs (10 mm), 1 μl of the 3′RACE adaptor (See Table 1; 10 mm) and DEPC-treated water for a total volume of 13 μl. Samples were incubated at 65 °C for 5 min and then transferred to ice. To the RNA mixture-3′RACE adaptor mixture, 4 μl of 5× First-Strand buffer (Invitrogen), 1 μl of 0.1 m DTT, and 1 μl of RNaseOUT (40 units/μl; Invitrogen) were added. Samples were incubated at 42 °C for 2 min before adding 1 μl of SuperScript III RT (200 units/μl; Invitrogen), briefly vortexing, and returning to 42 °C for 50 min. Following first strand synthesis, samples were heated to 70 °C for 15 min to denature the RT enzyme. For each PCR, 40 ng of cDNA was used along with the outer 3′RACE primer and a gene-specific inner primer (see Table 1) with the AmpliTaq Gold 360 master mix (Invitrogen), according to manufacturer's instructions. PCR products were resolved on a 2% agarose gel and confirmed by TOPO cloning (Invitrogen) and sequencing.
Biotin Pulldown
To assess direct binding of HuR to the PDCD4 transcript, DNA sequences corresponding to the 3′UTRs of c-Myc and GAPDH as well as various regions of the PDCD4 3′UTR (full length, 5′290, Δ290, 1–100, 101–200, and 201–290) were amplified by PCR and inserted into pGEM T-easy vectors (Promega) and linearized by digestion with SpeI (New England Biolabs). Biotinylated RNA probes were generated by in vitro transcription using the T7 Maxiscript kit (Ambion) with biotin-11-cytidine-5′-triphosphate (biotin-11-CTP; Roche Applied Science). Biotinylated CTP and normal CTP were used at a ratio of 1:4 to ensure adequate transcription yield and sufficient labeling. Nonincorporated nucleotides were removed with a G-25 column (GE Healthcare) followed by ethanol precipitation. RNA concentration was determined by A260 absorption, and the quality was examined by denatured RNA electrophoresis. MCF-7 cells were lysed in IP buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm MgCl2, 0.5 mm DTT, 1% Nonidet P-40 supplemented with cOmplete protease inhibitor tablets (Roche Applied Science) and RNase OUT (Invitrogen)) and spun at 13,000 rpm for 20 min at 4 °C, and ∼200 μg of purified cell lysate were rotated end-over-end with 400 ng of biotinylated RNA probe for 20 min at room temperature. To precipitate biotinylated RNA probes from MCF-7 cell lysates, NeutrAvidin beads (Thermo Scientific) pre-blocked with BSA (Roche Applied Science) were added and rotated end-over-end for 30 min at 4 °C. After precipitation, the beads were washed five times in IP buffer and then subjected to elution by boiling in reducing sample buffer. Bound proteins were analyzed by immunoblotting to detect HuR.
Nucleocytoplasmic Fractionation
To isolate distinct pools of RNA and protein from nuclear and cytoplasmic fractions, cells were collected on ice, spun down, and resuspended in ice-cold fractionation buffer (10 mm Tris-HCl, pH 7.4, 10 mm NaCl, 3 mm MgCl2, 0.5% (v/v) Nonidet P-40) supplemented with 1 mini cOmplete protease inhibitor tablet (Roche Applied Science; 1 tablet/10 ml of buffer) for 10 min on ice. Cell lysates were spun down, and the supernatant or cytoplasmic fraction was separated from the nuclear pellet. RNA was isolated from each fraction with TRIzol reagent (Invitrogen), and protein samples were prepared with RIPA-2 buffer (as described above).
mRNA Stability
To measure mRNA stability in MCF-7 cells, 5 μg/ml actinomycin D (Sigma) was added to the growth medium to inhibit transcription, and cells were harvested 30 min and 2, 4, 6, and 8 h later (fractionated cells were collected at 30 min and 3, 6, and 8 h after ActD addition). Nuclear and cytoplasmic RNA was isolated from cells (described above) and analyzed by qRT-PCR. Half-lives were determined by normalization to 18 S rRNA and to the 30-min time point.
Probe Synthesis
Probes were synthesized as described previously (53, 54). Briefly, FLAG-hyNic (Solulink) was added to 4FB-modified (Solulink) NeutrAvidin (Thermo Scientific Pierce) according to the manufacturer's protocol. For each probe, 2′-O-methyl RNA-DNA oligonucleotide chimeras complementary to the target sequence were designed with a 5′-biotin and dT-C6-NH2 internal modifications (Biosearch Technologies). Cy3B NHS ester fluorophores (GE Healthcare) or DyLight 650 NHS Ester fluorophores (Thermo Scientific Pierce) were conjugated to the oligonucleotide amino groups using the manufacturer's protocol. Free dye was removed using 30-kDa Amicon spin columns (EMD Millipore). The purified and labeled oligonucleotides were tetramerized by incubation for 1 h at room temperature with untagged or FLAG-tagged NeutrAvidin. Free ligands were removed using 30-kDa Amicon spin columns (EMD Millipore). Three probes each targeting different sequences in the 3′UTR of PDCD4 mRNA (Table 2) were assembled separately prior to delivery and combined during delivery. To deliver total 60 nm probes targeting PDCD4 mRNA, 20 nm of each probe was combined.
TABLE 2.
Probes used to detect PDCD4 mRNA
Boldface indicates 2′-O-methyl RNA; X indicates dT-C6-NH2; all others are DNA; underlines indicate the binding region.
| Target | Ligand | Location within transcript (from start of coding region) |
|---|---|---|
| PDCD4 3′UTR 1 | 5′-Biotin-TXTTTTAAGGAGXGGCAGXCAGACAXCAAU-3′ | 1,706–1,729 |
| PDCD4 3′UTR 2 | 5′-Biotin-TXTTTTAAXAAAGCUUUGUAXAAACCAGXGA-3′ | 1,761–1,785 |
| PDCD4 3′UTR 3 | 5′-Biotin-TXTTTTGUXCCAGCCACCXUUUACUXAACUG-3′ | 1,959–1,983 |
| PDCD4 3′UTR 4 | 5′-Biotin-TXTTTTACXCCCAAXAAAGAAUCAAUACXG-3′ | 2,057–2,080 |
Probe Delivery
For probe delivery, cells were washed in Dulbecco's phosphate-buffered saline without Ca2+ and Mg2+ (Lonza) and subsequently incubated with 0.15 units/ml activated streptolysin O (Sigma) in Opti-MEM (Invitrogen) containing 60 nm PDCD4 mRNA probes for 10 min at 37 °C. Delivery media were replaced with growth media for 15 min to restore membrane integrity before actinomycin D treatment or fixation. For actinomycin D treatment, cells were incubated for 90 min at 37 °C with 5 μg/ml actinomycin D (Sigma) in growth media and fixed at the end of the exposure.
Proximity Ligation Assay
The PLA was performed as described previously (54). Cells were fixed with 1% paraformaldehyde (Electron Microscopy Science) in PBS for 10 min, permeabilized with 0.2% Triton X-100 (Sigma) for 5 min, and blocked for 1 h with a blocking solution. The blocking solution consisted of 0.5% Tween 20 (Calbiochem), 0.1% Triton X-100, 0.1% gelatin (Aurion), 2% donkey serum (Sigma), and 1% bovine serum albumin (BSA) (EMD) in PBS. Cells were washed with PBS and incubated in two primary antibodies, one against the FLAG-tagged NeutrAvidin and one against the protein of interest. For anti-FLAG antibodies, either rabbit polyclonal (1:2,000; F7425, Sigma) or mouse monoclonal (1:2,000; M2, Sigma) anti-FLAG antibody was used. Mouse monoclonal anti-HuR (1:750; 3A2, Santa Cruz Biotechnology) was used to detect HuR, and rabbit polyclonal anti-TIA1 (1:1,500; AV40981, Sigma) was used to detect TIA1. After washing with Duolink wash solution (Olink Bioscience), the cells were incubated with species-corresponding oligonucleotide-labeled PLA probes (Olink Bioscience) diluted in 0.05% Tween 20 in PBS and washed with Duolink wash solution. The ligation and rolling circle amplification reaction (Olink Bioscience) were performed as instructed in the manufacturer's protocol. The cells were then immunostained and DAPI-stained (Invitrogen) and mounted on slides using Prolong (Invitrogen).
PLA Fluorescence Imaging
All the images were collected using an Axiovert 200 M microscope (Zeiss) with a ×63 NA 1.4 Plan-Apochromat objective and an ORCA-ER AG camera (Hamamatsu). The images were acquired using the Volocity acquisition software (PerkinElmer Life Sciences). Image stacks were recorded at 200-nm intervals to sample volumes for iterative deconvolution using Volocity's deconvolution algorithms. Probe and PLA signal quantification and Mander's coefficients were computed in Volocity and imported into Excel (Microsoft) and SigmaPlot (Systat) for further analysis and plotting. Images presented have been linearly contrast-enhanced for clarity. All calculations were performed directly on raw and deconvolved widefield data.
Image Quantification
The RNA volume and PLA frequency normalized to the RNA volume were measured using Volocity (PerkinElmer Life Sciences). Each cell was identified by the probe signal or immunofluorescence and analyzed individually. The Mander's coefficient calculation for probe colocalization was performed using Volocity. We analyzed three representative images. In SigmaPlot, an all pairwise multiple comparison procedure was performed with Tukey method to compare the Mander's coefficients for each probe. The RNA volume was determined based on standard deviation intensity of the probe. The PLA signal initially was identified as objects by their standard deviation intensity and then separated into individual punctae using the “separate touching objects” tool. The punctae were further filtered based on size and maximum intensity. For each experiment, we analyzed at least 10 images of each coverslip and three repeated experiments. In SigmaPlot, when comparing more than two groups, an all pairwise multiple comparison procedure was performed with Dunn's method to compare the RNA volume and PLA frequency. When comparing two groups, Mann-Whitney rank sum test was used.
Polysome Profiling
To analyze the translation of PDCD4 and GAPDH (control) mRNAs across various treatment groups, linear sucrose gradient fractionation of MCF-7 cell cytoplasmic extracts was performed as described previously (59, 60). Briefly, cells were incubated for 15 min with 100 μg/ml cycloheximide (VWR) to arrest translation prior to isolation. MCF-7 cells were lysed in polysome-preserving buffer (20 mm Tris-HCl, pH 7.5, 100 mm KCl, 5 mm MgCl2, 0.15% Triton X-100 (Sigma), 100 μg/ml cycloheximide, 200 units of RNase inhibitor (Promega), and protease inhibitors (Roche Applied Science)) or polysome-disrupting buffer (identical to polysome-preserving buffer except it lacks MgCl2 and contains 20 mm EDTA) and then centrifuged at 13,000 rpm for 30 min at 4 °C to remove cellular debris. The clarified lysates (0.8 ml) were loaded onto 15–45% (w/v) linear sucrose gradients containing 100 mm KCl, 20 mm Tris-HCl, pH 7.5, and 5 mm MgCl2 (polysome-disrupted lysates were loaded onto the same gradient lacking MgCl2 and containing 1 mm EDTA) and subjected to centrifugation in a Beckman SW41 rotor at 33,000 rpm for 90 min at 4 °C.
Each gradient was fractionated into 11 1-ml fractions by bottom displacement using a gradient fractionator (Isco) with the ribosomal profile monitored at A254. Total RNA from each fraction was isolated using standard phenol/chloroform extraction. Resulting RNA pellets were resuspended in 15 μl of RNase-free water and used for cDNA generation and subsequent qRT-PCR analysis using primers specific to PDCD4 and GAPDH transcripts. The % mRNA present in each fraction was calculated using the 2−Ct method.
RESULTS
PDCD4 mRNA Is Bound and Regulated by HuR in MCF-7 Breast Cancer Cells
Recent transcriptome-wide HuR PAR-CLIP analyses in HeLa and HEK293 cells reveal novel functions and potential targets of the RNA-binding protein, HuR (12, 31–33), including a number of candidate targets with relevance to cancer. One such candidate transcript is PDCD4, which encodes a novel tumor suppressor that inhibits neoplastic transformation by interfering with the helicase activity of the translation initiation factor, eIF4A (41). The PDCD4 transcript contains multiple predicted AREs (61) within the 3′UTR that overlap with CLIP-Seq tags from independent HuR CLIP studies, suggesting that it is a candidate target of HuR.
To assess whether HuR binds to PDCD4 mRNA in MCF-7 breast cancer cells, we performed RNA immunoprecipitation (RNA-IP) with endogenous HuR protein. As expected (Fig. 1A), HuR protein was detected in the bound fraction when purified with HuR antibody but not with IgG control antibody. The input and bound fractions were then analyzed for the presence of bound PDCD4 transcript using qRT-PCR. As shown in Fig. 1B, the PDCD4 transcript was robustly enriched with HuR pulldown. For controls, we also examined a known HuR target, ERα (38), as well as GAPDH, which is not bound by HuR (34). As expected, the GAPDH transcript showed no enrichment with HuR; however, the previously defined HuR target, ERα (38), showed enrichment. These results provide evidence that PDCD4 mRNA is bound by HuR in MCF-7 cells.
FIGURE 1.

HuR binds to the PDCD4 transcript and modulates steady-state PDCD4 mRNA and protein levels in MCF-7 cells. Endogenous HuR protein was immunoprecipitated from MCF-7 cells using either HuR antibody-coated protein A beads or isotype control mouse IgG-coated beads. A, proteins from the input, unbound (UB), and bound fractions were resolved on an SDS-polyacrylamide gel and subjected to immunoblotting with HuR antibody. HuR was detected in the HuR-bound fraction but not the IgG-bound fraction. B, RNA isolated from the HuR RNA-IP was subjected to qRT-PCR analyses with GAPDH, ERα, and PDCD4 primers. mRNA levels in HuR-bound fractions were normalized to input levels and then compared by fold-enrichment over IgG control. Significant enrichment of ERα and PDCD4 transcripts was observed with HuR IP. C, MCF-7 cells transiently transfected with Scramble or HuR siRNA (siScr. and siHuR, respectively) were subjected to immunoblot analysis with HSP90, HuR, or PDCD4 antibody. Steady-state PDCD4 protein levels were normalized to HSP90 loading control and siScramble set to 1.00. A decrease in PDCD4 steady-state protein levels upon HuR knockdown is quantified below the representative blot. D, qRT-PCR analysis of total RNA isolated from siRNA-treated cells with PDCD4 and RPLP0 primers demonstrates a significant decrease in PDCD4 steady-state mRNA levels upon HuR knockdown. Values represent the mean ± S.E. for n = 3 independent experiments. **, p ≤ 0.01.
The canonical role for HuR in the post-transcriptional regulation of target mRNA transcripts is as a positive regulator of mRNA stability (17); however, recent studies also reveal a role for HuR in modulating mRNA translation (18, 19, 21) as well as negatively regulating the expression of target transcripts via cooperative interactions with the miRNA machinery (20). To determine whether transient modulation of HuR levels has an impact on PDCD4 steady-state mRNA or protein levels, we performed siRNA-mediated knockdown of HuR in MCF-7 cells. As shown in Fig. 1C, immunoblotting revealed a robust knockdown of HuR with HuR siRNA but not with scramble control siRNA. Probing these same samples with PDCD4 antibody reveals a decrease in PDCD4 protein upon HuR knockdown but no change in a control protein, heat shock protein 90 (HSP90). Quantification of PDCD4 steady-state protein levels upon HuR knockdown confirmed a significant decrease in PDCD4 protein levels (Fig. 1C, quantification below representative blot).
To assess whether a change in mRNA transcript levels could underlie the observed decrease in PDCD4 protein levels, we examined RNA isolated from cells transfected with HuR siRNA. qRT-PCR analyses of these samples revealed a significant decrease in PDCD4 steady-state mRNA levels upon HuR knockdown (Fig. 1D). Together, these results demonstrate that HuR binds to the PDCD4 transcript in breast cancer cells and plays a role in regulating steady-state PDCD4 mRNA and protein levels.
HuR RRMs 1 and 2 Are Required for PDCD4 Binding
The HuR protein contains three RRMs (Fig. 2A). The two N-terminal RRMs are critical for ARE recognition, and the third RRM is thought to recognize polyadenosine sequences (13, 14). The crystal structure of RRMs 1 and 2 (residues 18–186) of HuR in complex with an AUUUUUUAUUUU RNA oligomer (Protein Data Bank code 4ED5) (11) is shown in Fig. 2A. Although RRM1 is the primary ARE recognition domain within HuR, the conformational change that takes place upon RNA binding leads to additional interactions between RRM2 and the target mRNA, ultimately increasing RNA binding affinity (11). This structure, along with other studies investigating the structural basis of HuR/RNA interactions (62), reveals a number of key residues that are predicted to be important for high affinity RNA binding by HuR (11, 62).
FIGURE 2.
Key residues in HuR RRMs 1 and 2 are required for binding to ARE-containing target mRNAs, including PDCD4. A, HuR protein contains three RRMs (RRM1, RRM2, and RRM3) and an HuR nucleocytoplasmic shuttling sequence (HNS), which mediates bidirectional transport of HuR between the nucleus and cytoplasm (16). The two N-terminal RRMs (green) are responsible for ARE recognition, and the C-terminal RRM (blue) is thought to bind to the poly(A) tail of mRNA transcripts. Shown below the linearized map of HuR is a recently solved co-crystal structure (Protein Data Bank code 4ED5) of HuR RRMs 1 and 2 (green) in complex with an AUUUUUUAUUUU RNA oligomer (yellow). Residues shown to be important for RNA recognition in in vitro binding studies are highlighted in red (N21, Y109, and R147). A putative HuR RNA-binding mutant (BM) was generated by changing these residues to alanine in a FLAG-tagged HuR expression construct. B, MCF-7 cells were transfected with vector control, FLAG-HuR(WT), or -HuR(BM) plasmids and subjected to immunoblotting with HuR and tubulin antibodies. FLAG-HuR proteins were detected only in FLAG-transfected cells and levels of BM are comparable with WT. C, transfected MCF-7 cells were analyzed by indirect immunofluorescence with FLAG antibody to detect FLAG-HuR(WT) or (BM). Colocalization with DAPI reveals steady-state nuclear localization of both FLAG-HuR proteins (WT and BM). Scale bar, 10 μm. D, cells expressing vector control or FLAG-HuR proteins were subjected to RNA-IP using FLAG antibody-conjugated beads. Immunoblot analysis of IP samples demonstrates specific enrichment of FLAG-HuR proteins in bound fractions. E, RNA that coprecipitated with FLAG-HuR proteins was subjected to qRT-PCR analyses with RPLP0, ERα, and PDCD4 primers to detect bound transcripts. ERα and PDCD4 transcripts were significantly enriched upon wild type HuR purification; however, a significant decrease in enrichment was observed upon purification of the HuR-binding mutant. mRNA levels in FLAG-HuR-bound fractions were normalized to input levels and then compared by fold-enrichment over vector control samples. Values represent the mean ± S.E. for n = 3. *, **, *** and **** represents p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, and p ≤ 0.0001, respectively.
To define specific residues within RRM1 and -2 that are critical for HuR binding to target RNA, we focused on three of the residues (asparagine 21 (Asn-21), tyrosine 109 (Tyr-109), and arginine 147 (Arg-147)) shown in vitro to be critical for RNA binding (62). We changed each of these residues to alanine in an N-terminal FLAG-tagged HuR expression vector to create a FLAG-HuR-binding mutant, which we termed FLAG-HuR(BM). To ensure that FLAG-HuR(BM) is expressed at comparable levels to the wild type FLAG-HuR, we transfected MCF-7 cells with the FLAG-HuR and -HuR(BM) constructs and analyzed expression by immunoblotting for HuR (Fig. 2B). HuR antibody detects both endogenous HuR (Fig. 2B, lower band) and FLAG-tagged HuR protein (upper band). The steady-state level of FLAG-HuR protein is comparable for HuR and HuR(BM), demonstrating that the amino acid changes within the RRM do not significantly impact the steady-state level of HuR protein. Importantly, the localization of HuR(BM) is also indistinguishable from HuR(WT) as assessed by indirect immunofluorescence (Fig. 2C).
To test whether the residues altered in HuR(BM) are critical for HuR binding to ARE-containing target mRNA, we expressed FLAG-HuR and -HuR(BM) in MCF-7 cells and subjected these cell lysates to RNA-IP analysis. To ensure specific purification of FLAG-tagged HuR, we performed the RNA-IP with FLAG antibody-conjugated protein A beads. As shown in Fig. 2C, we achieved robust purification of both FLAG-HuR and -HuR(BM) with no purification of the untagged, endogenous HuR protein. To identify RNA transcripts that copurify with the FLAG-tagged HuR proteins, qRT-PCR analysis was performed (Fig. 2D). As expected, the well studied HuR target, ERα (38), was robustly enriched upon purification of FLAG-HuR. Significantly reduced enrichment of the ERα transcript was detected with FLAG-HuR(BM) as compared with WT FLAG-HuR, providing the first evidence that these key residues within HuR RRM1 and RRM2 are important for interaction with HuR target RNA in cells and demonstrating the utility of this mutant for validating ARE-containing HuR target RNAs. As expected, the negative control transcript, RPLP0, did not enrich with either FLAG-HuR or -HuR(BM).
We next exploited the HuR(BM) to assess whether PDCD4 binding depends on HuR RRMs 1 and 2, as established for the ARE-containing ERα transcript. We analyzed PDCD4 mRNA enrichment with both FLAG-HuR and -HuR(BM). Consistent with the results from the endogenous HuR RNA-IP, we observed robust enrichment of the PDCD4 transcript upon purification of wild type HuR. In contrast, no significant enrichment of PDCD4 was detected in the HuR-binding mutant sample. The loss of enrichment of the ERα and PDCD4 transcripts with FLAG-HuR(BM) demonstrates that PDCD4 binding to HuR is similar to that of the classical ARE-containing target, ERα.
HuR Binds to Two Distinct Regions within the PDCD4 3′UTR
Previous CLIP-Seq studies examining HuR binding across the transcriptome reveal that HuR binds primarily within the 3′UTRs and introns of mRNAs (12, 31–33). Additional preliminary data from these CLIP-Seq studies show that the 3′UTR of the PDCD4 transcript contains multiple AU- and U-rich stretches that could be bound by HuR (12, 31–33). The UCSC Genome Browser defines the PDCD4 3′UTR as being 1,918 nt long; however, data from poly(A)-Seq analyses in various human tissues suggest that an evolutionarily conserved upstream polyadenylation site may be used for 3′ end processing of the PDCD4 transcript, resulting in a 3′UTR of ∼672 nt. To determine the precise length of the PDCD4 3′UTR in MCF-7 cells, we performed 3′RACE analysis using primers specific to the PDCD4 3′UTR. The GAPDH 3′UTR was used as a control. To define the size of the PDCD4 3′UTR in two other human cancer cell lines, we also performed 3′RACE analyses using cDNA from HeLa (cervical adenocarcinoma; ATCC CCL-2) and MDA-MB-231 (mammary adenocarcinoma; ATCC HTB-26) cells. As shown in Fig. 3A and verified by sequence analysis, the 3′UTR of PDCD4 is 672 nucleotides in all cell types analyzed. These results focus our analysis of post-transcriptional regulation of PDCD4 on the 672-nt 3′UTR present in MCF-7 cells.
FIGURE 3.
HuR binds to sites within the PDCD4 3′UTR. A, total RNA isolated from HeLa, MB-231, and MCF-7 cells was subjected to 3′RACE analysis using PDCD4 and GAPDH 3′UTR-specific primers as described under “Experimental Procedures.” PCR products were resolved on a 2% agarose gel and represent the 3′UTR plus the length of the primers used for amplification. The PDCD4 3′UTR is 672 nt and was verified by sequencing. The GAPDH 3′UTR is shown as a control. Molecular weight markers in base pairs (MW bp) are shown to the left of the gel. B, biotinylated probes corresponding to the 3′UTRs of the c-Myc, GAPDH, and PDCD4 transcripts were generated and used for biotin pulldown experiments in MCF-7 cells. The red asterisk denotes the well defined miR-21 seed region (46) in the PDCD4 3′UTR. The PDCD4 3′UTR was also dissected to generate various biotin probes that represent different regions of the transcript. C, proteins that coprecipitated with the avidin-bound biotinylated probes, as shown in B, were subjected to immunoblotting with HuR antibody. HuR protein coprecipitates with a probe corresponding to the c-Myc 3′UTR as well as the first two 100-nt regions of the PDCD4 3′UTR. Images are representative of n = 3 independent experiments.
To determine whether HuR binds to the 3′UTR of PDCD4, we generated a biotinylated RNA probe that corresponds to the 672-nt region of the PDCD4 3′UTR. For controls, we employed the c-Myc 3′UTR, which is a well defined target of HuR (10), and the GAPDH 3′UTR, which is not a HuR target (Fig. 3B). The biotinylated probes were incubated with MCF-7 cell lysates, precipitated with NeutrAvidin-coated beads, and then subjected to immunoblot analysis with HuR antibody to detect HuR bound to the probes. As shown in Fig. 3C, HuR protein was readily detected in the input fractions, verifying the presence of HuR protein in these cell lysates. We detected robust HuR binding to the full-length PDCD4 3′UTR probe, suggesting that HuR binds the 3′UTR of PDCD4. As expected, we detected HuR protein bound to the c-Myc 3′UTR probe but not samples without a probe or with the negative control GAPDH 3′UTR probe. We also tested biotinylated probes that correspond to the 5′UTR and coding region of the PDCD4 transcript in this assay. Consistent with preliminary data that suggests binding of HuR to the PDCD4 3′UTR specifically, we did not detect coprecipitation of HuR upon pulldown with the PDCD4 5′UTR and coding region probes (data not shown). These results confirm that HuR binds to the 3′UTR of PDCD4 mRNA.
To define specific regions of the PDCD4 3′UTR that confer HuR binding, we generated a set of biotinylated probes that correspond to the first 290 and final 382 nucleotides of the PDCD4 3′UTR (5′290 and Δ290 probes, respectively; Fig. 3B). As shown in Fig. 3C, we detected robust HuR binding to the 5′290 probe. We did not, however, detect binding of HuR to the Δ290 probe, suggesting that HuR binds specifically to the 5′290 nucleotides of the PDCD4 3′UTR. Interestingly, the 5′290 nucleotides of the PDCD4 3′UTR contain multiple AU- and U-rich stretches that could be bound by HuR (61). To determine whether HuR binds to one or more of these stretches, we generated additional biotinylated probes that correspond to 100 or 90 nucleotide regions of the PDCD4 5′290 3′UTR region (Fig. 3B; probes A, B, and C). We detected robust binding of HuR to the first two 100-nucleotide regions of the PDCD4 3′UTR but not to the third 90 nucleotides (Fig. 3C). These results show two distinct HuR-binding sites within the first 200 nucleotides of the PDCD4 3′UTR.
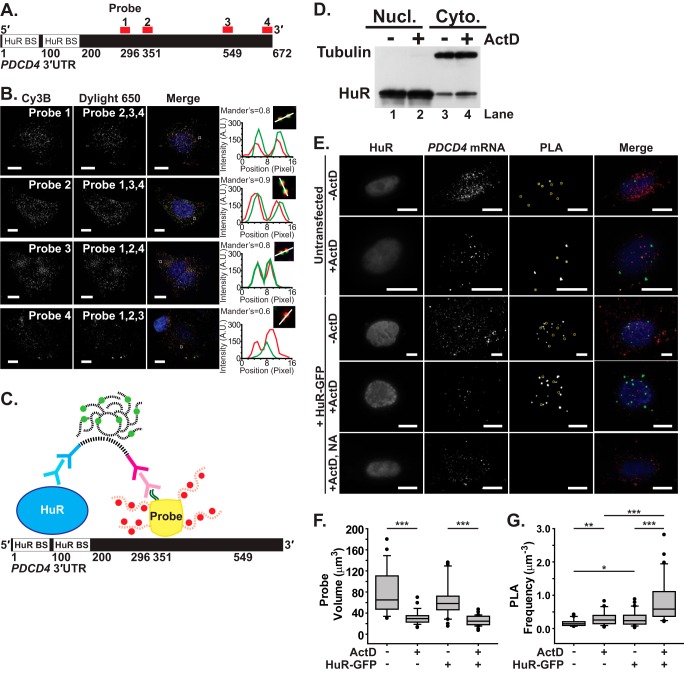
RNA Imaging Probes Can Be Used to Visualize PDCD4 mRNA in Live Cells
Recent studies demonstrate the ability to accurately visualize RNA granules and RNA/protein interactions at a single molecule level in live cells (53, 54, 63). To detect HuR/PDCD4 interactions in MCF-7 cells, we designed four imaging probes that target four different sequences in the PDCD4 3′UTR, avoiding the defined HuR-binding sites (Fig. 4A). To determine probe specificity, we delivered each probe, labeled with Cy3b fluorophores, along with the other three probes labeled with DyLight 650 fluorophores. For example, to examine the specificity of probe 1, probe 1 was labeled with Cy3b and probes 2, 3, and 4 were labeled with DyLight 650 (Fig. 4B). Therefore, colocalization of probe 1 with the other three probes would suggest specificity of probe 1 in targeting the PDCD4 3′UTR.
FIGURE 4.
Visualization of HuR/PDCD4 interactions in situ using FLAG-tagged probes. A, the 672-nt PDCD4 3′UTR contains two HuR-binding sites (HuR BS) within the first 200 nt. Four mRNA probes (1–4) were designed to target distinct positions within the PDCD4 3′UTR (shown in red). B, each of four PDCD4 3′UTR probes labeled with Cy3B fluorophores was delivered to MCF-7 cells along with the other three probes labeled with DyLight 650 fluorophores. Merge images of Cy3B-labeled probe (red), DyLight 650-labeled probe (green), and DAPI-stained nuclei (blue) are also shown. All image planes are represented. Magnified merge images of overlapping probes in the boxed region are shown with a profile plot of the fluorescence intensity of Cy3B (red) and DyLight 650 (green) along an intersection of the probes (white line). Scale bar, 10 μm. The mean Mander's coefficient of Cy3B and DyLight 650 probe colocalization is shown. C, schematic of the PLA, which measures the interaction between HuR and probes specific to the PDCD4 3′UTR, is shown. As described under “Experimental Procedures,” PLA was performed between HuR and FLAG-tagged (dark green lines) probes with four Cy3b-labeled (red) oligonucleotides (red dashed lines) and a NeutrAvidin core (yellow) in nucleotides 296–549 of the PDCD4 3′UTR. Anti-HuR mouse primary antibody (light blue) and anti-mouse PLA probe (dark blue) bind to HuR, whereas anti-FLAG rabbit primary antibody (light magenta) and anti-rabbit PLA probe (dark magenta) bind to FLAG. Once they are in proximity, the oligonucleotides (black dashed lines) attached to the PLA probe come together via ligation to form a template for DNA polymerase, which resulted in a coiled DNA product that can be labeled with Cy5-equivalent fluorophore (light green)-bound complementary DNA strands (black dash lines). D, MCF-7 cells treated with vehicle control or ActD for 90 min were subjected to subcellular fractionation (as described under “Experimental Procedures”) and subsequent immunoblotting with HuR and tubulin antibody. A distinct enrichment of HuR is observed in the cytoplasm upon ActD treatment. Nucl, nuclear; Cyto, cytoplasmic. E, HuR, PDCD4 mRNA and PLA between HuR and PDCD4 mRNA were imaged in untransfected and HuR-GFP-transfected MCF-7 cells exposed to (+ActD) or unexposed to (−ActD) actinomycin D. MCF-7 cells transfected with HuR-GFP and exposed to ActD received PDCD4 mRNA probes with NeutrAvidin lacking the FLAG tag (NA) and were used as a negative control. PLA punctae that are less than 2 μm in diameter have been marked with yellow circles. Merge images of HuR (blue), PDCD4 mRNA (red), and PLA between HuR and PDCD4 mRNA (green) are shown. All image planes are represented. Scale bar, 10 μm. F, probe volume (μm3) measured for each untransfected and HuR-GFP-transfected cell exposed or unexposed to ActD (untransfected, n = 46 cells; untransfected + ActD, n = 125; HuR-GFP-transfected, n = 51; HuR-GFP transfected + ActD, n = 62). G, PLA frequency normalized to the probe volume (μm−3) for each cell. * represents p ≥ 0.025, p < 0.05; ** represents p ≥ 0.001, p < 0.025; *** represents p < 0.001 (one-way ANOVA with Dunn's method).
For each probe, three images were obtained for analysis, and colocalization was quantified by Mander's coefficient. The mean Mander's coefficients for probes 1–3 were greater than 0.8 and were not statistically different from one another (p > 0.9), suggesting good colocalization. Probes 1–3 overlapped well with the other probes, as shown in the intensity profile of the representative mRNA punctae (Fig. 4B). However, the mean Mander's coefficient for probe 4 was 0.6 and was significantly less than the other three probes (p < 0.009). Generally, probe 4 did not overlap well with the other probes as shown in the intensity profile (Fig. 4B). Where the probe signal was bright, the signal of the other probes was dim or vice versa. Thus, probe 4 was deemed nonspecific and was not used for further analysis, and probes 1–3 were employed in all experiments. These results suggest that probes 1–3 specifically recognize the PDCD4 3′UTR and allow us to visualize PDCD4 RNA granules in MCF-7 cells.
Proximity Ligation Assay Can Detect HuR/PDCD4 mRNA Interactions
To visualize HuR protein/PDCD4 mRNA interactions in MCF-7 cells, we performed PLA between HuR and the FLAG peptides on the PDCD4 RNA probes. As schematized in Fig. 4C, primary antibodies of different species bind to HuR and the FLAG tag (mouse and rabbit, respectively), which can then be detected by species-specific PLA probes with oligonucleotides. The oligonucleotides attached to the PLA probe form a circularized DNA strand by enzymatic ligation, one of which can serve as a primer for rolling circle amplification. This amplification ultimately results in a coiled single-stranded DNA, or the PLA product, which is complementary to the circular DNA strand. The PLA product is then detected by hybridizing complementary fluorophore-labeled oligonucleotides (Fig. 4C) (54, 55).
Because of the cell permeabilization and probe delivery conditions, this PLA specifically detects cytoplasmic protein/RNA interactions. Previous work demonstrates that the steady-state localization of HuR in MCF-7 cells is primarily nuclear with a small amount located in the cytoplasm due to nucleocytoplasmic shuttling of HuR (35). To increase the cytoplasmic pool of HuR for analysis in the PLAs, cells were treated with the transcriptional inhibitor, actinomycin D, which increases the cytoplasmic localization of HuR (64). To confirm the steady-state nuclear localization of HuR in these cells as well as the cytoplasmic enrichment of HuR upon ActD treatment, we performed nucleocytoplasmic fractionation of MCF-7 cells subjected to a 90-min treatment with vehicle control (PBS) or ActD. As shown in Fig. 4D, fractionation of vehicle-treated cells confirms that the majority of the HuR protein is localized to the nucleus (compare lanes 1 and 3). Treatment with ActD results in an increase in the amount of HuR detected in the cytoplasm (Fig. 4D, compare lanes 2 and 4). Thus treatment of MCF-7 cells with ActD increases the cytoplasmic pool of HuR.
As shown in Fig. 4E, we observe endogenous HuR/PDCD4 interactions by PLA in untransfected and untreated cells (top row; quantified in Fig. 4G), which supports the HuR/PDCD4 binding data obtained from both RNA-IP (Figs. 1 and 2) and biotin pulldown analyses (Fig. 3). As expected, we observed no PLA signal in cells incubated with negative control probes lacking FLAG tags (NA; Fig. 4E). To validate the interaction of HuR with the PDCD4 transcript, we also modulated both HuR levels and localization and assessed the impact on the PLA signal. These experiments were carried out either in cells expressing endogenous HuR or where total levels of HuR were increased by expression of HuR-GFP. To assess changes in the frequency of HuR/PDCD4 mRNA interactions, we counted the number of HuR/PDCD4 PLA punctae and normalized to the amount of mRNA in each cell, which was quantified by measuring the volume of probe signal for each cell. The probe volume was the same in both untransfected and HuR-GFP-transfected cells with (p = 0.16) and without (p = 0.26) ActD (Fig. 4F). This analysis confirms that the probe delivery and binding as well as the amount of RNA granules are not significantly affected by transfection. As expected for treatment with a transcriptional inhibitor, incubation of cells with ActD reduced the total volume of PDCD4 mRNA by ∼40% (p < 0.001; quantified in Fig. 4F).
Treatment with ActD resulted in significantly more HuR/PDCD4 PLA punctae than untreated cells due to increased cytoplasmic HuR in both untransfected (p < 0.02) and HuR-GFP-transfected cells (p < 0.001; quantified in Fig. 4G). As experiments employed a low amount of HuR-GFP plasmid (0.8 μg) for transfection, HuR-GFP-transfected cells not treated with ActD showed only a slight increase in PLA frequency compared with the untransfected samples (p = 0.046). As demonstrated above, addition of ActD caused HuR to localize to the cytoplasm and resulted in a greater increase in HuR/PDCD4 interactions in the HuR-GFP-transfected cells than in untransfected cells (p < 0.001). These results demonstrate individual HuR/PDCD4 interactions in MCF-7 cells and further validate the interaction between HuR and the PDCD4 transcript.
Cytoplasmic PDCD4 mRNA Is Regulated Differently than Nuclear PDCD4 mRNA
As mentioned above, canonically, HuR binding to target mRNAs results in increased transcript stability (17). In fact, many HuR target mRNAs are inherently labile and responsive to cellular stimuli (65, 66). As shown in Fig. 1, C and D, knockdown of HuR in MCF-7 cells results in decreased steady-state PDCD4 mRNA and protein levels, suggesting that HuR could negatively regulate the stability of the PDCD4 transcript. To determine the half-life of the PDCD4 transcript in MCF-7 cells, we treated cells with the potent transcriptional inhibitor, ActD (67), and collected cells at 30 min and 2, 4, 6, and 8 h after drug addition. We performed qRT-PCR analysis with PDCD4 primers to determine the percent mRNA remaining over time following ActD treatment (Fig. 5A). For controls, we also analyzed the c-Myc transcript, which has a short half-life (66) as well as the very stable RPLP0 transcript (68). As shown in Fig. 5A, the PDCD4 transcript showed significant decay over the course of the experiment, with a calculated half-life of ∼6.1 h, which is less than the average mRNA half-life of ∼9 h (69). These results suggest that the PDCD4 transcript is labile. As expected, the c-Myc transcript levels decreased dramatically after ActD treatment with a calculated half-life of ∼1.6 h, which is comparable with the previously determined c-Myc mRNA half-life values (66). The RPLP0 transcript decayed a very small amount over the time course examined as expected for a highly stable transcript.
FIGURE 5.
Cytoplasmic pool of PDCD4 mRNA is highly stable compared with the nuclear pool. A, MCF-7 cells were treated with ActD and collected at the indicated time points after drug addition. Total RNA isolated from ActD-treated cells was subjected to qRT-PCR analysis with RPLP0, PDCD4, and c-Myc primers. mRNA levels are represented as % of amount present at 30 min of ActD exposure. The half-life of the c-Myc and PDCD4 transcripts was calculated to be 1.6 and 6.1 h, respectively. B, immunoblot analysis of total protein isolated from the samples in A to detect PDCD4, HSP90, tubulin, and HuR reveal a sharp decrease in PDCD4 protein upon treatment with ActD but not vehicle control. C, MCF-7 cells treated with vehicle control or ActD over the indicated time course were subjected to subcellular fractionation followed by immunoblotting with HSP90, HuR, TIA1, or PDCD4 antibody. Immunoblots reveal an accumulation of both HuR and TIA1 in the cytoplasm across the ActD time course, but no accumulation is observed during vehicle control treatment, as expected. PDCD4 is predominantly cytoplasmic in these cells and the cytoplasmic pool of PDCD4 demonstrates the robust decrease after ∼3 h of ActD treatment. RNA isolated from nuclear and cytoplasmic fractions from (C) were subjected to qRT-PCR analysis with β-actin (D), c-Myc (E), and PDCD4 (F) primers. The decay profiles of β-actin and c-Myc mRNA are similar between the nuclear and cytoplasmic compartments (D and E); however, the cytoplasmic pool of PDCD4 mRNA displays a significantly increased decay profile compared with the nuclear pool of PDCD4 mRNA (F). Ct values were normalized to 18 S rRNA, and decay profiles are represented as % of amount at 30 min of ActD exposure. Data points represent the mean ± S.E. for n = 3 independent experiments. Images are representative of n = 3 independent experiments.
Immunoblot analysis of protein samples isolated across the ActD time course reveals a sharp decrease in PDCD4 steady-state protein levels upon ActD treatment (Fig. 5B, lanes 6–10) but not vehicle control-treated cells (lanes 1–5). The observed decrease in PDCD4 protein levels is consistent with previous work that demonstrates activation (by phosphorylation of serine 70) of S6K1, the kinase responsible for phosphorylation and subsequent degradation of PDCD4 (50), upon treatment with actinomycin D (70). As expected, the steady-state levels of tubulin and HuR are unchanged across the time course of ActD treatment.
The observation that HuR interacts with PDCD4 mRNA in the cytoplasm (Fig. 4E) as well as the fact that cytoplasmic HuR protein levels increase upon ActD treatment (Fig. 4D) led us to question whether there may be differential regulation of the nuclear and cytoplasmic pools of PDCD4 mRNA. To isolate nuclear and cytoplasmic fractions from MCF-7 cells treated with ActD (or vehicle control), we performed cellular fractionation as described under “Experimental Procedures” and collected nuclear and cytoplasmic fractions at 30 min and 3, 6, and 8 h after drug treatment. Immunoblot analysis of fractionated cells reveals robust and clean fractionation of nuclear and cytoplasmic compartments as evidenced by distinct partitioning of steady-state nuclear (HuR) and cytoplasmic proteins (HSP90). As shown in Fig. 5C, we observe cytoplasmic localization of PDCD4, consistent with its role in translational regulation (41, 42). As expected, HSP90 levels remain unchanged in control and ActD-treated cells. Consistent with the results obtained from total cellular protein levels upon ActD treatment (Fig. 5B), we observe a decrease in PDCD4 protein levels upon ActD treatment in the cytoplasmic fractions.
As shown previously (Fig. 4D), the steady-state level of HuR protein increases in the cytoplasm upon ActD treatment with no change in the vehicle control-treated cells (Fig. 5C). Immunoblot analysis of another RNA-binding protein that shuttles between the nucleus and cytoplasm and shifts in localization upon ActD treatment (29), TIA1, reveals a sharp decrease in steady-state nuclear protein levels with a concomitant increase in cytoplasmic levels. Interestingly, in these cells, the shift to cytoplasmic localization for TIA1 may occur faster than HuR as evidenced by increased cytoplasmic levels of TIA1 at 30 min of ActD exposure (as compared with vehicle control-treated cells at 30 min).
To identify any differences in mRNA decay profiles between nuclear and cytoplasmic pools of mRNA, we performed qRT-PCR analysis of RNA isolated from each compartment with primers to detect β-actin, c-Myc and PDCD4 transcripts (Fig. 5, D–F). The highly stable β-actin transcript (71) and the extremely labile c-Myc transcript (66), both previously defined targets of HuR (10, 14, 20, 72), display similar decay profiles between the nuclear and cytoplasmic compartments (Fig. 5, D and E, respectively). The PDCD4 transcript, however, displays a striking difference in mRNA decay between the nucleus and cytoplasm. As shown in Fig. 5F, the cytoplasmic pool of PDCD4 mRNA is significantly more stable than the nuclear pool, coincident with changes in the localization of RNA-binding proteins (Fig. 5C) that may regulate this critically important transcript. These results suggest that PDCD4 mRNA may be regulated in a unique manner than other HuR target mRNAs.
The U-rich Element RNA-binding Protein, TIA1, Binds to PDCD4 mRNA in MCF-7 Cells
The observation that the cytoplasmic pool of PDCD4 mRNA is significantly more stable than nuclear PDCD4 mRNA (Fig. 5F) coupled with the shift in localization that occurs for many RNA-binding proteins, including two U-rich element binding proteins, HuR and TIA1 (Fig. 5C), led us to hypothesize that other factors may bind to and modulate the PDCD4 transcript via the 3′UTR. One well studied mode of regulation of the PDCD4 transcript occurs via an interaction between miR-21 and the PDCD4 3′UTR, which negatively regulates cellular PDCD4 levels (46, 47). Recent advances in sequencing technology have allowed for extensive characterization of candidate mRNA targets of many RNA-binding proteins. As shown in Fig. 6A, alignment of iCLIP-Seq tags from a recent transcriptome-wide TIA1 iCLIP study in HeLa cells (27) reveals two predicted TIA1-binding sites that overlap with the 3′UTR regions bound by HuR (Fig. 3C). These observations suggest that HuR and TIA1 could bind to similar or overlapping regions in the PDCD4 3′UTR. Interestingly, previous work has defined a cooperative relationship between HuR and TIA1 in regulating the translation of specific target RNAs (21), and therefore it provides precedence for interactions and/or competition between these two RNA-binding proteins on target mRNAs.
FIGURE 6.
RNA-binding protein, TIA1, interacts with PDCD4 mRNA. A, a recent TIA1 iCLIP study reveals two predicted TIA1-inding sites (shown here in blue) within the first 200 nt of the PDCD4 3′UTR (27) that overlap with the validated HuR-inding sites (green) defined here. The red asterisk denotes the well defined miR-21 seed region. B, endogenous TIA1 protein was immunoprecipitated from MCF-7 cells using TIA1 antibody-coated protein A beads alongside isotype control goat IgG-coated beads. Proteins from the input, unbound (UB), and bound fractions were subjected to immunoblotting with TIA1 antibody. TIA1 was detected in the TIA1-bound fraction but not the control IgG-bound fraction. TIA1 is alternatively spliced to generate two distinct protein products that correspond to the two bands detected. C, qRT-PCR analysis of RNA isolated from the TIA1 RNA-IP using PDCD4, CYCS, and PTMA primers reveals clear enrichment of these transcripts upon TIA1 pulldown. A control mRNA, GAPDH, did not coprecipitate with TIA1. Values represent the mean ± S.E. for n = 3 independent experiments. * and ** represent p ≤ 0.05 and p ≤ 0.01, respectively. D, PLAs performed with TIA1 antibody and PDCD4 3′UTR probes 1–3 reveal interactions between TIA1 and the PDCD4 3′UTR. Merge images of nuclei (blue), TIA1 (white), PDCD4 mRNA (red) and PLA between TIA1 and PDCD4 mRNA (green) are shown. All image planes are represented. Scale bar, 10 μm. E, probe volume (μm3) measured for each untransfected (n = 11 cells) and TIA1-GFP-transfected (n = 15) cell. F, PLA frequency normalized to the probe volume (μm−3) for each cell. *** represents p < 0.001 (Mann-Whitney rank sum test).
To assess whether TIA1 binds to PDCD4 mRNA in MCF-7 cells, we performed RNA-IP with endogenous TIA1 protein. As shown in Fig. 6B, TIA1 protein was detected in the bound fraction when purified with TIA1 antibody but not with IgG control antibody. The input and bound fractions were then analyzed for PDCD4 transcript using qRT-PCR. As shown in Fig. 6C, the PDCD4 transcript was robustly enriched with TIA1. For controls, we also examined two known TIA1 targets, cytochrome c (CYCS) (21) and prothymosin α (PTMA) (18), as well as GAPDH, which is not bound by TIA1. As expected, the GAPDH transcript showed no enrichment with TIA1; however, the previously defined TIA1 targets, CYCS and PTMA, demonstrated significant enrichment. These results confirm that PDCD4 mRNA is bound by TIA1 in MCF-7 cells.
To further validate the interaction between TIA1 and PDCD4 mRNA, we performed PLA between TIA1 and the FLAG-tagged probes designed for the PDCD4 3′UTR (see Fig. 4A) with and without TIA1-GFP transfection (Fig. 6D). As shown in Fig. 6E, we observed no significant difference in the amount of mRNA probes under any of the experimental conditions employed (p = 0.855), suggesting that probe delivery and binding were not affected by TIA1-GFP transfection. TIA1/PDCD4 mRNA interactions were observed by PLA with endogenous TIA1, and as expected, a significant increase in TIA1/PDCD4 interactions was observed by PLA upon increasing TIA1 levels via transfection of TIA1-GFP (p < 0.001), which demonstrates specific interaction between TIA1 and PDCD4 mRNA in these cells (Fig. 6F).
Given that the probes bind in this region and that we are able to detect TIA1 interactions readily via PLA, it is likely that TIA1 binds within the first ∼200 nt of the 3′UTR, in a similar region to where we map HuR binding (Fig. 3C). These results suggest that TIA1 represents another factor that binds to the PDCD4 transcript and could play a role in regulating PDCD4 steady-state levels.
Knockdown of HuR and/or TIA1 Decreases Steady-state PDCD4 mRNA and Protein Levels
Both binding and PLA studies reveal interactions between HuR and TIA1 with the PDCD4 transcript. As shown in Fig. 1, C and D, we observe a significant decrease in steady-state PDCD4 mRNA and protein levels upon knockdown of HuR in MCF-7 cells. To determine whether TIA1 also has an effect on PDCD4 steady-state levels, we transiently transfected MCF-7 cells with siRNA targeting TIA1. We also transfected a population of cells with HuR and TIA1 siRNA together to determine whether reduction of both factors had any additional effect on PDCD4 steady-state levels. As shown in Fig. 7A, we achieve robust knockdown of HuR and TIA1 (alone and in tandem) in these cells. Upon knockdown of HuR and/or TIA1, PDCD4 steady-state protein levels are reduced ∼30–40% (quantification below the representative blot). As expected, a control protein, HSP90, displays no difference in steady-state levels across all treatment groups. qRT-PCR analysis of RNA isolated from HuR and TIA1 knockdown samples with primers to PDCD4 and RPLP0 revealed a significant reduction of PDCD4 steady-state mRNA levels (Fig. 7B), consistent with the trend observed at the protein level (Fig. 7A).
FIGURE 7.
Knockdown of HuR and TIA1 leads to decreased PDCD4 steady-state mRNA and protein levels. A, MCF-7 cells transiently transfected with Scramble, HuR, and/or TIA1 siRNA (siScr, siHuR, siTIA1, and siBoth) were subjected to immunoblot analysis with HSP90, HuR, TIA1, or PDCD4 antibody. Steady-state PDCD4 protein levels were normalized to HSP90 loading control and siScramble set to 1.00. Decreased PDCD4 steady-state protein levels upon HuR and/or TIA1 knockdown is quantified below the representative blot. B, qRT-PCR analysis of total RNA isolated from siRNA-treated cells with PDCD4 and RPLP0 primers demonstrates a significant decrease in PDCD4 steady-state mRNA levels upon HuR and/or TIA1 knockdown. C, qRT-PCR analysis of steady-state miR-21 and RNU48 (loading control) levels upon knockdown of HuR and/or TIA1 reveals no significant difference in miR-21 levels across all treatment groups. D, polysome profiling was performed on MCF-7 cells as described previously (60). Cytoplasmic lysates from MCF-7 cells were treated with EDTA (to disrupt polyribosomes) or left untreated (no treatment, N.T.) and subjected to polysome fractionation. Untreated MCF-7 cells demonstrate active translation of PDCD4 mRNA (as represented by a sharp peak in fraction 8), whereas EDTA-treated cell lysates display a collapse of the polyribosomes on the PDCD4 transcript, as expected. Polysome profiling was performed on MCF-7 cell lysates transfected with scramble control, HuR, and/or TIA1 siRNA. Analysis of the GAPDH (E) and PDCD4 (F) transcripts reveals that PDCD4 mRNA is actively translated in MCF-7 cells, similar to GAPDH, and that knockdown of HuR and/or TIA1 has no effect on the translation of the PDCD4 transcript under these conditions. Polysome profiles are representative of two technical qRT-PCR replicates of a single fractionation experiment. Values represent the mean ± S.E. for n = 3 independent experiments. * and ** represents p ≤ 0.05 and p ≤ 0.01, respectively.
As discussed previously, miR-21 is a well studied regulator of PDCD4 levels in MCF-7 cells (47). Recent work has described a role for HuR in modulating miRNA loading onto target transcripts (20, 73) as well a potential role for HuR in miRNA biogenesis (32, 33, 74). To determine whether HuR and/or TIA1 knockdown impacts steady-state miR-21 levels in these cells, we performed qRT-PCR analysis with primers to detect miR-21 as well as a control small ncRNA, RNU48 (75). As shown in Fig. 7C, knockdown of HuR and/or TIA1 did not alter steady-state miR-21 levels; however, this finding does not exclude the possibility that HuR and/or TIA1 could modulate the loading of miR-21 onto the PDCD4 transcript.
The best characterized role of TIA1 in post-transcriptional processing is that of a negative regulator of translation (40). Although we observe a decrease in both the steady-state mRNA and protein level of PDCD4 upon TIA1 knockdown, we questioned whether TIA1 or HuR could also modulate translation of the PDCD4 transcript. To assess the translational profile of PDCD4 mRNA upon knockdown of TIA1 and/or HuR, we performed polysome profiling on MCF-7 cytoplasmic lysates via sucrose gradient fractionation. Cytoplasmic lysates were either untreated or treated with EDTA to ensure the identity of the polysomes. Analysis was also performed for cells treated with scramble control siRNA or siRNA targeting HuR, TIA1, or both. Total RNA isolated from each fraction was used for cDNA generation and subsequent qRT-PCR analysis with PDCD4 and GAPDH primers. The percent distribution of PDCD4 mRNA in each fraction was determined and plotted as shown in Fig. 7D. Untreated MCF-7 cells display a PDCD4 polysome profile characteristic of an actively translated mRNA, such as GAPDH (76), as indicated by a sharp peak in fraction 8, which corresponds with the polysomes (assessed by A254 absorbance, data not shown). As expected, treatment of cytoplasmic lysates with EDTA disrupted polysomes and resulted in a striking leftward shift on the profile (Fig. 7D).
As expected, polysome profiling of the actively translated mRNA, GAPDH, upon HuR and/or TIA knockdown reveals no difference in the translational profile of this message (Fig. 7E). As shown in Fig. 7F, analysis of PDCD4 translation across the same treatment groups also reveals no significant difference in PDCD4 translational profile in these cells. Together, these data support a role for HuR and TIA1 in positively regulating PDCD4 mRNA levels.
HuR and TIA1 Compete for Binding to PDCD4 mRNA
We have demonstrated by various methods that both HuR and TIA1 bind the PDCD4 transcript in MCF-7 cells. We also show that knockdown of HuR and/or TIA1 results in a steady-state decrease in PDCD4 mRNA and protein levels. However, we also observe a very interesting and seemingly dynamic mode of regulation of the PDCD4 transcript, specifically in the cytoplasm. To assess whether HuR and TIA1 cooperate or compete for binding to the PDCD4 transcript in the cytoplasm, we modulated the levels of TIA1 or HuR in MCF-7 cells and analyzed HuR/PDCD4 interactions using PLA (Fig. 8, A–C). No difference in the probe volume was observed between the treatment groups (p = 0.12; data not shown), demonstrating that probe delivery and binding as well as the level of PDCD4 mRNA were not affected by transfection. As expected, upon reduction of HuR, HuR/PDCD4 interactions decreased significantly (p < 0.001; quantified in Fig. 8C). A similar decrease in HuR/PDCD4 interactions was observed upon overexpression of TIA1 through transfection of TIA1-GFP (p < 0.001). In contrast, knockdown of TIA1 resulted in increased HuR/PDCD4 interactions comparable with HuR-GFP transfected cells (p = 0.76). These results suggest that increasing TIA1 may prevent HuR from binding to the PDCD4 3′UTR, but decreasing TIA1 facilitates HuR/PDCD4 interactions (Fig. 8, A–C), which suggests a competitive relationship between HuR and TIA1 for binding to the PDCD4 3′UTR.
FIGURE 8.
HuR and TIA1 compete for binding to the PDCD4 3′UTR. A, visualization of HuR protein, PDCD4 mRNA, and interaction between HuR and PDCD4 mRNA (PLA) in control cells transfected with scrambled siRNA (siScr), or siRNA directed against either HuR (siHuR), or TIA1 (siTIA1). Merged images of HuR (white), PDCD4 mRNA (red), PLA (green), and nuclei (blue) are also shown. PLA punctae that are less than 2 μm in diameter have been marked with yellow circles. All image planes are represented. Scale bar, 10 μm. B, visualization of GFP, PDCD4 mRNA and interaction between HuR and PDCD4 mRNA in TIA1-GFP-transfected cells. Merged images of GFP (white), PDCD4 mRNA (red), PLA (green), and nuclei (blue) are also shown. PLA punctae that are less than 2 μm in diameter have been marked with yellow circles. All image planes are represented. Scale bar, 10 μm. C, HuR/PDCD4 mRNA PLA frequency normalized to probe volume (μm−3) for untransfected (n = 46 cells), siScramble (n = 81), siHuR (n = 25), HuR-GFP (n = 51), siTIA1 (n = 97), and TIA1-GFP (n = 69) cells. *** represents p < 0.001 (one-way ANOVA with Dunn's method). D, visualization of TIA1 protein, PDCD4 mRNA, and PLA interaction between TIA1 and PDCD4 mRNA in siScramble (siScr), siHuR, and siTIA1-transfected cells. Merged images of TIA1 (white), PDCD4 mRNA (red), PLA (green), and nuclei (blue) are also shown. PLA punctae that are less than 2 μm in diameter have been marked with yellow circles. All image planes are represented. Scale bar, 10 μm. E, visualization of GFP, PDCD4 mRNA, and PLA interaction between TIA1 and PDCD4 mRNA in HuR-GFP-transfected cells. Merged images of HuR (white), PDCD4 mRNA (red), PLA (green), and nuclei (blue) are shown. PLA punctae that are less than 2 μm in diameter have been marked with yellow circles. All image planes are represented. Scale bar, 10 μm. F, TIA1-PDCD4 mRNA PLA frequency normalized to probe volume (μm−3) for untransfected (n = 55 cells), siScramble (n = 49), siHuR (n = 66), HuR-GFP (n = 46), siTIA1 (n = 44), and TIA1-GFP (n = 76) cells. *** represents p < 0.001 (one-way ANOVA with Dunn's method). G, model for the post-transcriptional regulation of PDCD4 mRNA in the cytoplasm. In MCF-7 cells, the steady-state localization of both HuR (green rectangle) and TIA1 (blue circle) is predominantly nuclear with a small pool that shuttles in and out of the cytoplasm (black bi-directional arrow). We observe a competitive mode of binding between HuR and TIA1 on the PDCD4 3′UTR in the cytoplasm, in close proximity to a well defined miR-21-binding site (red asterisk).
To determine whether changing HuR levels affects TIA1/PDCD4 interactions, we modulated HuR by siRNA or HuR-GFP transfection and assessed TIA1-PDCD4 PLA values. Again, we found no difference in the probe volume between different transfection groups (p = 0.21; data not shown), indicating that probe delivery and binding as well as the level of PDCD4 mRNA were not affected by transfection. As expected, knockdown and overexpression of TIA1 resulted in decreased and increased, respectively, TIA1/PDCD4 PLA interactions (Fig. 8, D and F). Consistent with our model where HuR and TIA1 compete for binding to PDCD4, upon reduction of HuR we observed an increase in TIA1 binding to PDCD4 mRNA (p < 0.001; Fig. 8, D and F). In contrast, overexpression of HuR did not have a significant effect on TIA1 binding to PDCD4 (p = 0.21), but the level of HuR overexpression was quite modest (Fig. 8, D–F). These results suggest that the PDCD4 transcript is competitively bound by HuR and TIA1 (schematized in Fig. 8G) and therefore may be regulated in a different manner than a previously described target mRNA that is cooperatively regulated by HuR and TIA1 (21).
DISCUSSION
In this study, we identified the PDCD4 mRNA transcript as a novel target of the RNA-binding proteins, HuR and TIA1. Our results show that HuR binds to the PDCD4 transcript via at least two distinct binding sites within the 3′UTR that overlap with TIA1-binding sites identified by CLIP-Seq (27). We demonstrate binding of TIA1 to the PDCD4 transcript and also show that modulation of TIA1 levels regulates HuR/PDCD4 interactions in MCF-7 cells, suggesting a competitive interaction between HuR and TIA1 on the PDCD4 transcript in the cytoplasm, specifically via the PDCD4 3′UTR. Nucleocytoplasmic fractionation reveals a significantly more stable pool of PDCD4 mRNA in the cytoplasm compared with the nucleus, suggesting that these pools of PDCD4 mRNA are regulated in a distinct manner. Knockdown of HuR and/or TIA1 results in significant reduction of PDCD4 mRNA, supporting a role for these factors in positively regulating PDCD4 mRNA levels. This work as well as recent studies implicating HuR and TIA1 as dynamic and coordinate regulators of target mRNA expression (21, 77) suggest a novel mode of regulation of PDCD4 by HuR and TIA1 and yield mechanistic insight into the post-transcriptional regulation of U-rich-containing mRNAs, such as PDCD4.
The PDCD4 protein is an inhibitor of the eIF4A helicase, which unwinds secondary structural elements located within the 5′UTR of mRNA transcripts to facilitate translation (41). PDCD4 expression is up-regulated after the initiation of programmed cell death (or apoptosis) (78), and a reduction in PDCD4 protein levels leads to neoplastic transformation (79). Because of the critical role for PDCD4 as a global regulator of protein synthesis and as a tumor suppressor, PDCD4 expression is regulated at multiple levels (43). PDCD4 is regulated at the transcriptional level by the transcription factor, v-Myb (80), as well as by epigenetic regulation through DNA methylation (81). With regard to post-transcriptional regulation of PDCD4, the only study beyond the extensive analysis of miR-21 (46, 47), examined the regulation of the PDCD4 transcript by the splicing factor, SRSF3 (82). SRSF3 binds to the 5′UTR of the PDCD4 transcript and modulates splicing and translational efficiency (82). The work presented here, which provides evidence for two additional RNA-binding proteins that bind to and regulate the PDCD4 transcript, expands our understanding of the post-transcriptional regulation of PDCD4.
Our results show that HuR binds to two independent regions within the first 200 nt of the PDCD4 3′UTR. The PDCD4 3′UTR is considerably AU- or U-rich (68 and 37%, respectively) and contains two striking U-rich stretches within the 5′290 nt region of the 3′UTR. Indeed, these two U-rich regions are found within the first 200 nt of the 3′UTR and are consistent with the mapped HuR-binding sites (Fig. 3). Preliminary secondary structure prediction analyses of the 672-nt 3′UTR of PDCD4 predict two single-stranded U-rich motifs within the first 200 nt, suggesting that these are the motifs bound by HuR (83). The predicted binding sites of TIA1 on the PDCD4 3′UTR (27) overlap with the HuR-binding sites and, due to the U-rich preference of TIA1, likely represent U-rich regions that are competitively bound by HuR and TIA1.
Previous studies demonstrate that competition between HuR and TIA1 for binding to 5′splice sites regulates alternative splicing in numerous cell types (84–86). However, there is no precedent for competition between HuR and TIA1 that influences mRNA stability and/or translation. Employing RNA imaging probes and PLA, we detected and quantified individual interactions between native PDCD4 mRNA and either endogenous HuR or TIA1 in single cells in situ. These PLA studies (Fig. 8) provide evidence that HuR and TIA1 compete for binding to the PDCD4 3′UTR. Overexpression of TIA1 reduced interactions between HuR and PDCD4, whereas knockdown of TIA1 increased HuR/PDCD4 interactions (Fig. 8). Similarly, knockdown of HuR increased TIA1 interactions with PDCD4 (Fig. 8). This competition between HuR and TIA1 could provide a way for cells to fine-tune the levels of PDCD4 to modulate programmed cell death.
Although the data presented in this study suggest a model where HuR and TIA1 compete for binding to overlapping cis-acting sequences, other RNAs are regulated through a mechanism where these RNA-binding proteins cooperate with one another. For example, post-transcriptional regulation of the cytochrome c gene, which encodes a key regulator of apoptosis (87), occurs through HuR and TIA1 binding to nonoverlapping binding sites in the AU-rich regions within the 3′UTR (21). This study reported that HuR binding promotes the translation of the cytochrome c mRNA without affecting mRNA levels, whereas TIA1 binding inhibits translation (21). In contrast to the competition between HuR and TIA1 that we detect (Fig. 8) on PDCD4, the study of the cytochrome c transcript reported that HuR and TIA1 bind cooperatively to the cytochrome c 3′UTR such that increasing TIA1 levels increased HuR binding; however, increasing HuR levels had no detectable effect on TIA1 binding (21). The caveat of this study, as the authors noted, is that the analysis of cytochrome c employed recombinant, exogenous proteins. In direct binding assays that employed RNA electrophoretic mobility shift assays, the authors did not detect simultaneous binding of HuR and TIA1 (21). These results indicate that coordinate regulation by RNA-binding proteins needs to be individually defined for each transcript. In this study, we provide evidence for overlapping binding sites for HuR and TIA1 on the PDCD4 3′UTR, which supports a model (Fig. 8G) where these two factors would compete for binding and subsequent regulation of the transcript.
Although the steady-state localization of HuR and TIA1 in MCF-7 cells is predominantly nuclear (Fig. 5C), both of these proteins shuttle in and out of the nucleus (15, 16, 28, 29). Previous studies demonstrate that HuR and TIA1 accumulate in the cytoplasm in response to various cues, including transcriptional inhibition (16, 29), creating two pools of protein that have the potential for distinct regulation of target transcripts. Consistent with this idea, nucleocytoplasmic fractionation of MCF-7 cells treated with ActD reveals a very stable pool of PDCD4 mRNA in the cytoplasm (Fig. 5F). Analysis of two other HuR target mRNAs (c-Myc and β-actin) (10, 14, 20, 72), however, revealed similar decay profiles between the nucleus and cytoplasm (Fig. 5, D and E), suggesting that the PDCD4 transcript is regulated in a specific manner. The observed stability of the PDCD4 transcript in the cytoplasm could be indicative of a stress response due to ActD treatment. In fact, ActD treatment results in a distinct increase in cytoplasmic localization of both HuR and TIA1 (Fig. 5C), consistent with other sources of cellular stress that result in shifts in the localization of RNA-binding proteins (65). We hypothesize that the enhanced stability of the PDCD4 transcript in the cytoplasm relative to the nucleus may be a result of the increased levels of HuR and TIA1, both of which play a role in positively regulating PDCD4 mRNA levels (Fig. 7B). However, we cannot rule out that stress could trigger other changes in these cells that could influence the regulation of PDCD4.
There is precedent for post-translational modification of PDCD4 in response to various cellular cues, including methylation of PDCD4 at an N-terminal arginine residue that accelerates tumor growth (51) and promotes tumor cell viability during nutrient deprivation (88). PDCD4 is also phosphorylated at serine 67 upon activation of Akt or S6K1, which leads to significantly decreased PDCD4 protein levels via increased proteasomal degradation (50, 89). We observe a rapid decrease in PDCD4 protein levels upon treatment with ActD (Fig. 5, B and C), consistent with increased activation of S6K1 (70), the kinase responsible for phosphorylation of PDCD4 at serine 67 (50). Alternatively, treatment with ActD could alter the translational profile of PDCD4, perhaps due to an increase in the cytoplasmic pool of the documented translational repressor, TIA1 (Fig. 5C) (40). Although PDCD4 protein levels are rapidly degraded in these cells, we observe an underlying stable pool of PDCD4 mRNA, specifically in the cytoplasm (Fig. 5, A and F). We hypothesize that this stable population of PDCD4 mRNA represents a pool of transcript that is readily available for translation upon extracellular cues and/or alleviation of cellular stress. This reserve would allow cells to fine-tune the expression of PDCD4 protein in response to various cellular environments. The observation that reduction of HuR and/or TIA1 results in reduced PDCD4 mRNA and protein levels (Fig. 7) suggests that HuR and TIA1 play a role in positively regulating steady-state PDCD4 mRNA levels and may therefore assist in modulating the dynamic cytoplasmic pool of PDCD4 mRNA.
Analysis of RNA regulons and the cooperation and/or competition of multiple RNA-binding proteins on target mRNAs suggests a complex and dynamic post-transcriptional regulatory program (2). The observation that RNA-binding proteins such as HuR interface with the miRNA machinery (90) adds additional complexity to the regulation of target mRNAs. In fact, recent studies demonstrate a competitive relationship between HuR and miRNA machinery at both the individual transcript (74, 91–94) and transcriptome-wide (32, 33) levels. The transcriptome-wide PAR-CLIP analyses that examined interplay between HuR and miRNAs suggested that transcripts with nonoverlapping HuR/miRNA-binding sites (such as PDCD4) are more likely regulated by the miRNA than by HuR (32, 33). Our results demonstrate that modulation of HuR and TIA1 impacts the steady-state mRNA and protein levels of PDCD4 and suggests a model where HuR modulates the stability of PDCD4. The mechanism by which HuR modulates stability is not well understood; however, recent studies demonstrate that direct competition between HuR and miRNAs can influence stability (91). Alternatively, HuR binding could influence the secondary structure of the 3′UTR to modulate miRNA binding/accessibility (90). This type of coordination is challenging to study and is therefore less understood than direct competition.
As mentioned previously, the PDCD4 3′UTR is a well studied target of the oncomiR, miR-21 (46, 47), which is overexpressed in many cancer types (45) and targets the PDCD4 transcript via a well studied seed region within the 3′UTR (46). The miR-21 seed region lies within the 290-nt region of the PDCD4 3′UTR that was identified in a HuR iCLIP study (12). However, the miR-21 seed region does not overlap with the HuR-binding sites identified here (Fig. 3) and instead is located more distal to the coding region (miRNA seed region designated by red asterisk in Fig. 3B). If miR-21 is involved in HuR-mediated regulation of PDCD4 stability, the location of the miR-21-binding site is more consistent with a model where HuR impacts the local RNA structure to modulate miRNA binding than direct competition.
In addition to possible modulation of miR-21 binding, growing evidence shows that HuR binds to and regulates the processing of primary miRNA (pri-miR) transcripts (32, 33, 74). A recent study demonstrated HuR binding to and destabilizing miR-7 (32), suggesting that this may represent another mode of regulation by HuR in post-transcriptional processing. Interestingly, another study identified HuR binding to the pri-miR-21 transcript (33), which could indicate direct regulation of the miR-21 transcript by HuR. We did not observe an effect on steady-state miR-21 levels upon knockdown of HuR (Fig. 7C); however, this result does not preclude the possibility that HuR may be involved in the loading of miR-21 onto the PDCD4 transcript. Further study is required to understand how direct interactions and feedback mechanisms between miR-21, as well as other miRNAs predicted to bind the PDCD4 3′UTR, and RNA-binding proteins, such as HuR and TIA1, function coordinately to control post-transcriptional regulation of biologically important transcripts such as PDCD4.
An important question moving forward is how HuR and TIA1 regulate PDCD4 mRNA and protein levels in various cellular contexts. Previous studies demonstrate that both HuR and TIA1 can modulate the post-transcriptional processing of target transcripts in diverse ways in response to stress (65). For instance, HuR and TIA1 contribute to decreased translation of the cytochrome c transcript, specifically in the context of endoplasmic reticulum stress (21). Additionally, exposure of HeLa cells to UV light results in a dramatic association between HuR and the apoptosome inhibitor, prothymosin α mRNA, resulting in increased translation (18). These previous studies as well as others (65) support a model where HuR and TIA1 can have alternative modes of regulation on target transcripts in response to stress. Further study is required to elucidate whether HuR and TIA1 differentially modulate PDCD4 levels in various stress conditions, specifically in the context of the tumor microenvironment.
Acknowledgments
We are grateful to the members of the Corbett and Santangelo laboratories for helpful discussions and contributions to this work. We thank Dr. Myriam Gorospe for supplying the HuR-GFP plasmid used for the PLA studies.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 GM058728 (to A. H. C.), R01 GM094198 and R21 CA147922 (to P. J. S.), and F31 CA168321 and T32 GM008367 (to C. P. W.).
- miRNA
- microRNA
- RACE
- rapid amplification of cDNA ends
- ARE
- AU- or U-rich RNA element
- ANOVA
- analysis of variance
- qRT
- quantitative RT
- ActD
- actinomycin D
- PLA
- proximity ligation assay
- ER
- estrogen receptor
- BM
- binding mutant
- RRM
- RNA recognition motif
- RNA-IP
- RNA-immunoprecipitation
- nt
- nucleotide.
REFERENCES
- 1. Moore M. J. (2005) From birth to death: the complex lives of eukaryotic mRNAs. Science 309, 1514–1518 [DOI] [PubMed] [Google Scholar]
- 2. Keene J. D. (2007) RNA regulons: coordination of post-transcriptional events. Nat. Rev. Genet. 8, 533–543 [DOI] [PubMed] [Google Scholar]
- 3. Cech T. R., Steitz J. A. (2014) The noncoding RNA revolution–trashing old rules to forge new ones. Cell 157, 77–94 [DOI] [PubMed] [Google Scholar]
- 4. Blackinton J. G., Keene J. D. (2014) Post-transcriptional RNA regulons affecting cell cycle and proliferation. Semin. Cell Dev. Biol. 34, 44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan Y. A., Hieter P., Stirling P. C. (2014) Mechanisms of genome instability induced by RNA-processing defects. Trends Genet. 30, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lukong K. E., Chang K. W., Khandjian E. W., Richard S. (2008) RNA-binding proteins in human genetic disease. Trends Genet. 24, 416–425 [DOI] [PubMed] [Google Scholar]
- 7. Abdelmohsen K., Gorospe M. (2010) Posttranscriptional regulation of cancer traits by HuR. Wiley Interdiscip. Rev. RNA 1, 214–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dixon D. A., Balch G. C., Kedersha N., Anderson P., Zimmerman G. A., Beauchamp R. D., Prescott S. M. (2003) Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1. J. Exp. Med. 198, 475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Subramaniam K., Ooi L. L., Hui K. M. (2010) Transcriptional down-regulation of IGFBP-3 in human hepatocellular carcinoma cells is mediated by the binding of TIA-1 to its AT-rich element in the 3′-untranslated region. Cancer Lett. 297, 259–268 [DOI] [PubMed] [Google Scholar]
- 10. Ma W. J., Cheng S., Campbell C., Wright A., Furneaux H. (1996) Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J. Biol. Chem. 271, 8144–8151 [DOI] [PubMed] [Google Scholar]
- 11. Wang H., Zeng F., Liu Q., Liu H., Liu Z., Niu L., Teng M., Li X. (2013) The structure of the ARE-binding domains of Hu antigen R (HuR) undergoes conformational changes during RNA binding. Acta Crystallogr. D Biol. Crystallogr. 69, 373–380 [DOI] [PubMed] [Google Scholar]
- 12. Uren P. J., Burns S. C., Ruan J., Singh K. K., Smith A. D., Penalva L. O. (2011) Genomic analyses of the RNA-binding protein Hu antigen R (HuR) identify a complex network of target genes and novel characteristics of its binding sites. J. Biol. Chem. 286, 37063–37066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abe R., Sakashita E., Yamamoto K., Sakamoto H. (1996) Two different RNA binding activities for the AU-rich element and the poly(A) sequence of the mouse neuronal protein mHuC. Nucleic Acids Res. 24, 4895–4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma W. J., Chung S., Furneaux H. (1997) The Elav-like proteins bind to AU-rich elements and to the poly(A) tail of mRNA. Nucleic Acids Res. 25, 3564–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doller A., Pfeilschifter J., Eberhardt W. (2008) Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell. Signal. 20, 2165–2173 [DOI] [PubMed] [Google Scholar]
- 16. Fan X. C., Steitz J. A. (1998) HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc. Natl. Acad. Sciences of the U.S.A. 95, 15293–15298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brennan C. M., Steitz J. A. (2001) HuR and mRNA stability. Cell. Mol. Life Sci. 58, 266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lal A., Kawai T., Yang X., Mazan-Mamczarz K., Gorospe M. (2005) Antiapoptotic function of RNA-binding protein HuR effected through prothymosin α. EMBO J. 24, 1852–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazan-Mamczarz K., Galbán S., López de Silanes I., Martindale J. L., Atasoy U., Keene J. D., Gorospe M. (2003) RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc. Natl. Acad. Sci. U.S.A. 100, 8354–8359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim H. H., Kuwano Y., Srikantan S., Lee E. K., Martindale J. L., Gorospe M. (2009) HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 23, 1743–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawai T., Lal A., Yang X., Galban S., Mazan-Mamczarz K., Gorospe M. (2006) Translational control of cytochrome c by RNA-binding proteins TIA-1 and HuR. Mol. Cell. Biol. 26, 3295–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu T. X., Rao J. N., Zou T., Liu L., Xiao L., Ouyang M., Cao S., Gorospe M., Wang J. Y. (2013) Competitive binding of CUGBP1 and HuR to occludin mRNA controls its translation and modulates epithelial barrier function. Mol. Biol. Cell 24, 85–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Del Gatto-Konczak F., Bourgeois C. F., Le Guiner C., Kister L., Gesnel M. C., Stévenin J., Breathnach R. (2000) The RNA-binding protein TIA-1 is a novel mammalian splicing regulator acting through intron sequences adjacent to a 5′ splice site. Mol. Cell. Biol. 20, 6287–6299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Förch P., Valcárcel J. (2001) Molecular mechanisms of gene expression regulation by the apoptosis-promoting protein TIA-1. Apoptosis 6, 463–468 [DOI] [PubMed] [Google Scholar]
- 25. Kawakami A., Tian Q., Duan X., Streuli M., Schlossman S. F., Anderson P. (1992) Identification and functional characterization of a TIA-1-related nucleolysin. Proc. Natl. Acad. Sci. U.S.A. 89, 8681–8685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dember L. M., Kim N. D., Liu K. Q., Anderson P. (1996) Individual RNA recognition motifs of TIA-1 and TIAR have different RNA binding specificities. J. Biol. Chem. 271, 2783–2788 [DOI] [PubMed] [Google Scholar]
- 27. Wang Z., Kayikci M., Briese M., Zarnack K., Luscombe N. M., Rot G., Zupan B., Curk T., Ule J. (2010) iCLIP predicts the dual splicing effects of TIA-RNA interactions. PLoS Biol. 8, e1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kedersha N., Cho M. R., Li W., Yacono P. W., Chen S., Gilks N., Golan D. E., Anderson P. (2000) Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151, 1257–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang T., Delestienne N., Huez G., Kruys V., Gueydan C. (2005) Identification of the sequence determinants mediating the nucleo-cytoplasmic shuttling of TIAR and TIA-1 RNA-binding proteins. J. Cell Sci. 118, 5453–5463 [DOI] [PubMed] [Google Scholar]
- 30. Anderson P., Kedersha N. (2002) Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation. Cell Stress Chaperones 7, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kishore S., Jaskiewicz L., Burger L., Hausser J., Khorshid M., Zavolan M. (2011) A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nat. Methods 8, 559–564 [DOI] [PubMed] [Google Scholar]
- 32. Lebedeva S., Jens M., Theil K., Schwanhäusser B., Selbach M., Landthaler M., Rajewsky N. (2011) Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. Cell 43, 340–352 [DOI] [PubMed] [Google Scholar]
- 33. Mukherjee N., Corcoran D. L., Nusbaum J. D., Reid D. W., Georgiev S., Hafner M., Ascano M., Jr., Tuschl T., Ohler U., Keene J. D. (2011) Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol. Cell 43, 327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. López de Silanes I., Zhan M., Lal A., Yang X., Gorospe M. (2004) Identification of a target RNA motif for RNA-binding protein HuR. Proc. Natl. Acad. Sci. U.S.A. 101, 2987–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo X., Hartley R. S. (2006) HuR contributes to cyclin E1 deregulation in MCF-7 breast cancer cells. Cancer Res. 66, 7948–7956 [DOI] [PubMed] [Google Scholar]
- 36. Lal A., Mazan-Mamczarz K., Kawai T., Yang X., Martindale J. L., Gorospe M. (2004) Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 23, 3092–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang W., Caldwell M. C., Lin S., Furneaux H., Gorospe M. (2000) HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 19, 2340–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pryzbylkowski P., Obajimi O., Keen J. C. (2008) Trichostatin A and 5 Aza-2′ deoxycytidine decrease estrogen receptor mRNA stability in ER positive MCF7 cells through modulation of HuR. Breast Cancer Res. Treat. 111, 15–25 [DOI] [PubMed] [Google Scholar]
- 39. Abdelmohsen K., Lal A., Kim H. H., Gorospe M. (2007) Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle 6, 1288–1292 [DOI] [PubMed] [Google Scholar]
- 40. Piecyk M., Wax S., Beck A. R., Kedersha N., Gupta M., Maritim B., Chen S., Gueydan C., Kruys V., Streuli M., Anderson P. (2000) TIA-1 is a translational silencer that selectively regulates the expression of TNF-α. EMBO J. 19, 4154–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang H. S., Jansen A. P., Komar A. A., Zheng X., Merrick W. C., Costes S., Lockett S. J., Sonenberg N., Colburn N. H. (2003) The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol. Cell. Biol. 23, 26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rogers G. W., Jr., Richter N. J., Merrick W. C. (1999) Biochemical and kinetic characterization of the RNA helicase activity of eukaryotic initiation factor 4A. J. Biol. Chem. 274, 12236–12244 [DOI] [PubMed] [Google Scholar]
- 43. Lankat-Buttgereit B., Göke R. (2009) The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biol. Cell 101, 309–317 [DOI] [PubMed] [Google Scholar]
- 44. Cmarik J. L., Min H., Hegamyer G., Zhan S., Kulesz-Martin M., Yoshinaga H., Matsuhashi S., Colburn N. H. (1999) Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc. Natl. Acad. Sci. U.S.A. 96, 14037–14042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pan X., Wang Z. X., Wang R. (2010) MicroRNA-21: a novel therapeutic target in human cancer. Cancer Biol. Ther. 10, 1224–1232 [DOI] [PubMed] [Google Scholar]
- 46. Asangani I. A., Rasheed S. A., Nikolova D. A., Leupold J. H., Colburn N. H., Post S., Allgayer H. (2008) MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27, 2128–2136 [DOI] [PubMed] [Google Scholar]
- 47. Frankel L. B., Christoffersen N. R., Jacobsen A., Lindow M., Krogh A., Lund A. H. (2008) Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J. Biol. Chem. 283, 1026–1033 [DOI] [PubMed] [Google Scholar]
- 48. Leupold J. H., Asangani I. A., Mudduluru G., Allgayer H. (2012) Promoter cloning and characterization of the human programmed cell death protein 4 (pdcd4) gene: evidence for ZBP-89 and Sp-binding motifs as essential Pdcd4 regulators. Biosci. Rep. 32, 281–297 [DOI] [PubMed] [Google Scholar]
- 49. Vikhreva P. N., Shepelev M. V., Korobko I. V. (2014) mTOR-dependent transcriptional repression of Pdcd4 tumor suppressor in lung cancer cells. Biochim. Biophys. Acta 1839, 43–49 [DOI] [PubMed] [Google Scholar]
- 50. Dorrello N. V., Peschiaroli A., Guardavaccaro D., Colburn N. H., Sherman N. E., Pagano M. (2006) S6K1- and βTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314, 467–471 [DOI] [PubMed] [Google Scholar]
- 51. Powers M. A., Fay M. M., Factor R. E., Welm A. L., Ullman K. S. (2011) Protein arginine methyltransferase 5 accelerates tumor growth by arginine methylation of the tumor suppressor programmed cell death 4. Cancer Res. 71, 5579–5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soule H. D., Vazguez J., Long A., Albert S., Brennan M. (1973) A human cell line from a pleural effusion derived from a breast carcinoma. J. Natl. Cancer Inst. 51, 1409–1416 [DOI] [PubMed] [Google Scholar]
- 53. Santangelo P. J., Lifland A. W., Curt P., Sasaki Y., Bassell G. J., Lindquist M. E., Crowe J. E., Jr. (2009) Single molecule-sensitive probes for imaging RNA in live cells. Nat. Methods 6, 347–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jung J., Lifland A. W., Zurla C., Alonas E. J., Santangelo P. J. (2013) Quantifying RNA-protein interactions in situ using modified-MTRIPs and proximity ligation. Nucleic Acids Res. 41, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K. J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L. G., Landegren U. (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 [DOI] [PubMed] [Google Scholar]
- 56. Towbin H., Staehelin T., Gordon J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76, 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 58. Jain R., Devine T., George A. D., Chittur S. V., Baroni T. E., Penalva L. O., Tenenbaum S. A. (2011) RIP-chip analysis: RNA-binding protein immunoprecipitation-microarray (Chip) profiling. Methods Mol. Biol. 703, 247–263 [DOI] [PubMed] [Google Scholar]
- 59. Feng Y., Absher D., Eberhart D. E., Brown V., Malter H. E., Warren S. T. (1997) FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol. Cell 1, 109–118 [DOI] [PubMed] [Google Scholar]
- 60. Irier H. A., Shaw R., Lau A., Feng Y., Dingledine R. (2009) Translational regulation of GluR2 mRNAs in rat hippocampus by alternative 3′ untranslated regions. J. Neurochem. 109, 584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gruber A. R., Fallmann J., Kratochvill F., Kovarik P., Hofacker I. L. (2011) AREsite: a database for the comprehensive investigation of AU-rich elements. Nucleic Acids Res. 39, D66–D69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang H., Li H., Shi H., Liu Y., Liu H., Zhao H., Niu L., Teng M., Li X. (2011) Preliminary crystallographic analysis of the RNA-binding domain of HuR and its poly(U)-binding properties. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 67, 546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Santangelo P. J., Alonas E., Jung J., Lifland A. W., Zurla C. (2012) Probes for intracellular RNA imaging in live cells. Methods Enzymol. 505, 383–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peng S. S., Chen C. Y., Xu N., Shyu A. B. (1998) RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 17, 3461–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. von Roretz C., Di Marco S., Mazroui R., Gallouzi I. E. (2011) Turnover of AU-rich-containing mRNAs during stress: a matter of survival. Wiley Interdiscip. Rev. RNA 2, 336–347 [DOI] [PubMed] [Google Scholar]
- 66. Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. (1984) Extreme instability of myc mRNA in normal and transformed human cells. Proc. Natl. Acad. Sci. U.S.A. 81, 7046–7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hurwitz J., Furth J. J., Malamy M., Alexander M. (1962) The role of deoxyribonucleic acid in ribonucleic acid synthesis. III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc. Natl. Acad. Sci. U.S.A. 48, 1222–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tani H., Mizutani R., Salam K. A., Tano K., Ijiri K., Wakamatsu A., Isogai T., Suzuki Y., Akimitsu N. (2012) Genome-wide determination of RNA stability reveals hundreds of short-lived noncoding transcripts in mammals. Genome Res. 22, 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schwanhäusser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., Chen W., Selbach M. (2011) Global quantification of mammalian gene expression control. Nature 473, 337–342 [DOI] [PubMed] [Google Scholar]
- 70. Loreni F., Thomas G., Amaldi F. (2000) Transcription inhibitors stimulate translation of 5′ TOP mRNAs through activation of S6 kinase and the mTOR/FRAP signalling pathway. Eur. J. Biochem. 267, 6594–6601 [DOI] [PubMed] [Google Scholar]
- 71. Yang E., van Nimwegen E., Zavolan M., Rajewsky N., Schroeder M., Magnasco M., Darnell J. E., Jr. (2003) Decay rates of human mRNAs: correlation with functional characteristics and sequence attributes. Genome Res. 13, 1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dormoy-Raclet V., Ménard I., Clair E., Kurban G., Mazroui R., Di Marco S., von Roretz C., Pause A., Gallouzi I. E. (2007) The RNA-binding protein HuR promotes cell migration and cell invasion by stabilizing the β-actin mRNA in a U-rich-element-dependent manner. Mol. Cell. Biol. 27, 5365–5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Simone L. E., Keene J. D. (2013) Mechanisms coordinating ELAV/Hu mRNA regulons. Curr. Opin. Genet. Dev. 23, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Young L. E., Moore A. E., Sokol L., Meisner-Kober N., Dixon D. A. (2012) The mRNA stability factor HuR inhibits microRNA-16 targeting of COX-2. Mol. Cancer Res. 10, 167–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Davoren P. A., McNeill R. E., Lowery A. J., Kerin M. J., Miller N. (2008) Identification of suitable endogenous control genes for microRNA gene expression analysis in human breast cancer. BMC Mol. Biol. 9, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Abdelmohsen K., Panda A. C., Kang M. J., Guo R., Kim J., Grammatikakis I., Yoon J. H., Dudekula D. B., Noh J. H., Yang X., Martindale J. L., Gorospe M. (2014) 7SL RNA represses p53 translation by competing with HuR. Nucleic Acids Res. 42, 10099–10111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pullmann R., Jr., Kim H. H., Abdelmohsen K., Lal A., Martindale J. L., Yang X., Gorospe M. (2007) Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol. Cell. Biol. 27, 6265–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shibahara K., Asano M., Ishida Y., Aoki T., Koike T., Honjo T. (1995) Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene 166, 297–301 [DOI] [PubMed] [Google Scholar]
- 79. Yang H. S., Jansen A. P., Nair R., Shibahara K., Verma A. K., Cmarik J. L., Colburn N. H. (2001) A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-κB or ODC transactivation. Oncogene 20, 669–676 [DOI] [PubMed] [Google Scholar]
- 80. Schlichter U., Burk O., Worpenberg S., Klempnauer K. H. (2001) The chicken Pdcd4 gene is regulated by v-Myb. Oncogene 20, 231–239 [DOI] [PubMed] [Google Scholar]
- 81. Fan H., Zhao Z., Quan Y., Xu J., Zhang J., Xie W. (2007) DNA methyltransferase 1 knockdown induces silenced CDH1 gene reexpression by demethylation of methylated CpG in hepatocellular carcinoma cell line SMMC-7721. Eur. J. Gastroenterol. Hepatol. 19, 952–961 [DOI] [PubMed] [Google Scholar]
- 82. Kim J., Park R. Y., Chen J. K., Kim J., Jeong S., Ohn T. (2014) Splicing factor SRSF3 represses the translation of programmed cell death 4 mRNA by associating with the 5′-UTR region. Cell Death Differ. 21, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zuker M. (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Izquierdo J. M. (2008) Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. J. Biol. Chem. 283, 19077–19084 [DOI] [PubMed] [Google Scholar]
- 85. Zhu H., Hasman R. A., Barron V. A., Luo G., Lou H. (2006) A nuclear function of Hu proteins as neuron-specific alternative RNA processing regulators. Mol. Biol. Cell 17, 5105–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhu H., Hinman M. N., Hasman R. A., Mehta P., Lou H. (2008) Regulation of neuron-specific alternative splicing of neurofibromatosis type 1 pre-mRNA. Mol. Cell. Biol. 28, 1240–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kulikov A. V., Shilov E. S., Mufazalov I. A., Gogvadze V., Nedospasov S. A., Zhivotovsky B. (2012) Cytochrome c: the Achilles' heel in apoptosis. Cell. Mol. Life Sci. 69, 1787–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fay M. M., Clegg J. M., Uchida K. A., Powers M. A., Ullman K. S. (2014) Enhanced arginine methylation of programmed cell death 4 protein during nutrient deprivation promotes tumor cell viability. J. Biol. Chem. 289, 17541–17552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schmid T., Jansen A. P., Baker A. R., Hegamyer G., Hagan J. P., Colburn N. H. (2008) Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res. 68, 1254–1260 [DOI] [PubMed] [Google Scholar]
- 90. Srikantan S., Tominaga K., Gorospe M. (2012) Functional interplay between RNA-binding protein HuR and microRNAs. Curr. Protein Pept. Sci. 13, 372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bhattacharyya S. N., Habermacher R., Martine U., Closs E. I., Filipowicz W. (2006) Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125, 1111–1124 [DOI] [PubMed] [Google Scholar]
- 92. Epis M. R., Barker A., Giles K. M., Beveridge D. J., Leedman P. J. (2011) The RNA-binding protein HuR opposes the repression of ERBB-2 gene expression by microRNA miR-331-3p in prostate cancer cells. J. Biol. Chem. 286, 41442–41454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Srikantan S., Abdelmohsen K., Lee E. K., Tominaga K., Subaran S. S., Kuwano Y., Kulshrestha R., Panchakshari R., Kim H. H., Yang X., Martindale J. L., Marasa B. S., Kim M. M., Wersto R. P., Indig F. E., Chowdhury D., Gorospe M. (2011) Translational control of TOP2A influences doxorubicin efficacy. Mol. Cell. Biol. 31, 3790–3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tominaga K., Srikantan S., Lee E. K., Subaran S. S., Martindale J. L., Abdelmohsen K., Gorospe M. (2011) Competitive regulation of nucleolin expression by HuR and miR-494. Mol. Cell. Biol. 31, 4219–4231 [DOI] [PMC free article] [PubMed] [Google Scholar]