Background: The role of Trk neurotrophin receptors in glioma is unknown.
Results: TrkB and TrkC are required for survival of brain tumor-initiating cells in the absence of EGF and FGF.
Conclusion: Trk receptors can control the survival of BTICs in the absence of EGF and FGF.
Significance: Trks may be important targets for treatment of malignant gliomas.
Keywords: Brain-derived Neurotrophic Factor (BDNF), Cancer Stem Cells, Extracellular Signal-regulated Kinase (ERK), Glioblastoma, Neurotrophic Factor, Neurotrophin 3, Neurotrophin Receptor, Trk Receptor
Abstract
Neurotrophins and their receptors are frequently expressed in malignant gliomas, yet their functions are largely unknown. Previously, we have shown that p75 neurotrophin receptor is required for glioma invasion and proliferation. However, the role of Trk receptors has not been examined. In this study, we investigated the importance of TrkB and TrkC in survival of brain tumor-initiating cells (BTICs). Here, we show that human malignant glioma tissues and also tumor-initiating cells isolated from fresh human malignant gliomas express the neurotrophin receptors TrkB and TrkC, not TrkA, and they also express neurotrophins NGF, BDNF, and neurotrophin 3 (NT3). Specific activation of TrkB and TrkC receptors by ligands BDNF and NT3 enhances tumor-initiating cell viability through activation of ERK and Akt pathways. Conversely, TrkB and TrkC knockdown or pharmacologic inhibition of Trk signaling decreases neurotrophin-dependent ERK activation and BTIC growth. Further, pharmacological inhibition of both ERK and Akt pathways blocked BDNF, and NT3 stimulated BTIC survival. Importantly, attenuation of BTIC growth by EGFR inhibitors could be overcome by activation of neurotrophin signaling, and neurotrophin signaling is sufficient for long term BTIC growth as spheres in the absence of EGF and FGF. Our results highlight a novel role for neurotrophin signaling in brain tumor and suggest that Trks could be a target for combinatorial treatment of malignant glioma.
Introduction
Neurotrophins are secreted proteins that control diverse processes in neurogenesis such as neuronal survival, growth, and differentiation depending on cellular context (1, 2). There are four mammalian neurotrophins, such as NGF, BDNF, neurotrophin 3 (NT3),4 and neurotrophin 4 (NT4), known to bind to two distinct types of receptors: Trk (tropomyosin receptor kinase) receptors and the p75 neurotrophin receptor (p75NTR). NGF has preferential affinity for TrkA, BDNF and NT4 for TrkB, and NT3 for TrkC. Binding of a neurotrophin to Trk receptors leads to promotion of cell survival, proliferation, and differentiation. Each neurotrophin also binds to p75NTR and activates multiple signaling pathways that can enhance neuronal survival or induce cell death depending on cellular context (1, 2).
Glioblastomas (GBMs) are aggressive brain tumors and highly resistant to treatment (3). How gliomas arise is not well known. Stem-like brain tumor-initiating cells (BTICs) within the tumors are implicated in tumor formation and progression (4). We recently showed that p75NTR is required for glioma stem cell invasion (5, 6) and proliferation (7). However, the roles of Trk receptors in malignant glioma are not well known. Here, we found that TrkB and TrkC are expressed in human glioma tissues and also in human GBM-derived BTICs. We took loss of function and pharmacologic approaches to show that Trk receptor signaling promotes BTIC viability through MAP kinase and Akt (serine/threonine-specific protein kinase) pathways. Furthermore, activation of Trk receptors in BTICs can circumvent the effects of pharmacologic EGFR inhibition or growth factor deprivation. Our results have implications for mechanisms of BTIC survival and therapeutic resistance and suggest that Trk receptor blockade may be of clinical utility in combination with other inhibitors.
EXPERIMENTAL PROCEDURES
Tumor Samples
Snap-frozen human glioma tissues (15 males and 8 females) were obtained from the Moffitt Cancer Center after obtaining informed consent and in accordance with guidelines and permission from the institutional review board. Samples were from human glioma patients who were either previously irradiated or newly diagnosed and were graded according to the World Health Organization.
Cell Culture and Reagents
BTIC lines isolated from human gliomas were cultured in Neurocult NS-A basal medium (human) with heparin solution and proliferation supplements (Stem Cell Technologies), 20 ng/ml EGF and FGF2 (Stem Cell Technologies) unless otherwise stated. Neurotrophins were obtained as follows: NGF from Alomone Labs (catalog no. N-245), BDNF from Peprotech (catalog no. 450-02), and NT3 from Millipore (catalog no. GF308). The inhibitors used were as follows: GW441756 (Tocris Bioscience, catalog no. 2238), erlotinib (generously provided by Dr. Narendran, University of Calgary), MEK inhibitor PD0325901 (Cayman Biochemical, catalog no. 13034), or Akt inhibitor MK2206 (Chemietek, catalog no. CT-MK2206).
RT-PCR
Total RNA was isolated with RNeasy mini kit (Qiagen) per the manufacturer's protocol and was reverse transcribed into cDNA with SuperScript VILO cDNA synthesis kit (Invitrogen). PCR was performed using Platinum PCR super mix high fidelity (Invitrogen) and specific primer sets against human NGFR/p75NTR (Qiagen, catalog no. QT00056756, NM_002507, 118 bp), NTRK1/TrkA (Qiagen, catalog no. QT00054110, NM_002529, 112 bp), NTRK2/TrkB (Qiagen, catalog no. QT00082033, NM_006180, 103 bp), NTRK3/TrkC (Qiagen, catalog no. QT00052906, NM_002530, 143 bp), NGF (Qiagen, catalog no. QT00001589, NM_002506, 73 bp), BDNF (Qiagen, catalog no. QT00235368, NM_001143805, 120 bp), NT3 (Qiagen, catalog no. QT00204218, NM_001102654, 104 bp), and human actin B (Qiagen, catalog no. QT01680476, NM_001101, 104 bp). PCR conditions were: 5 min at 95 °C for initial PCR activation, 35 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min, and 72 °C for 10 min for final extension. Human brain total RNA (Clontech) was used as a positive control. PCR products were run on a 2% agarose gel. Quantitative PCR for TrkA expression was performed with real time PCR detection system (Bio-Rad) using cDNA, SYBR green PCR master mix (Applied Biosystem, catalog no. 4309155), and primers for TrkA (Qiagen, catalog no. QT00054110, NM_002529, 112 bp) and actin (Qiagen, catalog no. QT01680476, NM_001101, 104 bp), and expression was calculated as ΔΔCt, which is normalized to actin.
TrkB and TrkC Knockdown
Lentiviral plasmids (pLKO.1) containing shRNA sequences to TrkB and TrkC and a nontargeting sequences were obtained from Sigma. Five shRNA sequences per gene were assessed for target knockdown. The most effective sequences used in this study were as follows: TrkB, CCACTCCATCACATCTCCAAT; and TrkC, CACGGACATCTCAAGGAATAT. Lentiviral particles were prepared according to standard protocols using HEK293METR cells transfected with the pLKO.1 plasmid of interest and the viral packaging plasmids VSVG and R8.91. Virus was added to BTICs, and after 48 h infected cells were selected with puromycin (1 μg/ml), and the polyclonal cell population expanded.
Growth Factor Stimulation
Dissociated cells were seeded into 48-well plates at 100,000 cells per well and cultured overnight in medium lacking additional growth factors such as EGF and FGF. The indicated growth factors/neurotrophic factors were added. Where indicated, cells were treated with inhibitors for 1 h prior to growth factor stimulation. Cells were harvested and lysed in radioimmune precipitation assay buffer (10 mm Tris-HCl, 1 mm EDTA, 0.4 mm EGTA, 0.1% SDS, 140 mm sodium chloride, 0.1% sodium deoxycholate, 1% Triton X-100, and added with 1 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, aprotinin, and leupeptin) for Western blotting.
Western Blotting
BTICs were cultured and treated with appropriately, cells were lysed in radioimmune precipitation assay buffer, and lysates were subjected to Western blotting analysis for using antibodies for TrkA, 14G6 rabbit mAb (Cell Signaling, catalog no. 2508), TrkB, 80E3, rabbit mAb (Cell Signaling, catalog no. 4603), TrkC, C44H5, rabbit mAb (Cell Signaling, catalog no. 3376), phospho-TrkC, p-Tyr-820 (Novus Biologicals, catalog no. NBP1-03448), phospho-ERK rabbit polyclonal antibody (Cell Signaling, catalog no. 9101), p-Akt, T308 (Cell Signaling, catalog no. 4056), and β-actin antibody (Cell Signaling, catalog no. 4967).
Viability Assay
Dissociated BTICs were seeded into 96-well plates at 10,000 cells in 100 μl per well in medium lacking added growth factors EGF and FGF. The indicated growth factors/neurotrophic factors and inhibitors were added. After 7 days, viability was measured using the Alamar Blue assay per manufacturer's instructions. Viability is expressed as a percentage increase over control cells not treated with growth factor/neurotrophic factor.
Gene Expression Analysis
Gene expression analyses from two different data sets were used in this study. One contains microarray expression from the Cancer Genome Atlas data portal, and level 3 expression data on Affymetrix U133A chip was used in this analysis. The other data set contains microarray expression data from Moffitt's Total Cancer Care program. A customized Affymetrix chip was used to generate the expression profile, and MAS5 was used to normalize the expression data. R package was used to analyze gene expression data in which gene expression level was categorized into three groups: up-regulated, intermediate and down-regulated according the quartile of normalized expression data.
RESULTS
Human Gliomas and BTICs Express Neurotrophin Receptors and Ligands
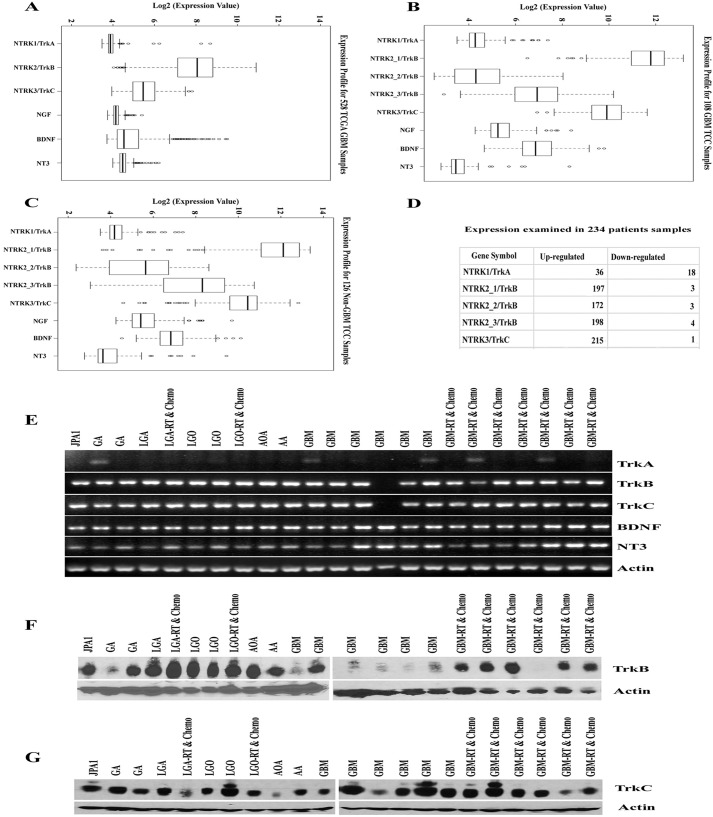
Previous studies using immunohistochemical analyses on limited numbers of gliomas have found that gliomas express neurotrophin receptors (8–10). To validate those findings and to further determine the frequency and type of neurotrophins expressed, we examined microarray gene expression data from 528 GBM tissues in the Cancer Genome Atlas data set (Ref. 11 and Fig. 1A) and 108 GBM (Fig. 1B) and 126 non-GBM tissues (Fig. 1C) patients from Moffitt Cancer Center (MCC) data set. In all data sets, TrkB and TrkC, levels were elevated, but TrkA expression levels were considerably low (Fig. 1, A–C). Further, individual patient microarray data analysis of 234 glioma patient samples from MCC data demonstrates that TrkB was up-regulated in 172–198 patient samples; TrkC was up-regulated in 215 patient samples. Interestingly, TrkA was up-regulated in only 36 and down-regulated in 18 patient samples (Fig. 1D). We further validated the expression of Trk receptors in glioma specimens using RT-PCR and Western blotting of tumors from 23 human glioma patients (15 males and 8 females) ranging from grade I to IV. As anticipated, TrkB and TrkC receptors and their ligands, BDNF and NT3, were expressed in almost every tumor samples analyzed (Fig. 1, E–G); these samples also express NGF and p75NTR (7). In contrast, TrkA was weakly expressed in only 5 of 23 tumor specimens (Fig. 1E). These results indicate that TrkB and Trk C are expressed nearly universally in human gliomas, spanning various types and grades of tumor.
FIGURE 1.
Trk receptors and their ligands are expressed in malignant glioma patient tumor specimens. A–C, we examined the microarray data set of 528 GBM patients from the Cancer Genome Atlas (A) and an independent data set from the MCC of 108 GBM patients (B) and 126 non-GBM patients (C) for expression analysis of TrkA/NTRK1, TrkB/NTRK2, TrkC/NTRK3, NGF, BDNF, and NT3. The MCC data set used three probe sets for NTRK2/TrkB. We considered that values above 2 of log2 expression value are significantly higher than the baseline expression. D, examination of up- and down-regulation of Trk receptors in 234 malignant gliomas using individual microarray expression data from the MCC data set. E, RT-PCR analysis for TrkA, TrkB, TrkC, BDNF, NT3, and actin from frozen glioma tissues using human specific primers. F and G, frozen tumor specimen were lysed and subjected to TrkB (F) and TrkC (G) Western blotting analysis and actin was used as a loading control. JPA1, juvenile pilocytic astrocytoma; GA, gemistocytic astrocytoma; LGA, low grade astrocytoma; LGO, low grade oligodendroglioma; AOA, anaplastic oligoastrocytoma; AA, anaplastic astrocytoma; GBM, glioblastoma multiforme; RT & Chemo, patient tumor treated with radiation and temozolomide (chemotherapy) before surgery.
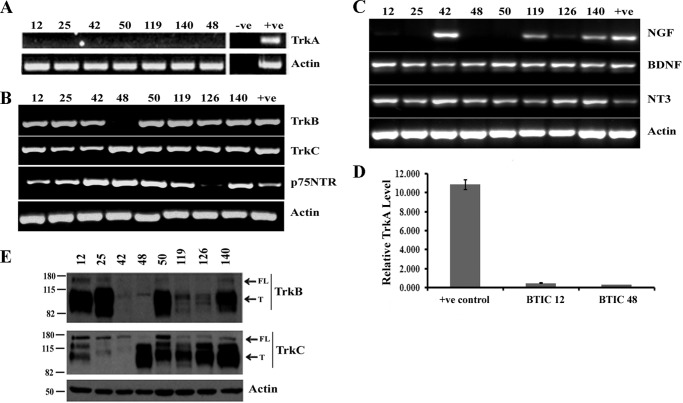
Because the stem cell-like compartment of gliomas has been implicated in tumor maintenance, progression, and recurrence (4, 12, 13), we therefore determined whether BTICs similarly express neurotrophin receptors. We have used GBM-derived BTICs, which were characterized previously (14), for examining neurotrophin receptors and their ligands. RT-PCR analysis showed that TrkC, p75NTR, BDNF, and NT3 were expressed in all BTIC lines, whereas TrkB was expressed in seven lines, and NGF was expressed in four lines (Fig. 2, B and C). None of the lines expressed TrkA (Fig. 2A) and even quantitative PCR analysis demonstrated that TrkA is not expressed in BTICs we examined (Fig. 2D). Western blotting analysis confirmed the expression of both the full-length and the truncated forms of TrkB and TrkC proteins (Fig. 2E). These data indicate that neurotrophins and their receptors are not only present in bulk tumor tissues but also commonly expressed in BTICs.
FIGURE 2.
Neurotrophins and their receptors are expressed in brain tumor-initiating cells. A–C, total RNA was isolated from BTICs, cDNA was synthesized, and RT-PCR analysis was performed for neurotrophin receptors, such as TrkA (A) or TrkB, TrkC, and p75NTR (B), and also for neurotrophic factors NGF, BDNF, and NT3 (C); actin was used as an internal control. Human brain total RNA was used as positive control (+ve). D, TrkA expression was not detected by RT-PCR, quantitative PCR analysis was conducted, and expression of TrkA levels was calculated as ΔΔCt against the actin, an internal control. G179 cells express TrkA were used as a positive control (7) (n = 3 independent experiments; p < 0.05). E, lysates from BTICs were subjected to Western blot analysis for TrkB and TrkC with actin as a loading control. T, truncated receptor; FL, full-length receptor. The data are representative of three independent experiments.
Neurotrophins Promote BTIC Viability and ERK Activation
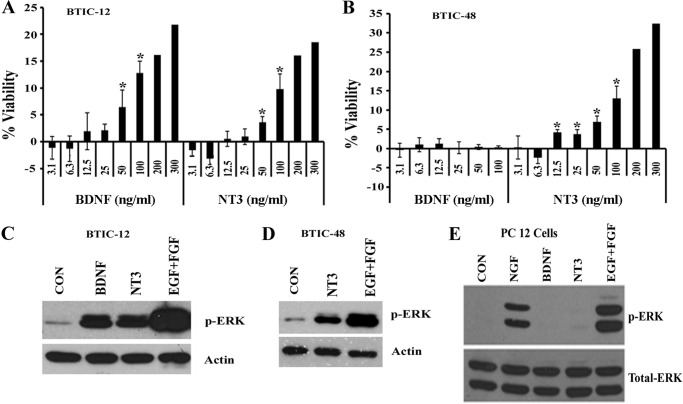
Neurotrophins and their receptors are known to regulate neuronal survival during development (2) and also in some cancers (15–17). Although we recently showed that neurotrophin receptor p75 promotes BTIC invasion (5, 6) and proliferation (7), the roles of Trk receptors in glioma stem cell biology are poorly understood. To address whether Trk receptor activation affects BTIC viability, we selected two BTIC lines that express both TrkB and TrkC (BTIC-12) or solely TrkC (BTIC-48) We treated dissociated BTIC-12 and BTIC-48 cultures with varying concentrations of neurotrophins BDNF and NT3 (in the absence of other growth factors such as EGF and FGF) and examined cell viability 7 days later using Alamar Blue assay (Fig. 3, A and B). As expected, BTIC-12 that expressed both TrkB and TrkC (Fig. 2B) showed increased viability in response to BDNF and NT3 (Fig. 3A). Similarly, BTIC-48, which expressed TrkC but not TrkB (Fig. 2B), showed increased viability in response to NT3 but not to BDNF (Fig. 3B). Thus, the growth stimulatory effects of each neurotrophin on BTICs correlated with the expression of specific Trk receptors.
FIGURE 3.
Neurotrophins BDNF and NT3 stimulate survival of brain tumor-initiating cells and phosphorylation of ERK. A and B, BTIC-12 (A) and BTIC-48 (B) were treated once with varying concentrations (ng/ml) of BDNF and NT3 for 7 days in the absence of other added growth factors (EGF and FGF). The effect on cell viability was measured using the Alamar Blue assay, and viability was expressed as a percentage increase over cells not treated with growth factors/neurotrophic factors. The data are means ± S.E. of two-four independent experiments (*, p < 0.05). C and D, BTIC-12 (C) and BTIC-48 (D) were deprived of growth factors overnight and then treated with BDNF, NT3, and EGF + FGF for 10 min and lysed; then p-ERK and actin Western blotting were performed. E, PC12 cells were serum-starved overnight and treated with NGF, BDNF and NT3, or EGF (100 ng/ml); cells were lysed; and ERK phosphorylation was measured. The data are representative of at least three independent experiments. CON, control.
A key pro-growth signaling downstream of Trk activation is the MAP kinase pathway (18). We therefore measured phospho-ERK levels in response to neurotrophin stimulation in BTICs. The addition of BDNF or NT3 to BTIC-12 increased p-ERK levels, correlating with the expression of TrkB and TrkC and their increased viability in response to BDNF and NT3 (Figs. 2B and 3, A and C). Similarly, BTIC-48, which expresses TrkC not TrkB (Fig. 2B), showed elevated p-ERK in response to NT3 (Fig. 3D). None of the BTICs we examined express TrkA, and NGF did not stimulate p-ERK levels in these lines (data not shown), although NGF did stimulate p-ERK in PC12 cells (Fig. 3E), which express receptor TrkA (19), suggesting that NGF is able to stimulate p-ERK when its receptor TrkA is expressed. These results suggest that, similar to neurons, Trk stimulation activates MAP kinase pathways in BTICs and promotes their viability.
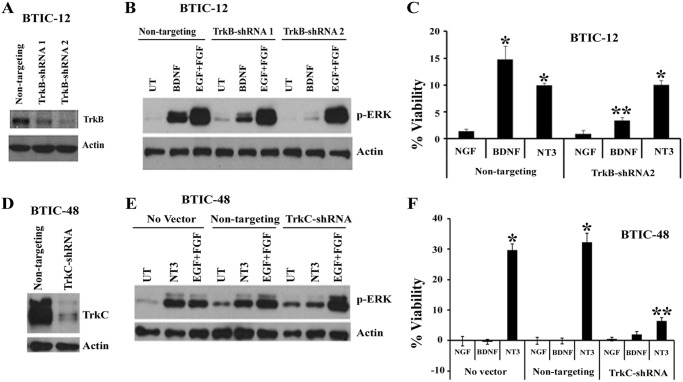
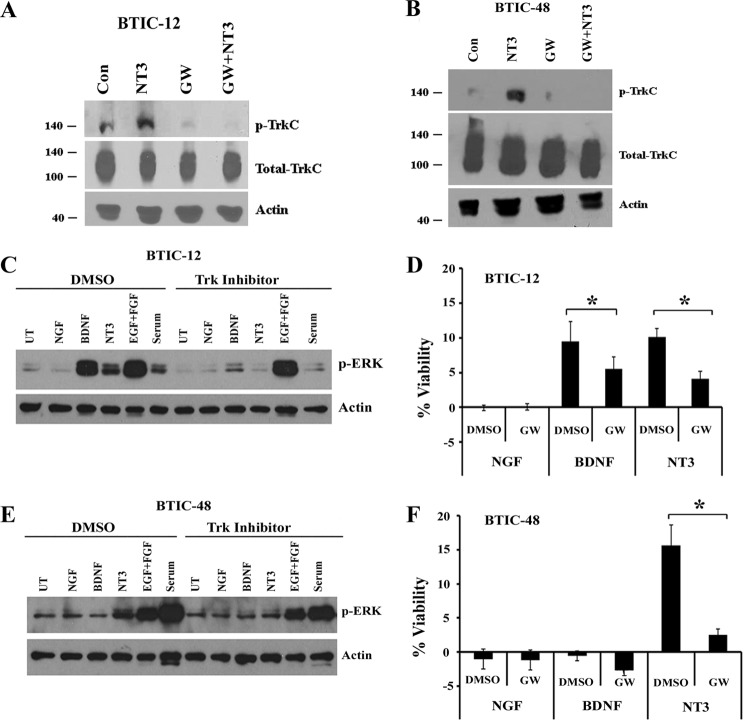
Knockdown of Trk Receptors or Inhibition of Its Signaling Attenuates Neurotrophin-dependent ERK Activation and BTIC Viability
To determine whether Trk receptors are necessary for BTIC growth in response to neurotrophins, we used shRNAs and a pharmacological inhibitor to down-regulate TrkB and TrkC receptor expression and to block their functions. First, we infected the BTICs with lentiviral particles expressing nontargeting control shRNA, TrkB-shRNA, or TrkC-shRNAs and examined the effect on knockdown; shRNAs dramatically knocked down the expression of TrkB and TrkC protein levels (Fig. 4, A and D). Then we examined the effect on neurotrophin-dependent ERK activation and BTIC viability. Knockdown of TrkB (Fig. 4A) and TrkC (Fig. 4D) completely attenuated the ERK phosphorylation and viability in response to neurotrophin treatment (Fig. 4, B–F). In BTIC-12, both BDNF and NT3 increased the cell viability, but knockdown of TrkB had no effect on NT3 stimulated viability, confirming the specificity of receptors and their ligands action in BTICs (Fig. 4C). Furthermore, to address whether kinase activity of the Trk receptor is necessary for BTIC growth, we treated the cells with the highly specific Trk inhibitor GW441756 (20) and then applied BDNF or NT3 and examined the effect on Trk phosphorylation, ERK activation, and cell viability. The Trk inhibitor GW441756 efficiently blocked NT3 stimulated TrkC phosphorylation in BTIC-12 and BTIC-48 (Fig. 5, A and B) and abolished both BDNF and NT3 stimulated ERK activation and BTIC growth in BTIC-12 (Fig. 5, C and D) and BTIC-48 (Fig. 5, E and F), with no effect on EGF- and FGF-mediated ERK signaling (Fig. 5, C and E). Together, these data support a critical role for TrkB and TrkC receptor activation in ERK stimulation and promoting BTIC growth in response to neurotrophins BDNF and NT3.
FIGURE 4.
Knockdown of TrkB and TrkC receptors attenuates ERK phosphorylation and BTIC survival. BTIC-12 (A–C) and BTIC-48 (D–F) were infected with lentivirus expressing nontargeting-shRNA, TrkB-shRNA or TrkC-shRNA and performed TrkB (A) and TrkC (D) Western blotting. TrkB (B) and TrkC (E) lentiviral-shRNA infected cells were starved of growth factors overnight and treated with BDNF or NT3 (100 ng/ml) for 10 min, and p-ERK levels were analyzed. Lentiviral-shRNA infected BTIC-12 (C) and BTIC-48 (F) were treated once with BDNF or NT3 (100 ng/ml) for 7 days, then viability was assessed, and viability was expressed as a percentage increase over cells not treated with growth factors/neurotrophic factors. The data are means ± S.E. of three independent experiments (*, p < 0.05). UT, untreated.
FIGURE 5.
Trk inhibitor blocks neurotrophin-dependent phosphorylation of Trk receptor and attenuates ERK phosphorylation and BTIC survival. BTIC-12 and BTIC-48 were withdrawn from growth factor overnight and treated with the Trk inhibitor GW441756 (1 μm), and 1 h later cells were added with NGF, BDNF, and NT3 (100 ng/ml) for 10 min. Then cells were lysed and measured phospho-TrkC (A and B) and p-ERK levels (C and E). Cell viability assays were also performed as previously in the presence or absence of a single treatment of GW441756 (1 μm) or NGF, BDNF, and NT3 (D and F) (n = 3 independent experiments; *, p < 0.050). ** indicates the significant difference between control shRNA and Trk-shRNA groups. Con, control; GW, GW441756; UT, untreated.
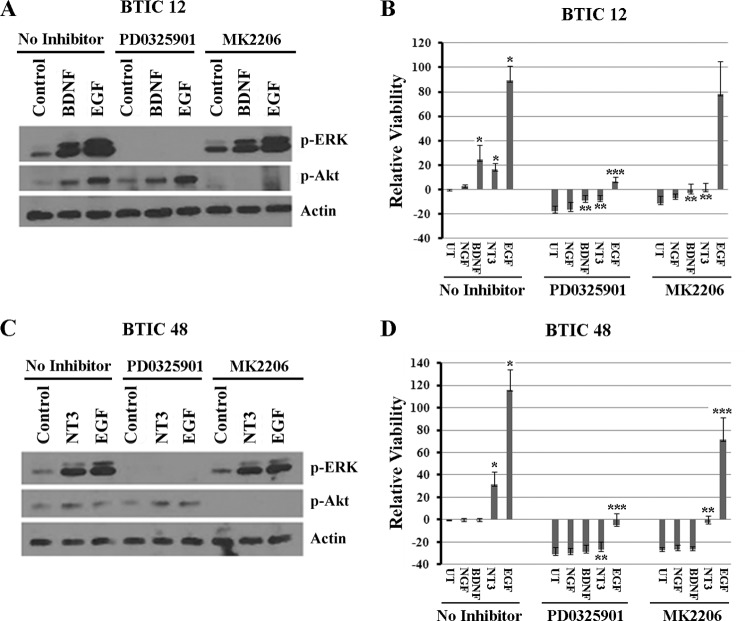
Inhibition of ERK and Akt Signaling Attenuates Neurotrophin-dependent BTIC Viability
We have shown that stimulation of Trk receptors by neurotrophins BDNF and NT3 leads to activation of ERK pathway (Figs. 3–5). In addition, PI3K/Akt pathway is also shown to be important for glioma stem cell survival and tumor development (21, 22). To determine whether ERK and Akt activation are required for BDNF- and NT3-mediated BTIC growth, we used MEK inhibitor PD0325901 and Akt inhibitor MK2206. We pretreated the BTIC lines 12 and 48 with the PD0325901 or MK2206 for 1 h and then stimulated the cells with BDNF or NT3 and examined the effect on ERK and Akt phosphorylation and BTIC viability. The MEK inhibitor PD0325901 and Akt inhibitor MK2206 completely blocked the BDNF- and NT3-stimulated ERK and Akt phosphorylation (Fig. 6, A and C) and cell viability that was measured 4 days after treatment (Fig. 6, B and D) in both BTIC-12 and 48. In case of EGF treatment, however, MK2206 completely blocked the Akt phosphorylation, but the effect on cell viability was not significant (Fig. 6). This could be due to very strong activation of MAP kinase pathway by EGF, which is sufficient for cell survival in the absence of Akt pathway activation. These results indicate that neurotrophin mediated BTIC growth requires both ERK and Akt pathways.
FIGURE 6.
Trk inhibitor blocks neurotrophin-dependent phosphorylation of Trk receptor and attenuates ERK phosphorylation and BTIC survival. BTIC-12 (A and B) and BTIC-48 (C and D) were withdrawn of growth factors overnight and treated with the MEK inhibitor PD0325901 (100 nm) or Akt inhibitor MK2206 (1 μm); 1 h later cells were added with BDNF and NT3 (100 ng/ml) for 10 min. Then cells were lysed, and p-ERK and p-Akt levels were measured (A and C). The effects of these inhibitors on cell viability were also measured in the presence or absence of a single treatment of PD0325901 (100 nm) or MK2206 (1 μm) and neurotrophins NGF, BDNF, and NT3, and EGF for 4 days (B and D). * indicates statistical significance between neurotrophin and EGF treatment compared with untreated; ** shows the difference between neurotrophin treatment with and without MEK and Akt inhibitors; and *** indicates the difference between EGF treatment with and without MEK and Akt inhibitors (p < 0.05). UT, untreated.
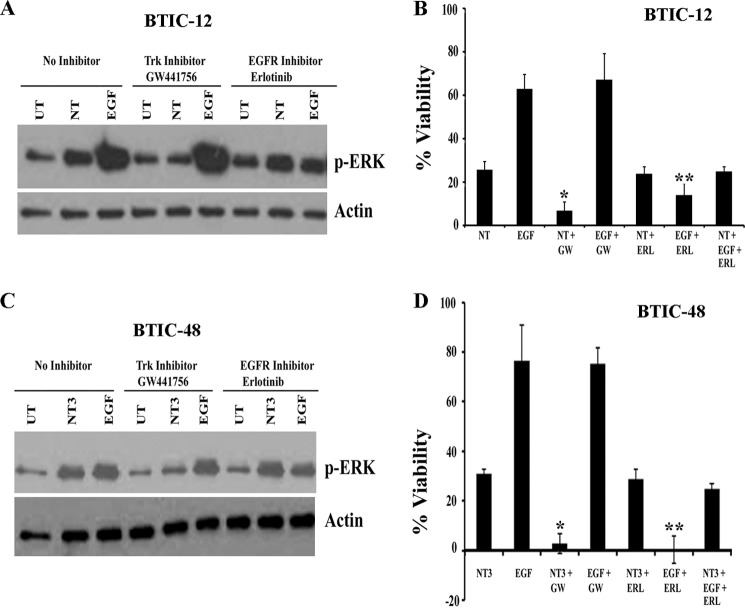
Neurotrophin Signaling Maintains BTIC Growth in the Absence of EGF and FGF Signaling
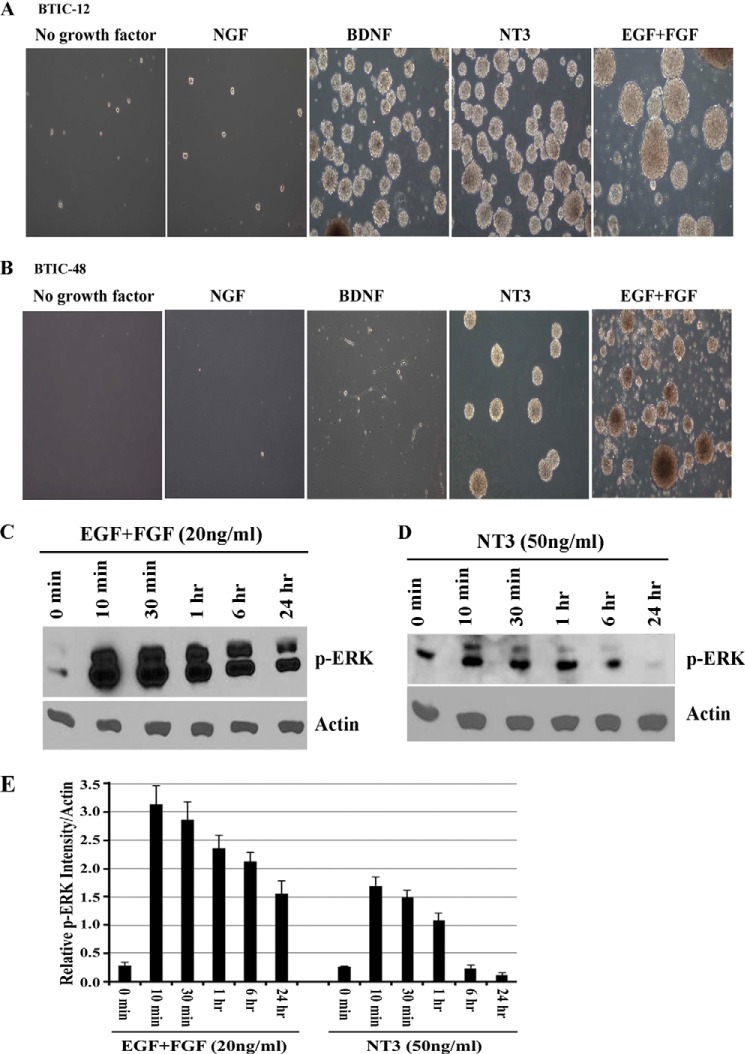
Because activation of receptor tyrosine kinase (RTK) pathways such as the EGFR pathway is common in GBM (23), several RTK inhibitors such as erlotinib have been developed and tested for efficacy in GBM with the aim of targeting specific growth factor receptor dependence. However, GBM responses to erlotinib and other RTK inhibitors have been disappointing (24, 25). Compensatory signaling via alternative RTK pathways is one explanation for the poor clinical performance of such inhibitors (26). Neurotrophin signaling via Trk receptors mediates BTIC viability (Figs. 3–5). We hypothesized that neurotrophin signaling through Trk receptors might sustain growth of BTICs when EGFR signaling was blocked or these cells were deprived of EGF and FGF signaling. To test this concept, we pretreated BTIC-12 and BTIC-48 with the EGFR inhibitor erlotinib or the Trk inhibitor GW441756, then stimulated with exogenous EGF, NT3+BDNF (for BTIC-12) or NT3 (for BTIC-48), and monitored ERK activation and BTIC growth. Measurement of p-ERK levels showed that although erlotinib effectively inhibited EGF-mediated ERK stimulation, it had no effect on neurotrophin-mediated stimulation (Fig. 7, A and C). In contrast, Trk inhibitor GW441756 had no effect on EGF stimulated p-ERK levels (Fig. 7, A and C). Erlotinib attenuated the growth effects of EGF, but not on neurotrophin-induced growth (Fig. 7, B and D). Conversely, GW441756 blocked the growth-promoting effect of neurotrophins but had no effect on EGF-stimulated growth. Importantly, neurotrophin treatment stimulates BTIC viability when EGFR signaling was blocked by erlotinib (Fig. 7, B and D). To determine the role of Trk signaling on long term growth and sphere formation, BTIC lines 12 and 48 were cultured for extended periods in the presence of EGF + FGF, NGF, BDNF, NT3, or no exogenous growth factors. BTICs cultured in the absence of growth factors had greatly reduced viability, and addition of NGF had no effect on the cells (Fig. 8, A and B). In contrast, BTIC-12, which expresses both TrkB and TrkC (Fig. 2B), could be cultured as spheres for several weeks in the presence of BDNF, NT3, or EGF + FGF (Fig. 8A), whereas BTIC-48 expressing only TrkC receptor could only be maintained as spheres in media containing NT3 or EGF + FGF (Fig. 8B). However, BTICs with EGF and FGF had to be passaged more frequently than with BDNF or NT3, and sphere sizes are larger in the BTICs treated with EGF and FGF compared with those treated with neurotrophins (Fig. 8, A and B). This difference could be due to differential activation of ERK by EGF + FGF and neurotrophins. Therefore, we examined the kinetics of ERK activation by EGF + FGF and neurotrophin treatment. We starved BTIC-48 overnight and then treated with 20 ng/ml of EGF and FGF or 50 ng/ml of NT3 for various time points from 10 min to 24 h or left untreated. We found that EGF and FGF cause robust and sustained activation of ERK at 10 min until 24 h; however, NT3-mediated ERK activation is relatively weak and transient compared with EGF and FGF (Fig. 8, C–E), suggesting that differential response to the growth factors is determined by the magnitude and duration of signaling downstream of their respective receptors. Overall, these data indicate that neurotrophin signaling via Trk receptors can circumvent the effects of EGFR-targeted glioma therapies.
FIGURE 7.
Neurotrophin signaling through Trk receptors can sustain the growth of BTICs in the absence of EGF and FGF signaling. BTIC-12 and BTIC-48 were starved of growth factors overnight and pretreated with Trk inhibitor GW441756 (GW) or 1 μm of EGFR inhibitor erlotinib (ERL) for 1 h and then treated with NT3 + BDNF (A and B), NT3 alone (C and D), or EGF; 10 min later cells were lysed, and p-ERK levels were measured (A and C). Cells were treated with the indicated combinations of EGF, NT3 + BDNF, NT3 alone, or erlotinib and GW441756 as above, and viability was assessed after 4 days by Alamar Blue (B and D). Viability was expressed as percentage increase over cells not treated with growth factors/neurotrophic factors (n = 3 independent experiments). *, difference between NT3 with and without GW441756; **, difference between EGF with and without erlotinib (p < 0.05).
FIGURE 8.
Neurotrophin signaling maintains long term BTICs growth and sphere formation in the absence of EGF and FGF signaling. A and B, to assess the effect of neurotrophins on long term growth of BITCs, BTIC-12 (A) and BTIC-48 (B) were maintained with 20 ng/ml of growth factors EGF and FGF or 50 ng/ml of neurotrophic factors NGF, BDNF, NT3, or with no added growth factor for multiple passages and analyzed for sphere formation. Representative images were taken at 10× objective from cultures 6 weeks after commencement of treatment. The data are representative of three independent experiments. BTIC-48 were deprived of growth factors overnight and then treated with 20 ng/ml of EGF + FGF (C) or 50 ng/ml of NT3 (D) for various time points, lysed, and analyzed for p-ERK and actin by Western blotting. E, quantitation of phospho-ERK Western blots expressed as relative expression level with actin (n = 3 independent experiments; p < 0.05).
DISCUSSION
Several lines of evidence suggest that glioma stem cells/BTICs are important in glioma biology (4, 13) and may be important novel targets of therapy (22, 27, 28). Our identification of the neurotrophin receptors TrkB and TrkC as nearly universally expressed in gliomas and glioma-derived BTICs raises the possibility that these receptors may be novel therapeutic targets. Indeed, comprehensive genomic sequencing of human gliomas has recently identified numerous alterations in Trk receptors. Specifically, 40% of non-brain stem pediatric high grade gliomas and a proportion of pediatric low grade gliomas and adult glioblastomas (29–31) harbor fusion genes containing the NTRK1, NTRK2, or NTRK3 kinase domains. Here, we demonstrate that as an alternative to activating mutations, ligand-mediated activation of the highly expressed endogenous TrkB and TrkC receptors is capable of promoting BTIC survival.
Inhibiting Trk signaling in combination with other RTK inhibitors may be an attractive approach as a glioma therapy for several reasons. First, the neurotrophin-rich milieu of the brain and the release of neurotrophins by tumor cells (acting in a paracrine fashion) may support glioma growth and treatment resistance in patients. Second, although Trk signaling is vital to neuronal survival during development (2), the genetic targeting of Trk receptors suggests that loss in the mature brain is not unduly deleterious to health (32, 33). Third, pharmacologic inhibitors of Trks penetrate the blood-brain barrier in mice at therapeutic concentrations (34). The emerging crystal structures of the different Trk receptors suggest the possibility of identifying Trk inhibitors that are specific for each Trk family member (34, 35). Finally, monotherapy with EGFR inhibitors (25, 26) has been unsuccessful clinically in glioma patients. Here, our results showed that the combination of Trk inhibition with EGFR inhibition may overcome treatment resistance to erlotinib alone.
Although we focused on ERK and Akt activation, other signaling pathways downstream of Trk activation could also play a role in the Trk-mediated BTIC growth and survival. In neurons, neurotrophin-dependent MAP kinase activation is mediated by SoS-Ras-MAP pathway and also by Frs2/ARMS-Crk pathway, and Akt activation is mediated by SoS-Ras-PI3K (2). Downstream of MAP kinase and Akt activation regulates RSK kinase, CREB phosphorylation, and NFκB activation, which promote transcription of genes necessary for neuronal survival (2, 18, 36–39). Activation of these pathways in the context of BTICs has not yet been extensively examined but could point to additional therapeutic targets in the future. In conclusion, we have identified neurotrophin signaling as a stimulus for BTIC viability and suggest that Trk receptors could be a target for combinatorial inhibition of RTKs for the treatment of malignant gliomas.
Acknowledgments
We thank Dr. Samuel Weiss and Dr. J. Gregory Cairncross (University of Calgary) for BTIC lines and Dr. A Narendran (University of Calgary) for providing erlotinib. We also thank Dr. Fortenbery of Moffitt Cancer Center for assistance in some experiments.
This work was supported by the Moffitt Foundation.
- NT
- neurotrophin
- BTIC
- brain tumor-initiating cells
- GBM
- glioblastoma multiforme
- MCC
- Moffitt Cancer Center
- RTK
- receptor tyrosine kinase.
REFERENCES
- 1. Chao M. V. (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 4, 299–309 [DOI] [PubMed] [Google Scholar]
- 2. Reichardt L. F. (2006) Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R., Hegi M. E., Mason W. P., van den Bent M. J., Taphoorn M. J., Janzer R. C. (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 [DOI] [PubMed] [Google Scholar]
- 4. Singh S. K., Hawkins C., Clarke I. D., Squire J. A., Bayani J., Hide T., Henkelman R. M., Cusimano M. D., Dirks P. B. (2004) Identification of human brain tumour initiating cells. Nature 432, 396–401 [DOI] [PubMed] [Google Scholar]
- 5. Johnston A. L., Lun X., Rahn J. J., Liacini A., Wang L., Hamilton M. G., Parney I. F., Hempstead B. L., Robbins S. M., Forsyth P. A., Senger D. L. (2007) The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol. 5, e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang L., Rahn J. J., Lun X., Sun B., Kelly J. J., Weiss S., Robbins S. M., Forsyth P. A., Senger D. L. (2008) γ-Secretase represents a therapeutic target for the treatment of invasive glioma mediated by the p75 neurotrophin receptor. PLoS Biol. 6, e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forsyth P. A., Krishna N., Lawn S., Valadez J. G., Qu X., Fenstermacher D. A., Fournier M., Potthast L., Chinnaiyan P., Gibney G. T., Zeinieh M., Barker P. A., Carter B. D., Cooper M. K., Kenchappa R. S. (2014) p75 neurotrophin receptor cleavage by α- and γ-secretases is required for neurotrophin-mediated proliferation of brain tumor-initiating cells. J. Biol. Chem. 289, 8067–8085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y., Hagel C., Hamel W., Müller S., Kluwe L., Westphal M. (1998) Trk A, B, and C are commonly expressed in human astrocytes and astrocytic gliomas but not by human oligodendrocytes and oligodendroglioma. Acta Neuropathol. 96, 357–364 [DOI] [PubMed] [Google Scholar]
- 9. Wadhwa S., Nag T. C., Jindal A., Kushwaha R., Mahapatra A. K., Sarkar C. (2003) Expression of the neurotrophin receptors Trk A and Trk B in adult human astrocytoma and glioblastoma. J. Biosci. 28, 181–188 [DOI] [PubMed] [Google Scholar]
- 10. Assimakopoulou M., Kondyli M., Gatzounis G., Maraziotis T., Varakis J. (2007) Neurotrophin receptors expression and JNK pathway activation in human astrocytomas. BMC Cancer 7, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bao S., Wu Q., McLendon R. E., Hao Y., Shi Q., Hjelmeland A. B., Dewhirst M. W., Bigner D. D., Rich J. N. (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 [DOI] [PubMed] [Google Scholar]
- 13. Chen J., Li Y., Yu T. S., McKay R. M., Burns D. K., Kernie S. G., Parada L. F. (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488, 522–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly J. J., Stechishin O., Chojnacki A., Lun X., Sun B., Senger D. L., Forsyth P., Auer R. N., Dunn J. F., Cairncross J. G., Parney I. F., Weiss S. (2009) Proliferation of human glioblastoma stem cells occurs independently of exogenous mitogens. Stem Cells. 27, 1722–1733 [DOI] [PubMed] [Google Scholar]
- 15. Thiele C. J., Li Z., McKee A. E. (2009) On Trk: the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin. Cancer Res. 15, 5962–5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marchetti D., Denkins Y., Reiland J., Greiter-Wilke A., Galjour J., Murry B., Blust J., Roy M. (2003) Brain metastatic melanoma: a neurotrophic perspective. Pathol. Oncol. Res. 9, 147–158 [DOI] [PubMed] [Google Scholar]
- 17. Nakagawara A. (2001) Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett. 169, 107–114 [DOI] [PubMed] [Google Scholar]
- 18. Bonni A., Brunet A., West A. E., Datta S. R., Takasu M. A., Greenberg M. E. (1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358–1362 [DOI] [PubMed] [Google Scholar]
- 19. Barker P. A., Shooter E. M. (1994) Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA in PC12 cells. Neuron 13, 203–215 [DOI] [PubMed] [Google Scholar]
- 20. Wang J., Hancock M. K., Dudek J. M., Bi K. (2008) Cellular assays for high-throughput screening for modulators of Trk receptor tyrosine kinases. Curr. Chem. Genomics 1, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallia G. L., Tyler B. M., Hann C. L., Siu I. M., Giranda V. L., Vescovi A. L., Brem H., Riggins G. J. (2009) Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol. Cancer Ther. 8, 386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eyler C. E., Foo W. C., LaFiura K. M., McLendon R. E., Hjelmeland A. B., Rich J. N. (2008) Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells 26, 3027–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verhaak R. G., Hoadley K. A., Purdom E., Wang V., Qi Y., Wilkerson M. D., Miller C. R., Ding L., Golub T., Mesirov J. P., Alexe G., Lawrence M., O'Kelly M., Tamayo P., Weir B. A., Gabriel S., Winckler W., Gupta S., Jakkula L., Feiler H. S., Hodgson J. G., James C. D., Sarkaria J. N., Brennan C., Kahn A., Spellman P. T., Wilson R. K., Speed T. P., Gray J. W., Meyerson M., Getz G., Perou C. M., Hayes D. N., and Cancer Genome Atlas Research Network (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hegi M. E., Diserens A. C., Bady P., Kamoshima Y., Kouwenhoven M. C., Delorenzi M., Lambiv W. L., Hamou M. F., Matter M. S., Koch A., Heppner F. L., Yonekawa Y., Merlo A., Frei K., Mariani L., Hofer S. (2011) Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib: a phase II trial. Mol. Cancer Ther. 10, 1102–1112 [DOI] [PubMed] [Google Scholar]
- 25. Prados M. D., Chang S. M., Butowski N., DeBoer R., Parvataneni R., Carliner H., Kabuubi P., Ayers-Ringler J., Rabbitt J., Page M., Fedoroff A., Sneed P. K., Berger M. S., McDermott M. W., Parsa A. T., Vandenberg S., James C. D., Lamborn K. R., Stokoe D., Haas-Kogan D. A. (2009) Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J. Clin. Oncol. 27, 579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stommel J. M., Kimmelman A. C., Ying H., Nabioullin R., Ponugoti A. H., Wiedemeyer R., Stegh A. H., Bradner J. E., Ligon K. L., Brennan C., Chin L., DePinho R. A. (2007) Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 318, 287–290 [DOI] [PubMed] [Google Scholar]
- 27. Fan X., Matsui W., Khaki L., Stearns D., Chun J., Li Y. M., Eberhart C. G. (2006) Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 66, 7445–7452 [DOI] [PubMed] [Google Scholar]
- 28. Sarangi A., Valadez J. G., Rush S., Abel T. W., Thompson R. C., Cooper M. K. (2009) Targeted inhibition of the Hedgehog pathway in established malignant glioma xenografts enhances survival. Oncogene 28, 3468–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu G., Diaz A. K., Paugh B. S., Rankin S. L., Ju B., Li Y., Zhu X., Qu C., Chen X., Zhang J., Easton J., Edmonson M., Ma X., Lu C., Nagahawatte P., Hedlund E., Rusch M., Pounds S., Lin T., Onar-Thomas A., Huether R., Kriwacki R., Parker M., Gupta P., Becksfort J., Wei L., Mulder H. L., Boggs K., Vadodaria B., Yergeau D., Russell J. C., Ochoa K., Fulton R. S., Fulton L. L., Jones C., Boop F. A., Broniscer A., Wetmore C., Gajjar A., Ding L., Mardis E. R., Wilson R. K., Taylor M. R., Downing J. R., Ellison D. W., Zhang J., Baker S. J., and St. Jude Children's Research Hospital–Washington University Pediatric Cancer Genome Project (2014) The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat. Genet. 46, 444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones D. T., Hutter B., Jäger N., Korshunov A., Kool M., Warnatz H. J., Zichner T., Lambert S. R., Ryzhova M., Quang D. A., Fontebasso A. M., Stütz A. M., Hutter S., Zuckermann M., Sturm D., Gronych J., Lasitschka B., Schmidt S., Seker-Cin H., Witt H., Sultan M., Ralser M., Northcott P. A., Hovestadt V., Bender S., Pfaff E., Stark S., Faury D., Schwartzentruber J., Majewski J., Weber U. D., Zapatka M., Raeder B., Schlesner M., Worth C. L., Bartholomae C. C., von Kalle C., Imbusch C. D., Radomski S., Lawerenz C., van Sluis P., Koster J., Volckmann R., Versteeg R., Lehrach H., Monoranu C., Winkler B., Unterberg A., Herold-Mende C., Milde T., Kulozik A. E., Ebinger M., Schuhmann M. U., Cho Y. J., Pomeroy S. L., von Deimling A., Witt O., Taylor M. D., Wolf S., Karajannis M. A., Eberhart C. G., Scheurlen W., Hasselblatt M., Ligon K. L., Kieran M. W., Korbel J. O., Yaspo M. L., Brors B., Felsberg J., Reifenberger G., Collins V. P., Jabado N., Eils R., Lichter P., Pfister S. M., and International Cancer Genome Consortium PedBrain Tumor Project (2013) Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat. Genet. 45, 927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frattini V., Trifonov V., Chan J. M., Castano A., Lia M., Abate F., Keir S. T., Ji A. X., Zoppoli P., Niola F., Danussi C., Dolgalev I., Porrati P., Pellegatta S., Heguy A., Gupta G., Pisapia D. J., Canoll P., Bruce J. N., McLendon R. E., Yan H., Aldape K., Finocchiaro G., Mikkelsen T., Privé G. G., Bigner D. D., Lasorella A., Rabadan R., Iavarone A. (2013) The integrated landscape of driver genomic alterations in glioblastoma. Nat. Genet. 45, 1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bergami M., Rimondini R., Santi S., Blum R., Götz M., Canossa M. (2008) Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc. Natl. Acad. Sci. U.S.A. 105, 15570–15575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Y., Luikart B. W., Birnbaum S., Chen J., Kwon C. H., Kernie S. G., Bassel-Duby R., Parada L. F. (2008) TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59, 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cazorla M., Prémont J., Mann A., Girard N., Kellendonk C., Rognan D. (2011) Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J. Clin. Invest. 121, 1846–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bertrand T., Kothe M., Liu J., Dupuy A., Rak A., Berne P. F., Davis S., Gladysheva T., Valtre C., Crenne J. Y., Mathieu M. (2012) The crystal structures of TrkA and TrkB suggest key regions for achieving selective inhibition. J. Mol. Biol. 423, 439–453 [DOI] [PubMed] [Google Scholar]
- 36. Riccio A., Ahn S., Davenport C. M., Blendy J. A., Ginty D. D. (1999) Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 286, 2358–2361 [DOI] [PubMed] [Google Scholar]
- 37. Xing J., Ginty D. D., Greenberg M. E. (1996) Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 273, 959–963 [DOI] [PubMed] [Google Scholar]
- 38. Xing J., Kornhauser J. M., Xia Z., Thiele E. A., Greenberg M. E. (1998) Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol. Cell. Biol. 18, 1946–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Atwal J. K., Massie B., Miller F. D., Kaplan D. R. (2000) The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 27, 265–277 [DOI] [PubMed] [Google Scholar]