Abstract Abstract
As with many other regions of the world, significant collecting, curation, and taxonomic efforts will be needed to complete the inventory of China’s ant fauna. This is especially true for the highly diverse tropical regions in the south of the country, where moist tropical forests harbor high species richness typical of the Southeast Asian region. We inventoried ants in the Xingshuangbanna prefecture, Yunnan, in June 2013, using a variety of methods including Winkler extraction and hand collection to sample ant diversity. We identified 213 species/morphospecies of ants from 10 subfamilies and 61 genera. After identification of 148 valid species of the 213 total species collected, 40 species represent new records for Yunnan province and 17 species are newly recorded for China. This increases the total number of named ant species in Yunnan and China to 447 and 951 respectively. The most common species collected were Brachyponera luteipes and Vollenhovia emeryi. Only one confirmed exotic species Strumigenys membranifera, was collected, although several others were potentially introduced by humans. These results highlight the high biodiversity value of the region, but also underscore how much work remains to fully document the native myrmecofauna.
Keywords: China, Formicidae, new records, Xishuangbanna, Yunnan
Introduction
The understanding of regional and global patterns of insect diversity is limited by our incomplete accounting of Earth’s species, especially for tropical regions where species richness peaks in most taxonomic groups. This is also true for Formicidae, an ecologically dominant insect family comprising at least 15,000 described species (Bolton 2014). Despite the ubiquity and ecological importance of ants (Hölldobler and Wilson 1990), many tropical regions remain undersampled even at the generic level (Guénard and Dunn 2012). Compiling and curating complete and accurate species checklists for all regions of the world should be a priority in biodiversity research, especially for diverse insect groups.
Towards that end, here we present the results of an ant survey conducted during the summer of 2013 in the area of Xishuangbanna, Yunnan Province, in the south of China. In particular, our goal here is to document new records of ant species detected in Yunnan, and some new records for China as a whole. The geographic location of Yunnan (ranging from 21.15°N to 29.20°N of latitude) and its topography (elevation range from < 100m to 6740m) render it the most diverse province of China in terms of ant diversity (406 species) (Guénard and Dunn 2012). The same is true for other taxa, such as plants (Li and Walker 1986, Mutke and Barthlott 2005), tiger beetles (Wu and Shook 2007), butterflies (Xie et al. 2009), or amphibians (Chen and Bi 2007). Xishuangbanna prefecture is located in the tropical southwestern region of Yunnan province, bordering Laos and Myanmar, and has been identified as the most diverse region of Yunnan (Long 1995). The ant fauna of Xishuangbanna has been the subject of three studies (Xu 1998, 1999, 2002) and new species are regularly described from this prefecture (e.g. Guénard et al. 2013, Xu et al. 2014a, b). According to Xu’s survey (2002), the myrmecofauna of Xishuangbanna consists of approximately 262 species, which constitute about 65% of the total number of species recorded for Yunnan province.
While elements of China’s ant fauna may be undocumented due to a lack of sampling in certain geographic regions, there are many taxa likely hidden in areas that have been sampled historically. In particular, methods targeting specifically subterranean or leaf litter ants have been rarely used in China, which as a result might bias our detection of ant species from specific strata. One of the most successful sampling techniques for collecting leaf litter ants, Winkler extraction, which is now commonly used for ant fauna surveys all over the world (Olson 1991, Fisher 1999, Agosti et al. 2000, Martelli et al. 2004, Vasconcelos and Lopes 2008, Ivanov and Keiper 2009), has only been used once in China (Hong Kong in Fellowes 1996) to the best of our knowledge. In this study we used Winkler extraction as a standardized collection technique for the first time in order to survey the leaf litter ant fauna of Xishuangbanna. Based predominantly on this highly successful sampling technique, our diversity survey revealed 40 new species records for Yunnan including 17 new records for China. Here we present those new records, as well as their known global distributions by using data information aggregated by the Global Ant BioInformatics project (GABI, Guénard et al. in prep).
Material and methods
Ant specimens were collected from primary forest, secondary forest and rubber plantation habitats near Menglun town, Xishuangbanna Prefecture, Yunnan Province, China during a survey in June 2013. Ants from leaf litter of multiple sites were collected and extracted by mini Winkler extractors for 72 hours using the shuffling method as described in Guénard and Lucky (2011). Ants were also collected by hand on the ground, lower vegetation, and tree trunks.
Samples were first sorted to morphospecies in alcohol, and up to three representatives of each morphospecies per sample were point-mounted. Each mounted specimen was assigned a unique specimen code, in this case a CASENT number, and traditional locality and collection labels. All mounted and alcohol-preserved ant specimens are currently located in EPE’s lab at the Okinawa Institute of Science and Technology Graduate University. Extended depth of field specimen images were taken with an incorporated Leica DFC400 digital camera mounted on a Leica M205C stereomicroscope through the Leica Application Suite V4 software. All specimens were identified to genus using Bolton’s key (Bolton 1994), and then identified to species using available keys (see results section) as well as the digital resources on AntWeb (http://www.antweb.org). All the specimen data are freely available on AntWeb.
Species distributions presented in the following maps are based on records reported here at the country level, or for the larger countries (China, India, Japan), at the first administration level. For large islands (e.g. Borneo, Sumatra, New Guinea) that form natural biogeographic units, we used the island boundary instead of political boundaries similar to a previous study (Guénard and Dunn 2012).
Results
Over 20000 specimens from 61 genera and 213 valid species and morphospecies were collected during this ant diversity survey (Table 1). A total of 40 new species records are presented for Yunnan province. Seventeen of these are recorded for the first time from China. The newly detected species belong to 15 genera from eight subfamilies. It is beyond the scope of the current paper to perform a comprehensive review/revision of the ant fauna of Yunnan Province, which would require much more geographically comprehensive sampling. Here, we present species accounts for the described ant species found during our survey that were previously unknown to Yunnan, supplementing other recently published checklists of the myrmecofauna of the region (Guénard and Dunn 2012).
Table 1.
Ant species (Formicidae) collected from Xishuangbanna, Yunnan in 2013.
| Species | Collection record1 |
|---|---|
| Aenictinae | |
| Aenictus artipus Wilson, 1964 | N* |
| Aenictus hodgsoni Forel, 1901 | N |
| Aenictus maneerati Jaitrong & Yamane, 2013 | N* |
| Aenictus paradentatus Jaitrong, Yamane & Tasen, 2012 | N* |
| Aenictus thailandianus Terayama & Kubota, 1993 | N |
| Aenictus clm01 | |
| Aenictus clm04 | |
| Amblyoponinae | |
| Bannapone scrobiceps Guénard, Blanchard, Liu, Yang & Economo, 2013 | N* |
| Mystrium camillae Emery, 1889 | |
| Cerapachyinae | |
| Cerapachys clm01 | |
| Cerapachys sulcinodis Emery, 1889 | |
| Cerapachys typhlus (Roger, 1861) | |
| Dolichoderinae | |
| Chronoxenus wroughtonii (Forel, 1985) | |
| Dolichoderus affinis Emery, 1889 | |
| Dolichoderus laotius Santschi, 1920 | N* |
| Dolichoderus squamanodus Xu, 2001 | |
| Dolichoderus thoracicus (Smith, 1860) | |
| Iridomyrmex anceps (Roger, 1863) | |
| Tapinoma indicum Forel, 1895 | |
| Tapinoma melanocephalum (Fabricius, 1793) | |
| Tapinoma clm04 | |
| Technomyrmex albipes (Smith, 1861) | |
| Technomyrmex horni Forel, 1912 | |
| Technomyrmex pratensis (Smith, 1860) | N |
| Ectatomminae | |
| Gnamptogenys costata (Emery, 1989) | N* |
| Gnamptogenys bicolor (Emery, 1989) | |
| Gnamptogenys treta Lattke, 2004 | N* |
| Formicinae | |
| Acropyga nipponensis Terayama, 1985 | |
| Anoplolepis gracilipes (Smith, 1857) | |
| Camponotus lasiselene Wang & Wu, 1994 | |
| Camponotus mitis (Smith, 1858) | |
| Camponotus parius Emery, 1889 | |
| Camponotus singularis Smith, 1858 | |
| Camponotus clm02 | |
| Camponotus clm03 | |
| Camponotus clm04 | |
| Camponotus clm07 | |
| Camponotus clm08 | |
| Camponotus clm09 | |
| Echinopla cherapunjiensis Bharti & Gul, 2012 | N |
| Gesomyrmex kalshoveni Wheeler, W.M. 1929 | N* |
| Lepisiota opaca (Forel, 1892) | |
| Lepisiota rothneyi (Forel, 1894) | |
| Myrmoteras binghamii Forel, 1893 | |
| Myrmoteras cuneonodum Xu, 1998 | |
| Nylanderia clm01 | |
| Nylanderia clm02 | |
| Nylanderia clm03 | |
| Nylanderia clm04 | |
| Nylanderia clm05 | |
| Nylanderia clm06 | |
| Oecophylla smaragdina (Fabricius, 1775) | |
| Paraparatrechina clm01 | |
| Paraparatrechina clm02 | |
| Paraparatrechina clm03 | |
| Paraparatrechina clm04 | |
| Plagiolepis clm01 | |
| Polyrhachis armata (Le Guillou, 1842) | |
| Polyrhachis bicolor Mayr, 1862 | |
| Polyrhachis bihamata (Drury, 1773) | |
| Polyrhachis furcata Emery, 1889 | |
| Polyrhachis halidayi Emery, 1889 | |
| Polyrhachis hippomanes Smith, 1861 | |
| Polyrhachis illaudata Walker, 1859 | |
| Polyrhachis illaudata pauperata Emery, 1889 | |
| Prenolepis naoroji Forel, 1902 | |
| Prenolepis sphingthoraxa Zhou & Zheng, 1998 | N |
| Pseudolasius cibdelus Wu & Wang, 1992 | |
| Pseudolasius emeryi Forel, 1915 | |
| Pseudolasius silvestrii Wheeler, 1927 | |
| Myrmicinae | |
| Acanthomyrmex luciolae Emery, 1893 | |
| Aphaenogaster beccarii Emery, 1887 | |
| Aphaenogaster feae Emery, 1889 | |
| Aphaenogaster clm05 | |
| Cardiocondyla wroughtonii (Forel, 1890) | |
| Carebara affinis (Jerdon, 1851) | |
| Carebara altinoda (Xu, 2003) | |
| Carebara bruni (Forel, 1913) | |
| Carebara diversa (Jerdon, 1851) | |
| Carebara melasolena (Zhou & Zheng, 1997) | N |
| Carebara clm01 | |
| Carebara clm05 | |
| Carebara clm06 | |
| Carebara clm07 | |
| Carebara clm08 | |
| Carebara clm09 | |
| Carebara clm10 | |
| Carebara clm11 | |
| Carebara clm12 | |
| Carebara clm13 | |
| Cataulacus granulatus (Latreille, 1802) | |
| Crematogaster dohrni Mayr, 1879 | |
| Crematogaster ferrarii Emery, 1888 | |
| Crematogaster millardi Forel, 1902 | |
| Crematogaster osakensis Forel, 1900 | |
| Crematogaster politula Forel, 1902 | |
| Crematogaster rothneyi Mayr, 1879 | |
| Crematogaster clm05 | |
| Crematogaster clm09 | |
| Crematogaster clm10 | |
| Crematogaster clm11 | |
| Dilobocondyla fouqueti Santschi, 1910 | |
| Kartidris ashima Xu & Zheng, 1995 | |
| Lophomyrmex quadrispinosus (Jerdon, 1851) | |
| Lordomyrma idianale Taylor, 2012 | |
| Meranoplus laeviventris Emery, 1889 | |
| Monomorium chinense Santschi, 1925 | |
| Monomorium pharaonis (Linnaeus, 1758) | |
| Monomorium clm01 | |
| Monomorium clm02 | |
| Monomorium clm05 | |
| Monomorium clm06 | |
| Myrmecina curvispina Zhou, Huang & Ma L., 2008 | N |
| Myrmecina guangxiensis Zhou, 2001 | N |
| Pheidole hongkongensis Wheeler, 1928 | N |
| Pheidole noda Smith, 1874 | |
| Pheidole pieli Santschi, 1925 | |
| Pheidole plagiaria Smith, 1860 | N |
| Pheidole planifrons Santschi, 1920 | N |
| Pheidole roberti Forel, 1902 | |
| Pheidole rugithorax Eguchi, 2008 | N |
| Pheidole sagei Forel, 1902 | |
| Pheidole smythiesii Forel, 1902 | N |
| Pheidole tumida Eguchi, 2008 | N |
| Pheidole vieti Eguchi, 2008 | N* |
| Pheidole zoceana Santschi, 1925 | N |
| Pheidole clm03 | |
| Pheidole clm04 | |
| Pheidole clm07 | |
| Pheidole clm12 | |
| Pheidole clm13 | |
| Pheidole clm16 | |
| Pheidole clm18 | |
| Pheidole clm22 | |
| Pheidole clm13 | |
| Pristomyrmex brevispinosus Emery, 1887 | |
| Pristomyrmex hamatus Xu & Zhang, 2002 | |
| Pristomyrmex punctatus (Smith, 1860) | |
| Recurvidris recurvispinosa (Forel, 1890) | |
| Recurvidris kemneri (Wheeler & Wheeler, 1954) | N* |
| Solenopsis jacoti Wheeler, 1923 | |
| Strumigenys ailaoshana (Xu & Zhou, 2004) | |
| Strumigenys dyschima (Bolton, 2000) | N* |
| Strumigenys exilirhina Bolton, 2000 | |
| Strumigenys feae Emery, 1895 | |
| Strumigenys kichijo (Terayama, Lin & Wu, 1996) | N |
| Strumigenys lyroessa (Roger, 1862) | |
| Strumigenys membranifera Emery, 1869 | |
| Strumigenys mitis (Brown, 2000) | N |
| Strumigenys mutica (Brown, 1949) | |
| Strumigenys nanzanensis Lin & Wu, 1996 | |
| Strumigenys nepalensis Baroni Urbani & De Andrade, 1994 | N* |
| Strumigenys rallarhina Bolton, 2000 | N |
| Strumigenys sauteri (Forel, 1912) | N |
| Tetramorium aptum Bolton, 1977 | |
| Tetramorium ciliatum Bolton, 1977 | |
| Tetramorium difficile Bolton, 1977 | N* |
| Tetramorium flavipes Emery, 1893 | N* |
| Tetramorium kheperra (Bolton, 1976) | |
| Tetramorium kraepelini Forel, 1905 | |
| Tetramorium nipponense Wheeler, 1928 | |
| Tetramorium parvispinum (Emery, 1893) | N |
| Tetramorium polymorphum Yamane & Jaitrong, 2011 | N* |
| Tetramorium tonganum Mayr, 1870 | N |
| Tetramorium clm03 | |
| Tetramorium clm10 | |
| Tetramorium clm18 | |
| Tetramorium clm19 | |
| Vollenhovia emeryi Wheeler, 1906 | |
| Ponerinae | |
| Anochetus graeffei Mayr, 1870 | |
| Anochetus mixtus Radchenko, 1993 | |
| Anochetus myops Emery, 1893 | |
| Anochetus clm04 | |
| Brachyponera luteipes (Mayr, 1862) | |
| Diacamma clm01 | |
| Ectomomyrmex astutus (Smith, 1858) | |
| Ectomomyrmex leeuwenhoeki (Forel, 1886) | |
| Ectomomyrmex lobocarenus (Xu, 1995) | |
| Ectomomyrmex clm01 | |
| Ectomomyrmex clm02 | |
| Ectomomyrmex clm03 | |
| Ectomomyrmex clm04 | |
| Emeryopone melaina Xu, 1998 | |
| Hypoponera clm01 | |
| Hypoponera clm02 | |
| Hypoponera clm03 | |
| Hypoponera clm04 | |
| Hypoponera clm05 | |
| Hypoponera clm06 | |
| Hypoponera clm07 | |
| Leptogenys birmana Forel, 1900 | |
| Leptogenys chinensis (Mayr, 1870) | |
| Leptogenys crassicornis Emery, 1895 | |
| Leptogenys diminuta (Smith, 1857) | |
| Leptogenys lucidula Emery, 1895 | |
| Leptogenys mengzii Xu, 2000 | |
| Leptogenys clm01 | |
| Leptogenys clm02 | |
| Leptogenys clm09 | |
| Myopias hania Xu & Liu, 2011 | |
| Odontomachus clm01 | |
| Odontoponera denticulata (Smith, 1858) | N |
| Platythyrea parallela (Smith, 1859) | |
| Pseudoneoponera rufipes (Forel, 1911) | |
| Proceratinae | |
| Discothyrea clavicornis Emery, 1897 | N* |
| Discothyrea kamiteta Kubota & Terayama, 1999 | N |
| Probolomyrmex longiscapus Xu & Zeng, 2000 | |
| Proceratium deelemani Perrault, 1981 | N* |
| Pseudomyrmecinae | |
| Tetraponera amargina Xu & Chai, 2004 | |
| Tetraponera allaborans (Walker, 1859) | |
| Tetraponera attenuata Smith, 1877 | |
| Tetraponera concava Xu & Chai, 2004 |
N = New to Yunnan province; N* = New to China.
Species accounts
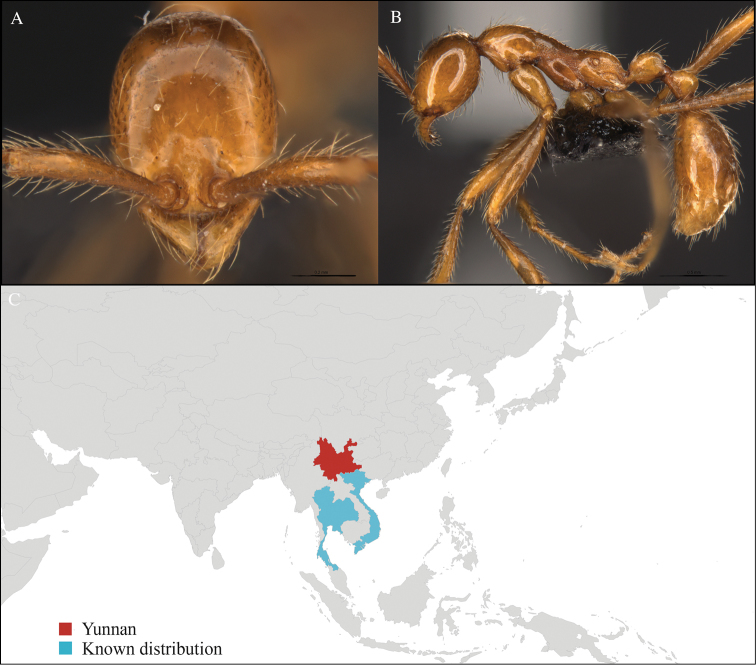
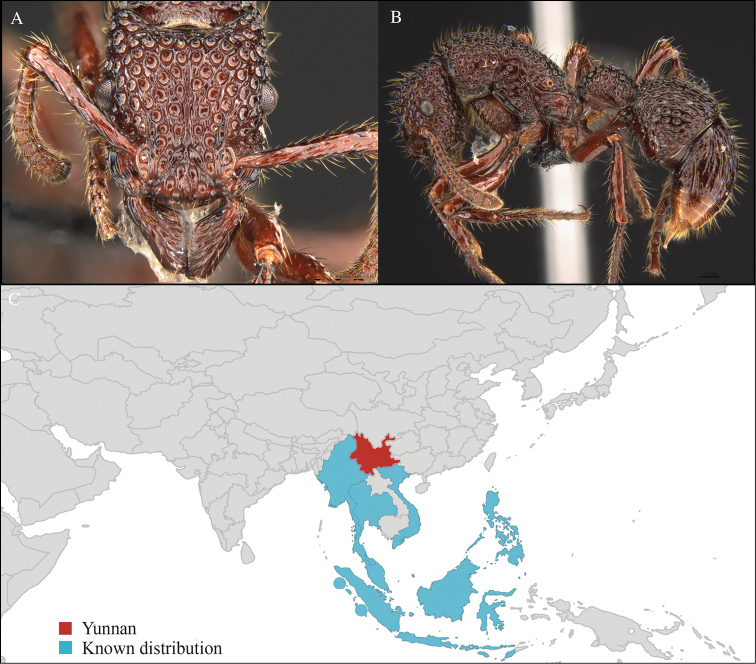
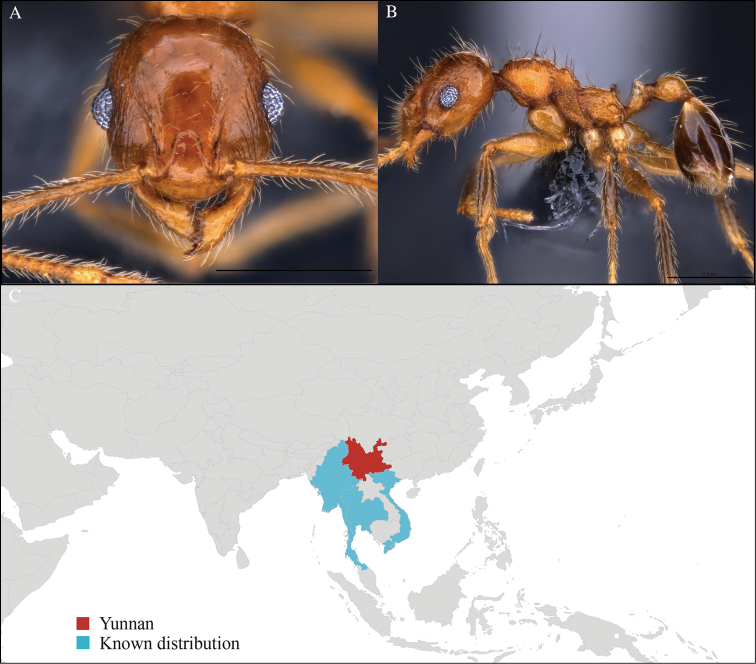
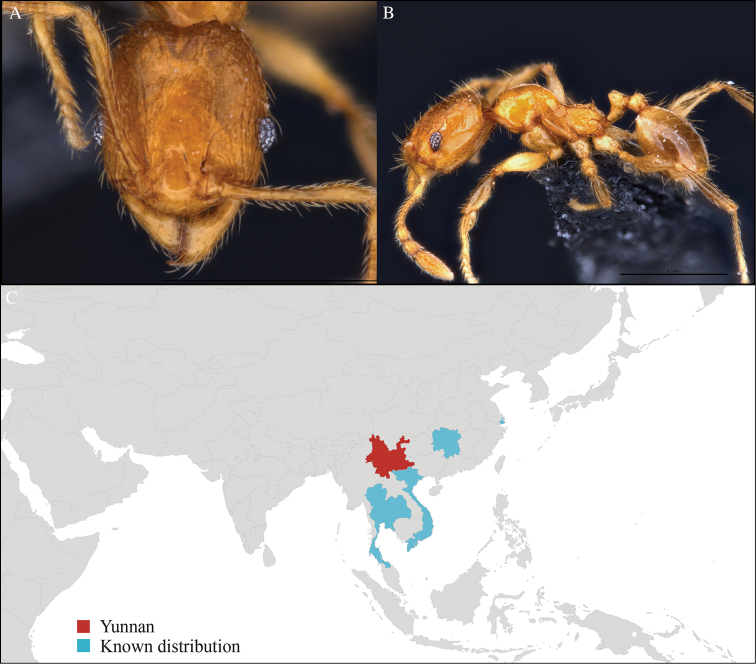
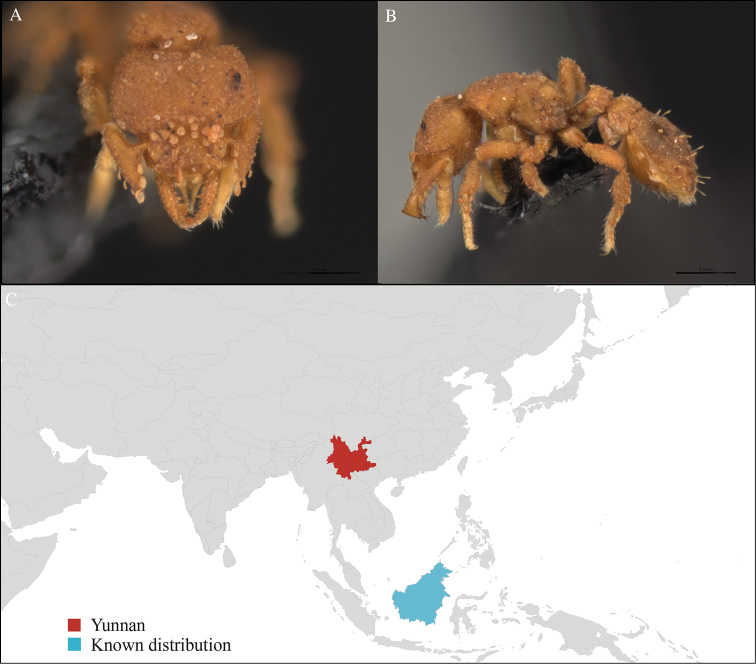
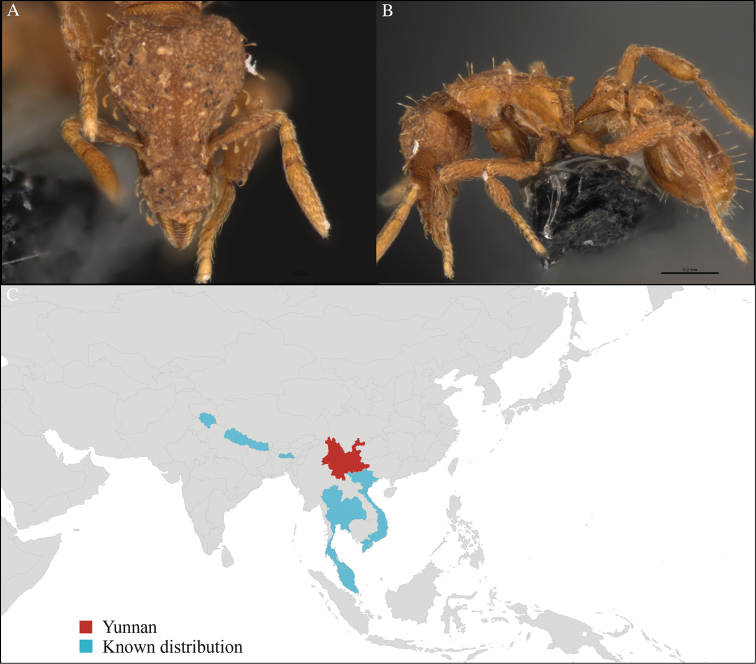
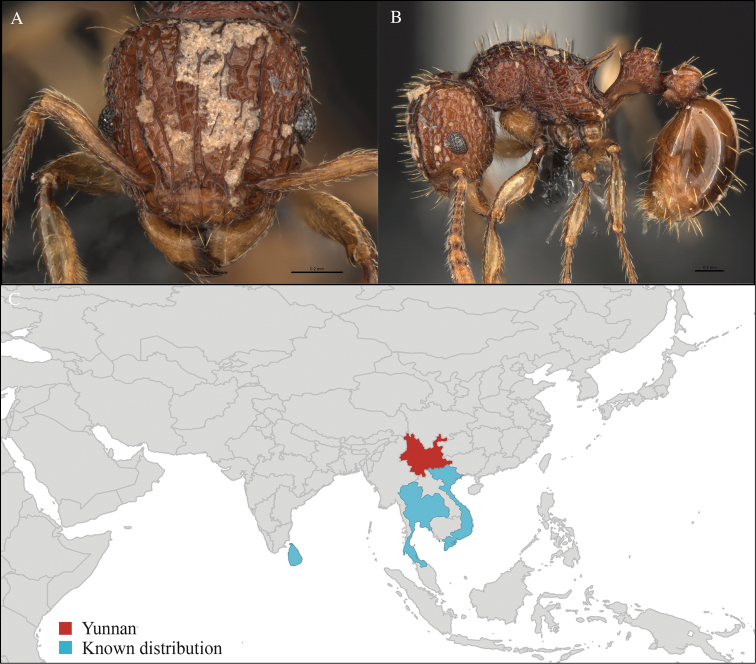
Aenictus artipus
Wilson, 1964
Figure 1.
Aenictus artipus worker, CASENT0717199. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: Man Sai village (21.858°N, 101.277°E), Rubber plantation, 12.vi.2013, 5 workers, 705m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.860°N, 101.278°E), Secondary forest, 12.vi.2013, 18 workers, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record), Vietnam and Thailand (Figure 1C). This collection represents the northern-most record of Aenictus artipus.
Taxonomic note.
Aenictus artipus belongs to the Aenictus wroughtonii species group and can be easily identified with the identification key provided by Jaitrong et al. (2010).
Natural history.
Aenictus artipus has been collected from leaf litter in various habitats such as secondary forest and rubber plantation located near natural secondary forest. In addition, Aenictus artipus has also been found in different habitats such as montane evergreen forest, savanna forest, evergreen forest and disturbed forest (Jaitrong et al. 2010).
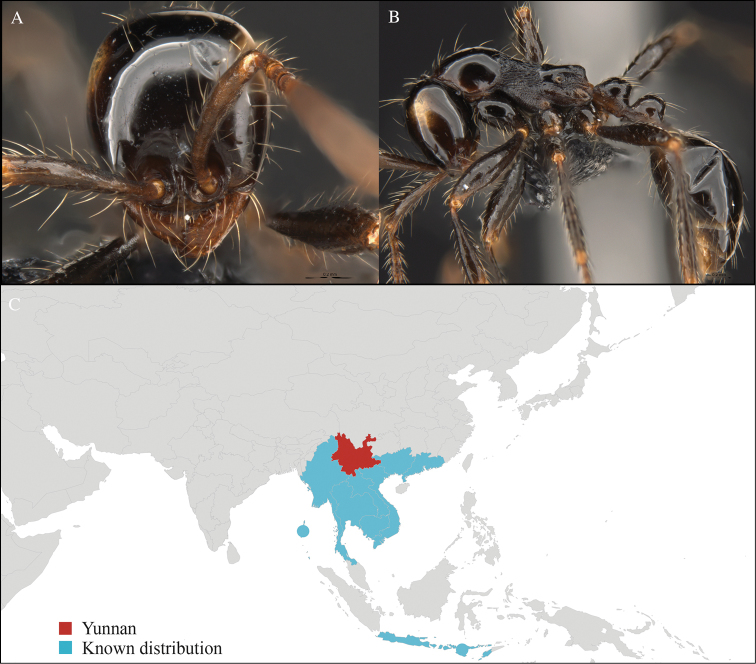
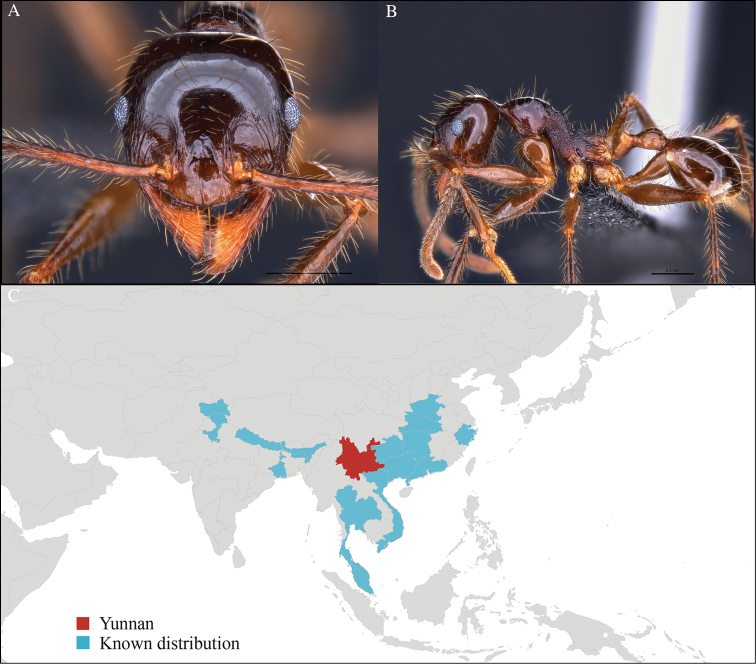
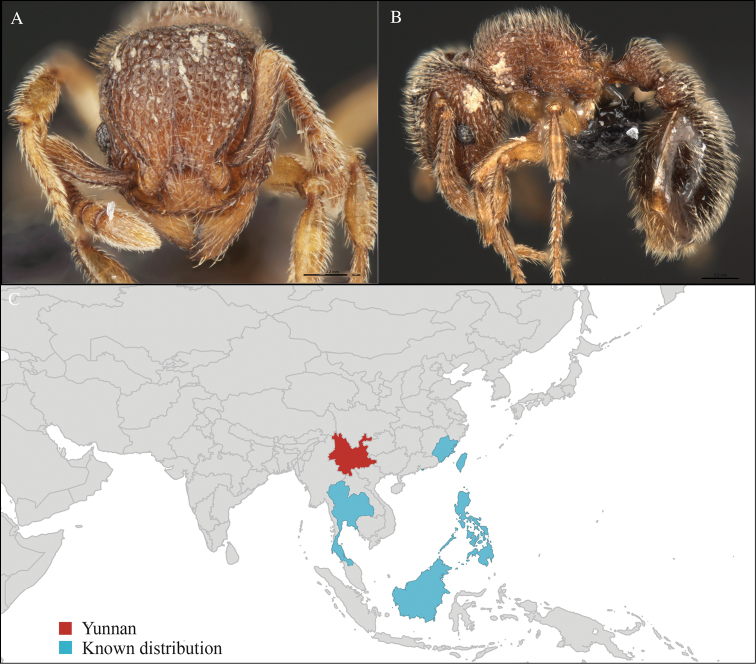
Aenictus hodgsoni
Forel, 1901
Figure 2.
Aenictus hodgsoni worker, CASENT0716190. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: Xishuangbanna Tropical Botanical Garden (known as ‘XTBG’) (21.919°N, 101.270°E), Secondary forest, 08.vi.2013, 12 workers, 610m, Hand collection, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Secondary forest, 13.vi.2013, 40 workers, 825m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in the Indo-Malayan subregions (Figure 2C).
Taxonomic note.
Aenictus hodgsoni belongs to the Aenictus laeviceps s species group and can be easily identified with the identification key provided by Jaitrong and Yamane (2011).
Natural history.
Aenictus hodgsoni has been collected from leaf litter and foraging columns on the forest ground in secondary forest. This species has also been found from lowland to highland in varied forest types (hill evergreen forest, dry evergreen forest, evergreen rain forest, mixed deciduous forest, and savanna) (Jaitrong and Yamane 2011).
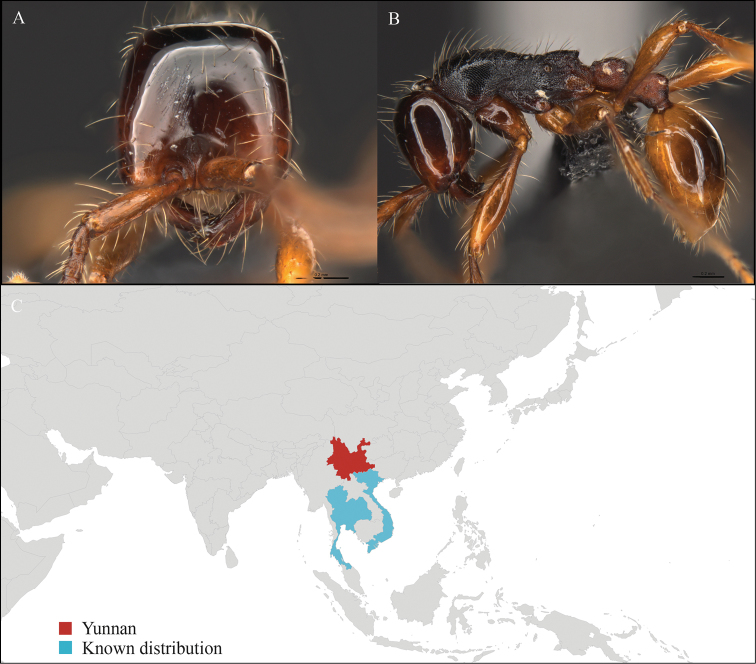
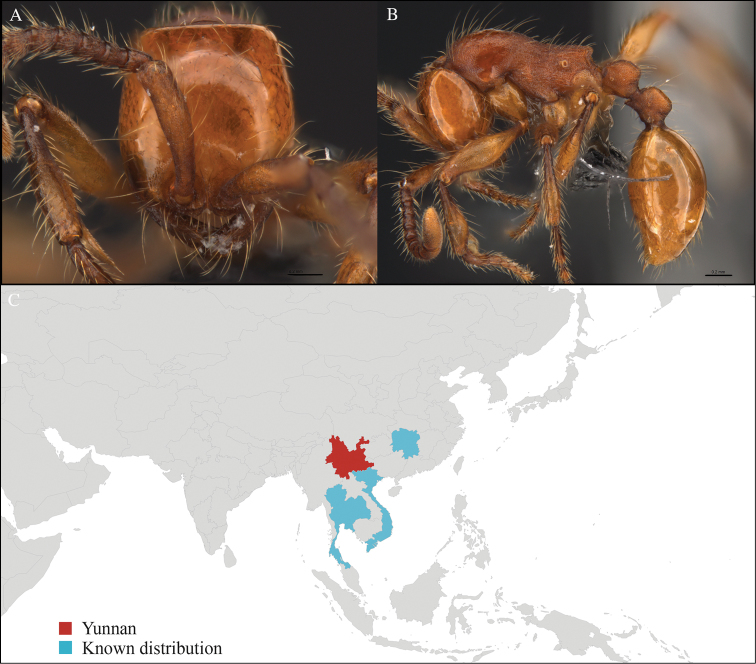
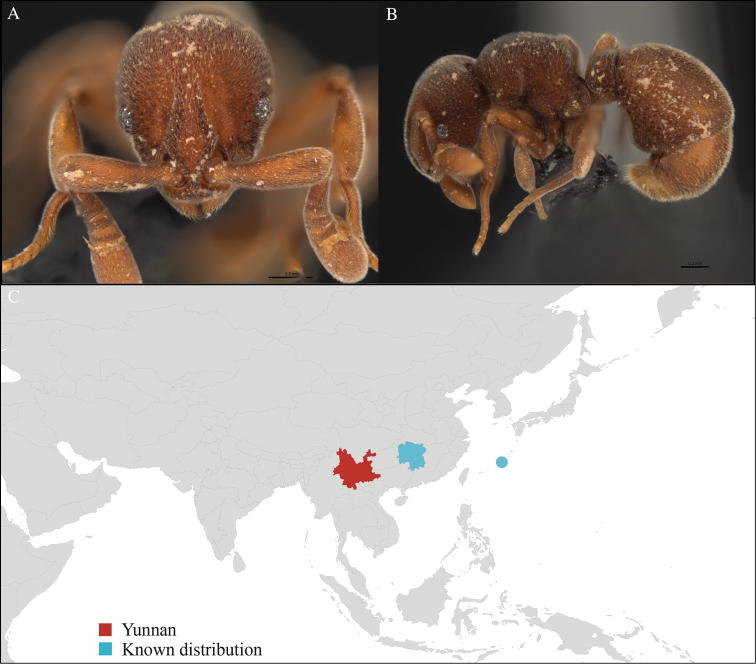
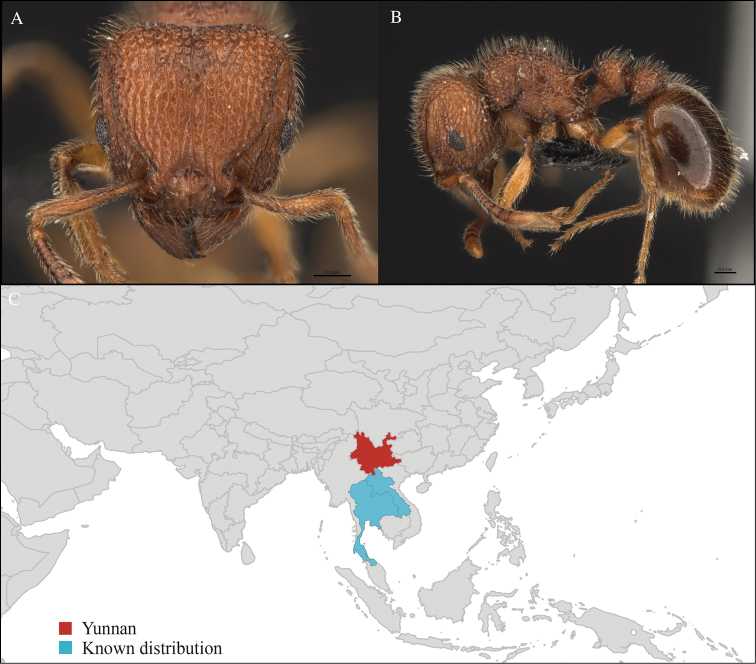
Aenictus maneerati
Jaitrong & Yamane, 2013
Figure 3.
Aenictus maneerati worker, CASENT0717211. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.916°N, 101.274°E), Secondary forest, 08.vi.2013, 1 worker, 615m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record), Vietnam and Thailand (Figure 3C). Our material represents the northern-most record of Aenictus hodgsoni.
Taxonomic note.
Aenictus hodgsoni belongs to the Aenictus ceylonicus species group and can be easily identified with the identification key provided by Jaitrong and Yamane (2013).
Natural history.
Little is known about the bionomics of Aenictus hodgsoni. Before our survey, it has been only collected from primary forest (Jaitrong and Yamane 2013). We collected it from leaf litter in secondary forest.
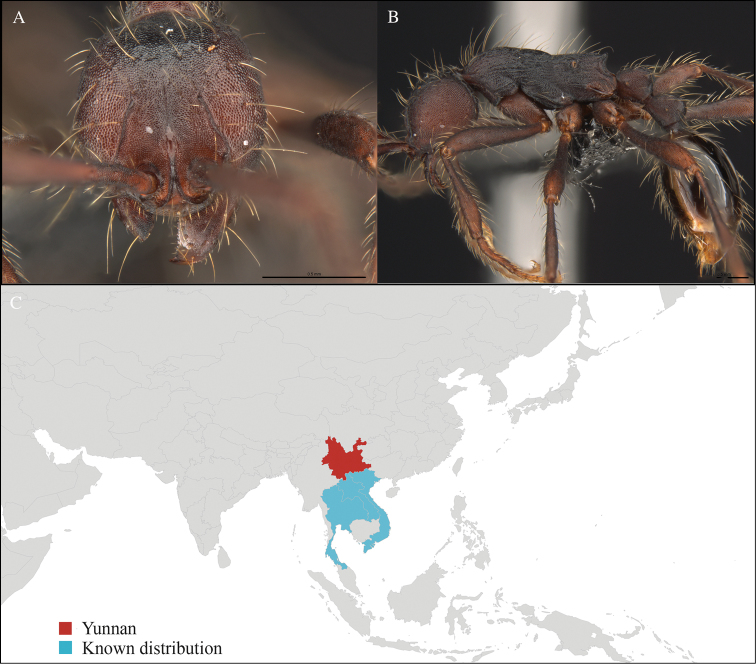
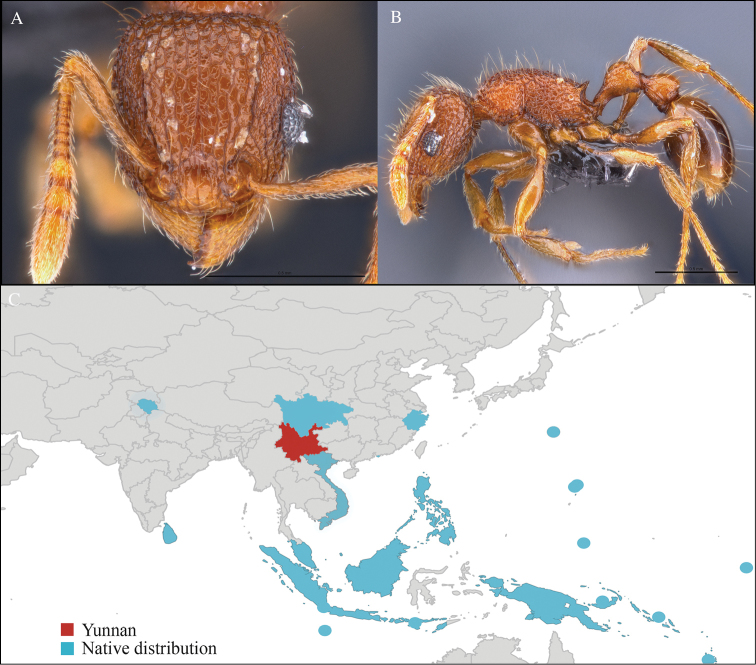
Aenictus paradentatus
Jaitrong, Yamane & Tasen, 2012
Figure 4.
Aenictus paradentatus worker, CASENT0716195. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.911°N, 101.281°E), Limestone forest, 06.vi.2013, 46 workers, 655m, Hand collection, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record), Vietnam, Laos, and Thailand (Figure 4C). This collection represents the northern-most record of Aenictus paradentatus.
Taxonomic note.
Aenictus paradentatus is very similar to Aenictus dentatus Forel, 1911, and can be easily identified with the key of Jaitrong et al. (2012).
Natural history.
Aenictus paradentatus has been collected from foraging columns on the ground in limestone forest, but was also reported to be found in other forest habitats, ranging from primary forest to disturbed forest (Jaitrong et al. 2012).
Aenictus thailandianus
Terayama & Kubota, 1993
Figure 5.
Aenictus thailandianus worker, CASENT0717202. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.919°N, 101.274°E), Secondary forest, 11.vi.2013, 19 workers, 590m, Hand collection, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.857°N, 101.277°E), Rubber plantation, 12.vi.2013, 19 workers, 680m, Hand collection, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.857°N, 101.277°E), Rubber plantation, 12.vi.2013, 254 workers, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record), Hunan, Vietnam and Thailand (Figure 5C).
Taxonomic note.
Aenictus thailandianus belongs to the Aenictus ceylonicus species group and can be easily identified with the identification key presented by Jaitrong and Yamane (2013).
Natural history.
Aenictus thailandianus has only been found at higher elevations (1000–1500m) in primary and secondary forest (Jaitrong and Yamane 2013). We collected it from leaf litter and foraging columns on the ground in secondary forest and rubber plantations at lower elevations (under 1000m).
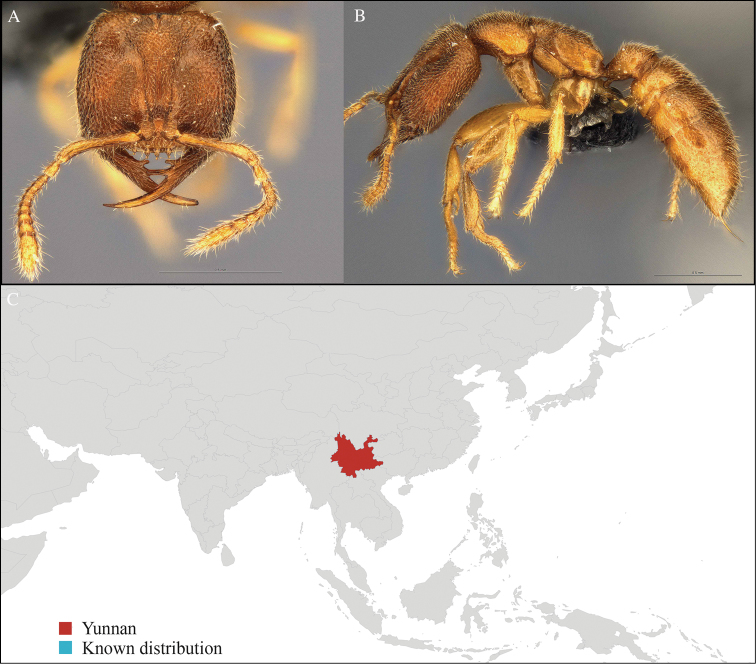
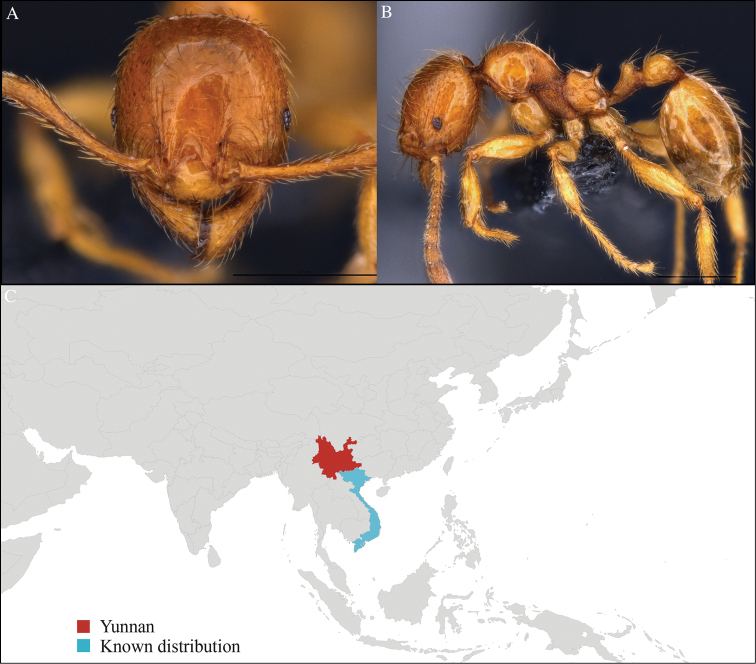
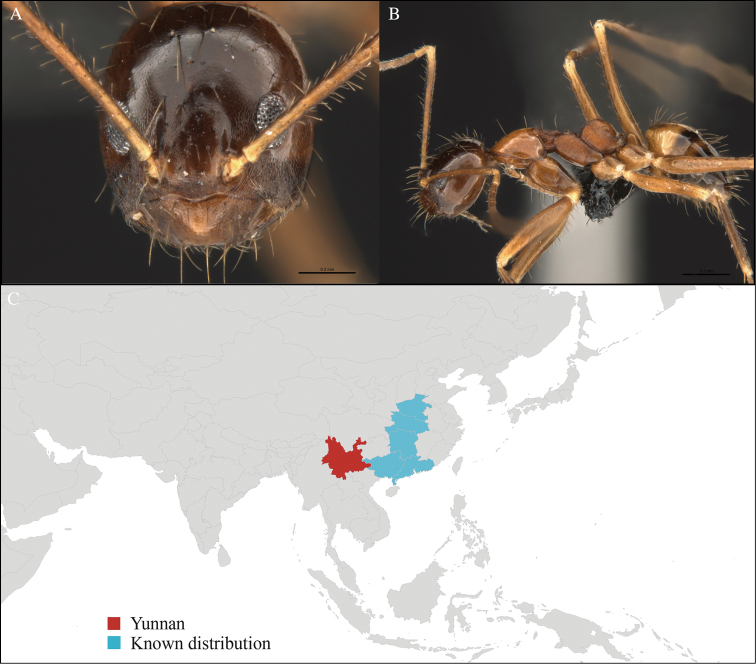
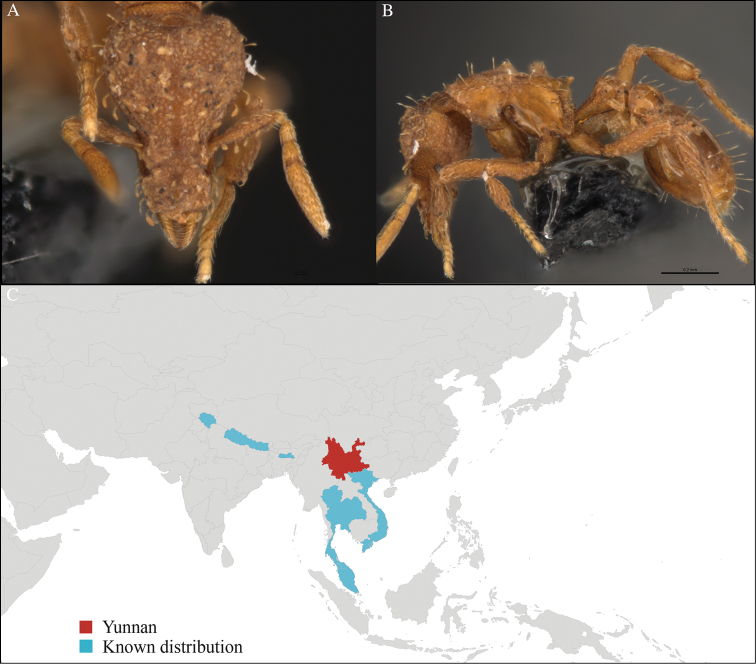
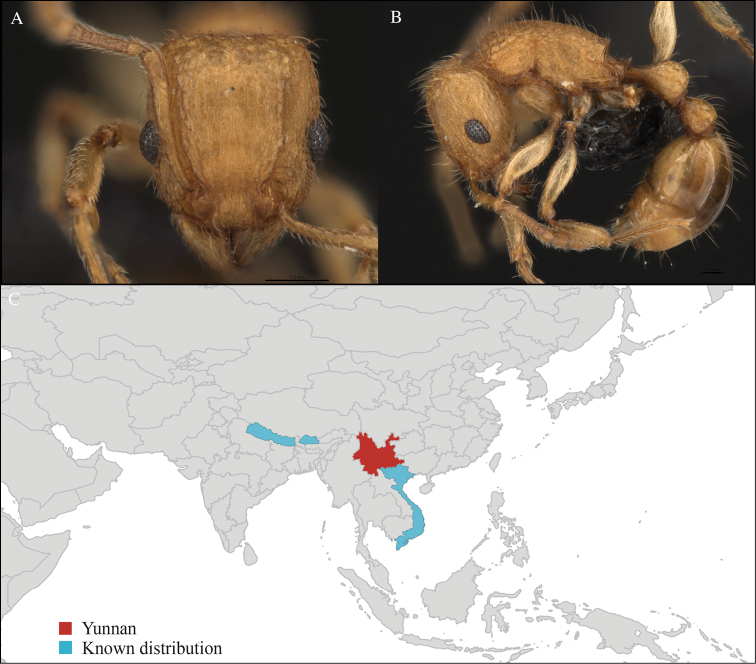
Bannapone scrobiceps
Guénard, Blanchard, Liu, Yang & Economo, 2013
Figure 6.
Bannapone scrobiceps worker, CASENT0339957. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 2 workers, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record) (Figure 6C).
Taxonomic note.
Bannapone scrobiceps was described recently (Guénard et al. 2013).
Natural history.
Little is known about the bionomics of Bannapone scrobiceps. The species was collected from leaf litter in secondary forest located at 550 meters elevation (Guénard et al. 2013).
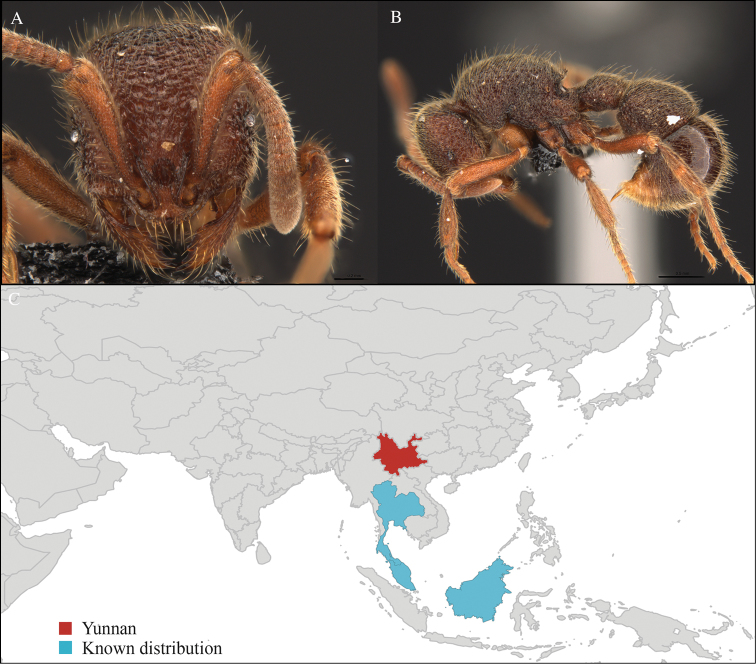
Carebara melasolena
(Zhou & Zheng, 1997)
Figure 7.
Carebara melasolena worker, CASENT0714818. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: Kilometer 55 station (21.960°N, 101.199°E), Rain forest, 10.vi.2013, 23 workers, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in Middle and South China (Figure 7C).
Taxonomic note.
Carebara melasolena can be identified with the key provided by Zhou and Zheng (1997; treated as Pheidologeton melasolenus)
Natural history.
Carebara melasolena has been collected from leaf litter in primary forest.
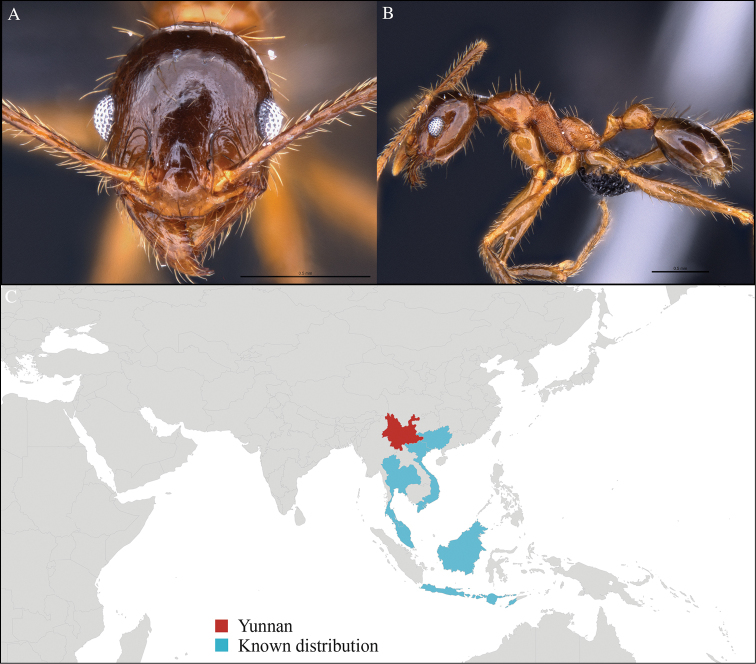
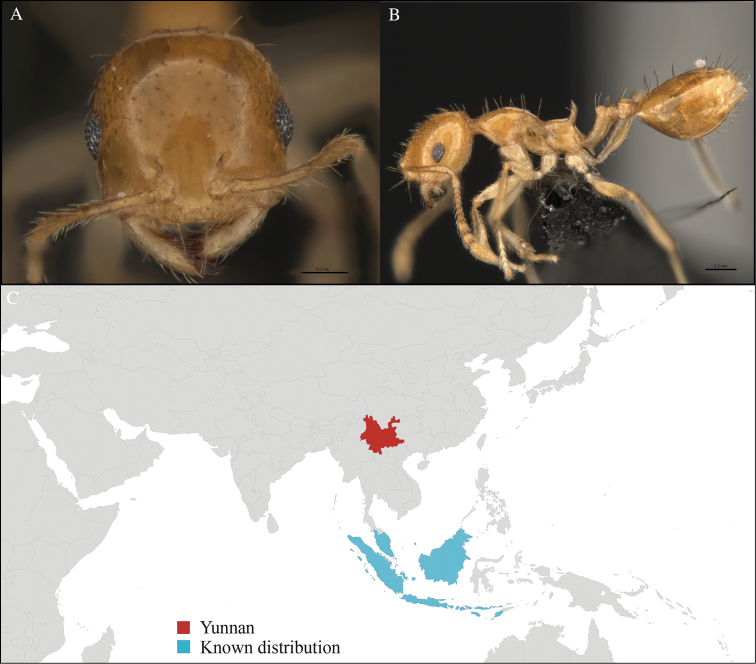
Discothyrea clavicornis
Emery, 1897
Figure 8.
Discothyrea clavicornis worker, CASENT0735814. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 10.vi.2013, 1 worker, 830m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 13.vi.2013, 8 workers, 805m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.201°E), Rain forest, 13.vi.2013, 1 worker, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.964°N, 101.202°E), Rain forest, 13.vi.2013, 3 workers, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.270°E), Rubber plantation, 09.vi.2013, 12 workers, 645m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 6 worker, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.912°N, 101.285°E), Limestone forest, 06.vi.2013, 3 workers, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.274°E), Limestone forest, 05.vi.2013, 15 workers, 552m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.284°E), Limestone forest, 06.vi.2013, 1 worker, 690m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.918°N, 101.271°E), Rain forest, 05.vi.2013, 3 workers, 581m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.281°E), Limestone forest, 05.vi.2013, 1 worker, 650m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.916°N, 101.274°E), Rain forest, 08.vi.2013, 3 workers, 615m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.917°N, 101.274°E), Rain forest, 08.vi.2013, 2 workers, 625m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.922°N, 101.268°E), Rubber plantation, 14.vi.2013, 3 workers, 620m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Discothyrea clavicornis is a very widespread and common species encountered throughout most of the Austral-Asian and Indo-Malayan subregions (Figure 8C). This new record represents an important extension of the northern range in the distribution of this species.
Taxonomic note.
There is no available key for Discothyrea clavicornis. Our identification is based on the original description (Emery 1897), comparison with reference material, and montage images of the holotype provided by AntWeb.
Natural history.
Discothyrea clavicornis has been collected from leaf litter in various habitats such as primary forest, limestone forest and rubber plantation.
Discothyrea kamiteta
Kubota & Terayama, 1999
Figure 9.
Discothyrea kamiteta worker, CASENT0717828. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: Kilometer 55 station (21.963°N, 101.201°E), Rain forest, 13.vi.2013, 1 worker, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.283°E), Limestone forest, 06.vi.2013, 1 worker, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.917°N, 101.274°E), Secondary forest, 08.vi.2013, 1 worker, 625m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 1 worker, 690m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record), Hunan, Okinawa (Figure 9C). This new record represents an important western-most extension in the known distribution of this species.
Taxonomic note.
Discothyrea kamiteta is very similar to the recently described Discothyrea banna Xu, Burwell & Nakamura, 2014. Both species seem to be very close morphologically, and their separation is based on minor differences, which could also be attributed to intraspecific variation. The identification is based on the original description of Discothyrea kamiteta, comparison with Discothyrea kamiteta material from the type locality (Okinawa), and Xu’s key (Xu et al. 2014)
Natural history.
Discothyrea kamiteta has been collected from leaf litter in various habitats, such as primary forest, limestone forest and secondary forest.
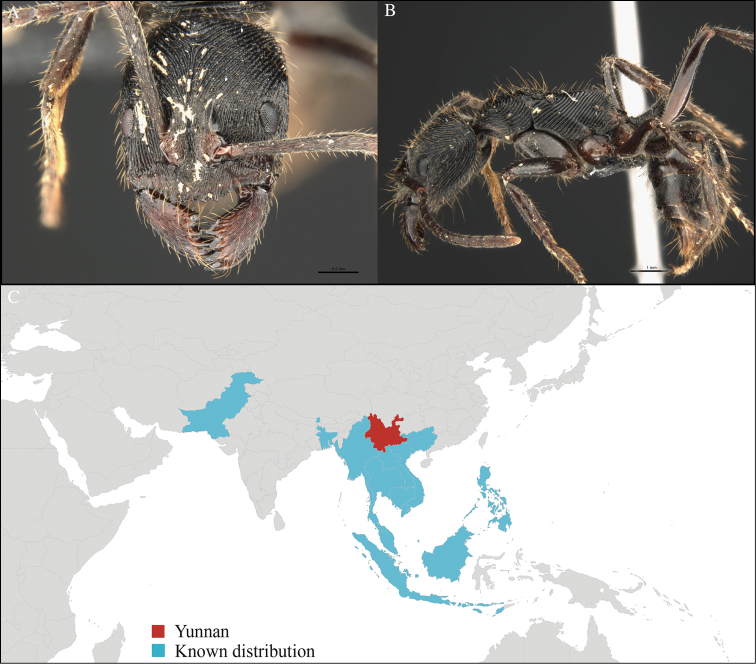
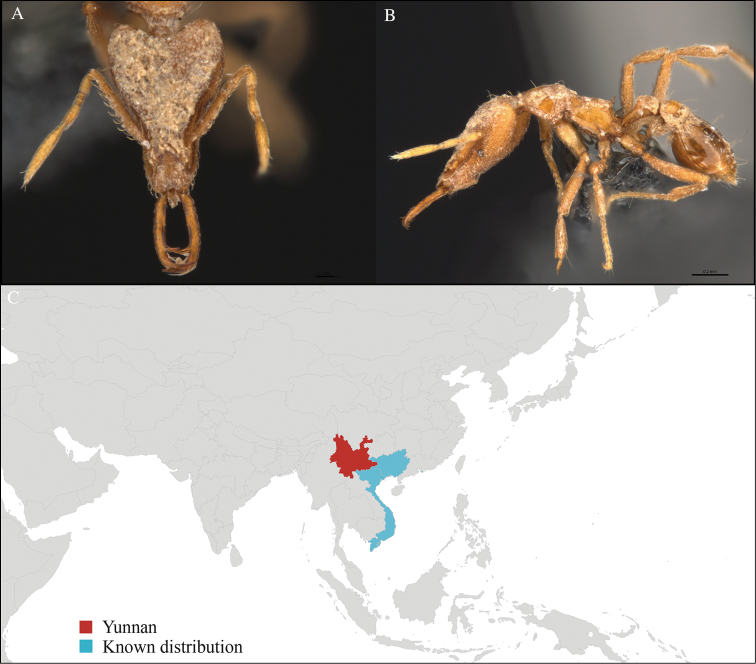
Dolichoderus laotius
Santschi, 1920
Figure 10.
Dolichoderus laotius worker, CASENT0716164. A Head in front view B Alitrunk in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 5 worker, 690m, Hand collection, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record), Laos, Thailand (Figure 10C). This collection represents the northern-most record of Dolichoderus laotius.
Taxonomic note.
There is no available key for the genus in the region. Our identification is based on the description provided by Dill et al. (2002).
Natural history.
Little is known about the bionomics of Dolichoderus laotius. This species has been collected on a tree trunk in secondary forest.
Echinopla cherapunjiensis
Bharti & Gul, 2012
Figure 11.
Echinopla cherapunjiensis worker, CASENT0716524. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.919°N, 101.273°E), Secondary forest, 08.vi.2013, 1 worker, 615m, Hand collection, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record) and Meghalaya (Figure 11C). This new record represents an important northern and western extension in the distribution of Echinopla cherapunjiensis.
Taxonomic note.
There is no available key for this genus. Identification is based on the original description (Bharti and Gul 2012).
Natural history.
Little is known about the bionomics of Echinopla cherapunjiensis. This species has been collected on a tree trunk in secondary forest.
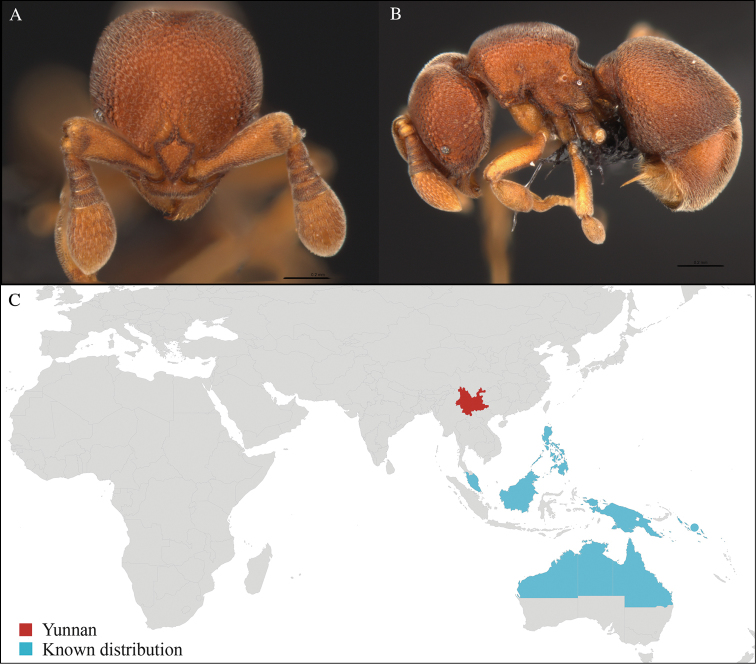
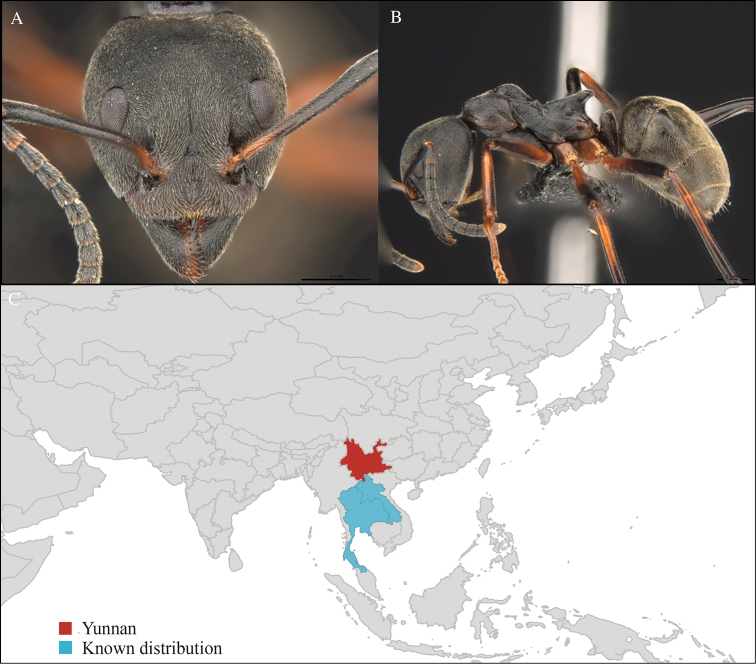
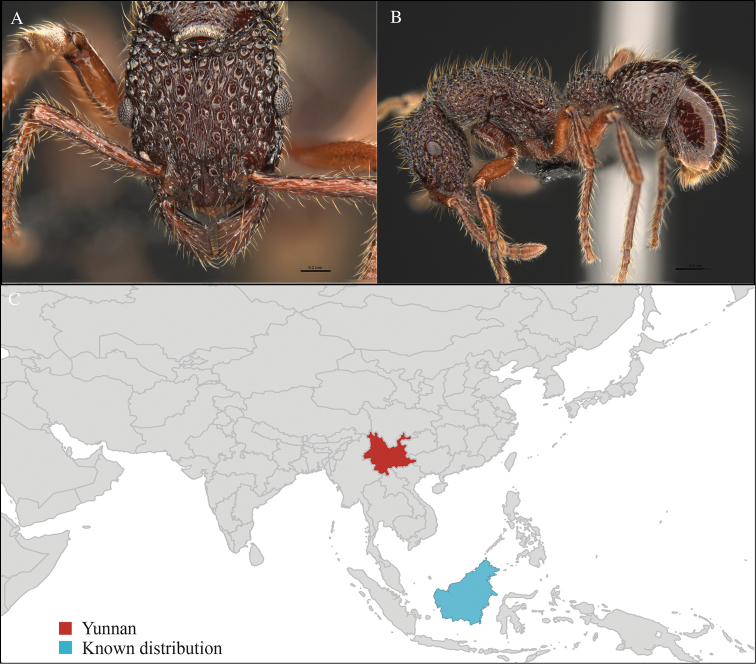
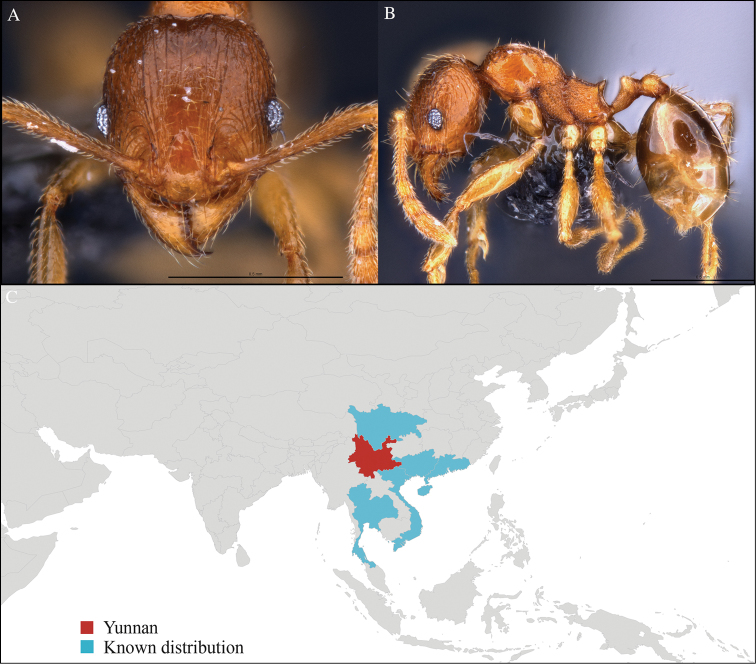
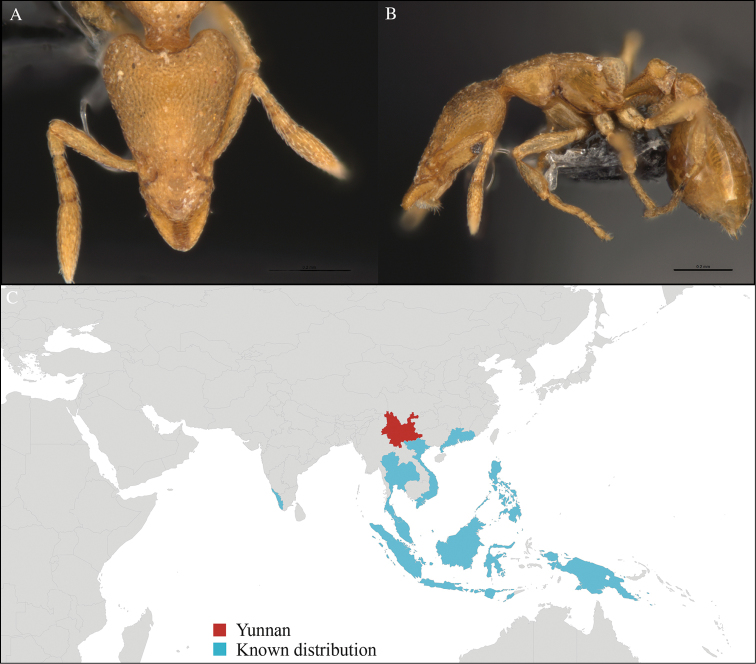
Gesomyrmex kalshoveni
Wheeler, 1929
Figure 12.
Gesomyrmex kalshoveni worker, CASENT0716525. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.925°N, 101.270°E), Forest fragment, 08.vi.2013, 1 worker, 615m, Hand collection, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record), Malaysia and Indonesia (Figure 12C). This new record represents an important extension in the northern range of the distribution of this species and the first occurrence of the genus Gesomyrmex from Yunnan province.
Taxonomic note.
There is no available key for this genus. The identification is based on the original description (Wheeler 1929) and comparison with reference material from Borneo. Identification in Gesomyrmex is generally very difficult due to the high degree of worker polymorphism. However, our single specimen is a minor worker and fits the minor workers of Gesomyrmex kalshoveni very well.
Natural history.
Little is known about the bionomics of Gesomyrmex kalshoveni. It has been collected from a small branch of a tree on the side of road.
Gnamptogenys costata
(Emery, 1889)
Figure 13.
Gnamptogenys costata worker, CASENT0715692. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.911°N, 101.281°E), Limestone forest, 06.vi.2013, 1 worker, 655m, Hand collection, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.274°E), Rain forest, 08.vi.2013, 2 workers, 615m, Hand collection, B. Guénard, B. Blanchard and C. Liu
Distribution.
Widely distributed in the Austral-Asian and Indo-Malayan subregions (Figure 13C).
Taxonomic note.
The identification is based on the key provided by Lattke (2004). The material from Yunnan shows some minor variation in the development of gastral sculpture, which we consider as geographic, intraspecific variation.
Natural history.
Gnamptogenys costata has been collected from foraging columns on the ground in rain forest and limestone forest.
Gnamptogenys treta
Lattke, 2004
Figure 14.
Gnamptogenys treta worker, CASENT0715166. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.912°N, 101.285°E), Limestone forest, 06.vi.2013, 1 worker, 655m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; “Holy Hills” (21.920°N, 101.240°E), Rain forest, 07.vi.2013, 1 worker, 665m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.271°E), Rubber plantation, 09.vi.2013, 7 workers, 645m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.277°E), Secondary forest, 12.vi.2013, 2 workers, 690m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 13.vi.2013, 10 workers, 865m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu
Distribution.
Known from Yunnan (new record) and Borneo (Figure 14C). This collection represents the northern-most record in the distribution of Gnamptogenys treta.
Taxonomic note.
The identification is based on the key provided by Lattke (2004). Our material fits the holotype very well, except for the shape of the ventral process of the petiole, which is more rectangular in the material from Yunnan, whereas in the material from Borneo it is more triangular. Since this is the only difference we were able to observe, we treat it as intraspecific variation.
Natural history.
Gnamptogenys treta has been collected from the leaf litter in rain forest, secondary forest and limestone forest and rubber plantation.
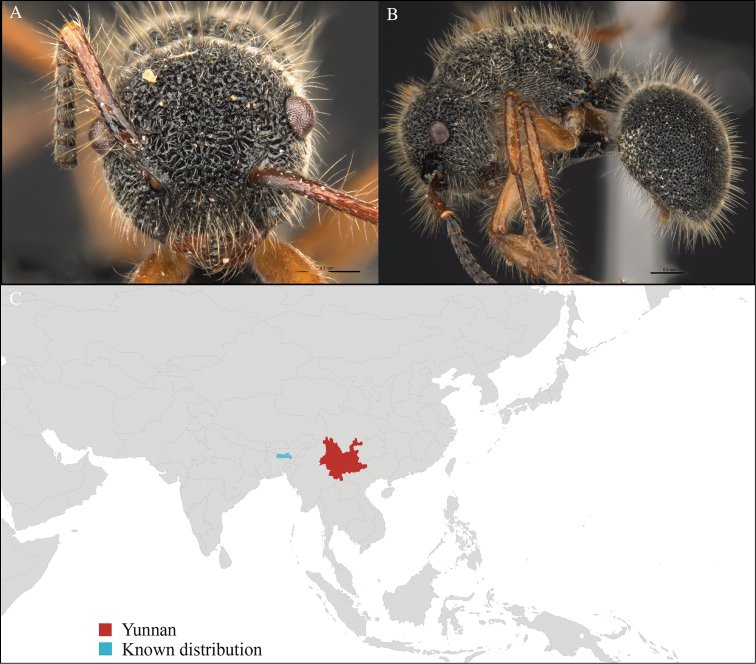
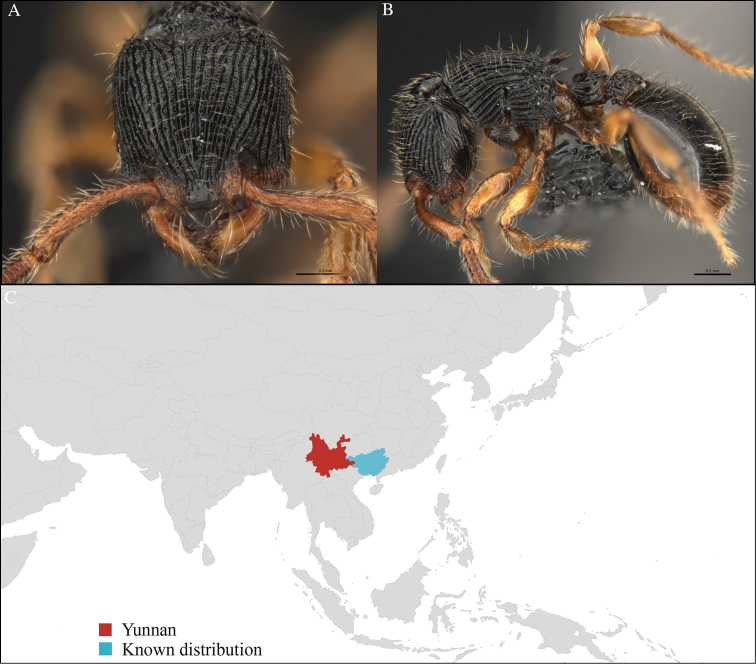
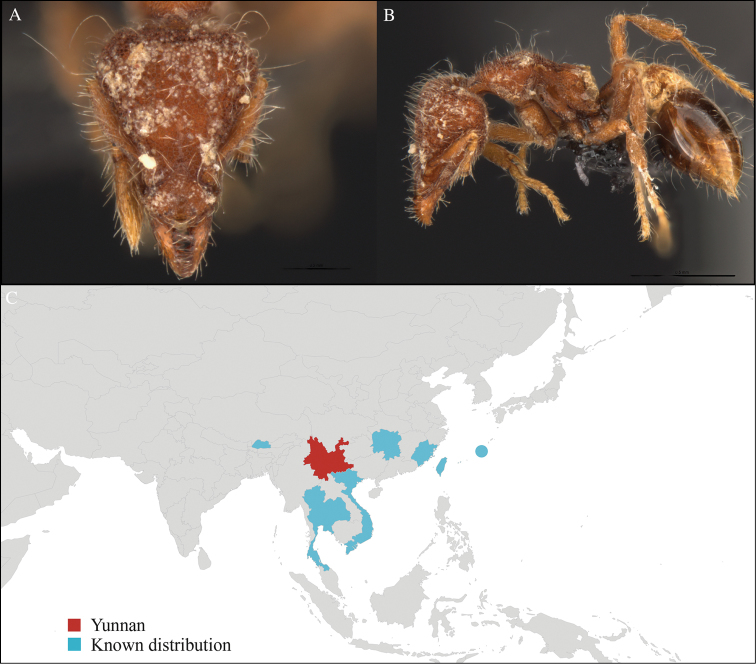
Myrmecina curvispina
Zhou, Huang & Ma L., 2008
Figure 15.
Myrmecina curvispina worker, CASENT0713308. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: “Holy Hills” (21.920°N, 101.240°E), Rain forest, 07.vi.2013, 1 worker, 655m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; “Holy Hills” (21.919°N, 101.239°E), Rain forest, 07.vi.2013, 1 worker, 670m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.961°N, 101.200°E), Rain forest, 10.vi.2013, 1 worker, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Secondary forest, 13.vi.2013, 12 workers, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.963°N, 101.201°E), Rain forest, 13.vi.2013, 14 workers, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.277°E), Rubber plantation, 12.vi.2013, 1 worker, 705m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu;: Man Sai village (21.907°N, 101.273°E), Rubber plantation, 12.vi.2013, 2 workers, 635m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu;: Man Sai village (21.858°N, 101.277°E), Secondary forest, 12.vi.2013, 2 workers, 685m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 3 workers, 690m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.860°N, 101.278°E), Secondary forest, 12.vi.2013, 2 workers, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.271°E), Rubber plantation, 09.vi.2013, 3 workers, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 1 worker, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.912°N, 101.182°E), Limestone forest, 06.vi.2013, 9 workers, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.888°N, 101.266°E), Rubber Plantation, 14.vi.2013, 7 workers, 600m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.889°N, 101.267°E), Rubber Plantation, 14.vi.2013, 3 workers, 630m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.890°N, 101.267°E), Rubber Plantation, 14.vi.2013, 2 workers, 620m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record) and Guangxi (Figure 15C). This new record represents the western-most occurrence in the distribution of Myrmecina curvispina.
Taxonomic note.
The identification is based on the original description and the identification key given by Zhou et al. (2008).
Natural history.
Myrmecina curvispina has been collected from the leaf litter of various habitats such as rain forest, secondary forest and rubber plantation.
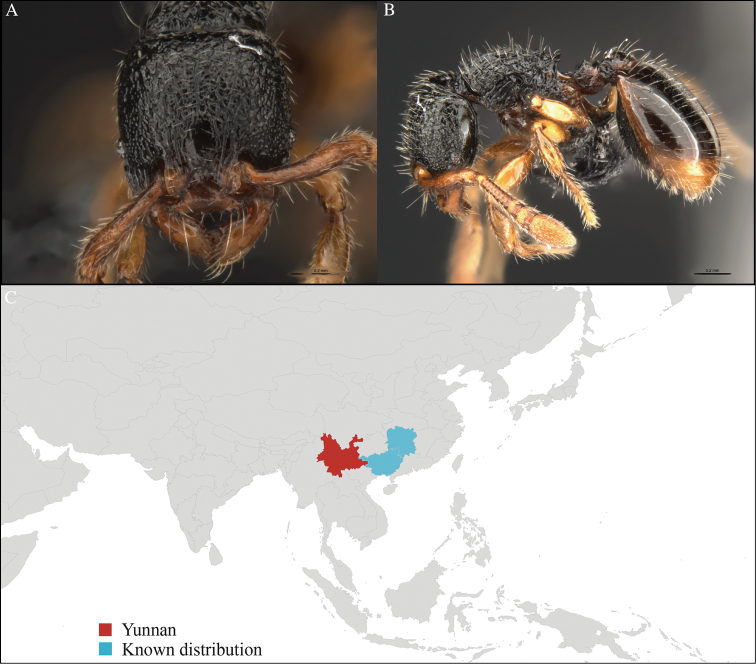
Myrmecina guangxiensis
Zhou, 2001
Figure 16.
Myrmecina guangxiensis worker, CASENT0713314. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.912°N, 101.285°E), Secondary forest, 05.vi.2013, 4 workers, 552m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.274°E), Limestone forest, 06.vi.2013, 1 worker, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.281°E), Limestone forest, 06.vi.2013, 2 workers, 650m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.960°N, 101.199°E), Rain forest, 10.vi.2013, 1 worker, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.963°N, 101.201°E), Rain forest, 13.vi.2013, 9 workers, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record), Guangxi and Hunan (Figure 16C). This new record represents the western-most occurrence in the distribution of Myrmecina guangxiensis.
Taxonomic note.
Identification is based on the key provided by Zhou et al. (2008).
Natural history.
Myrmecina guangxiensis has been collected from leaf litter in rain forest, secondary forest and limestone forest.
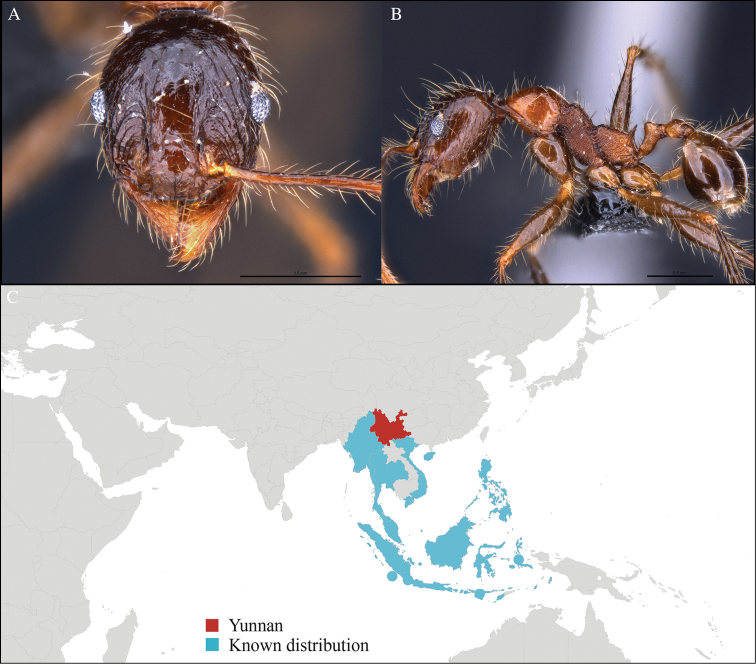
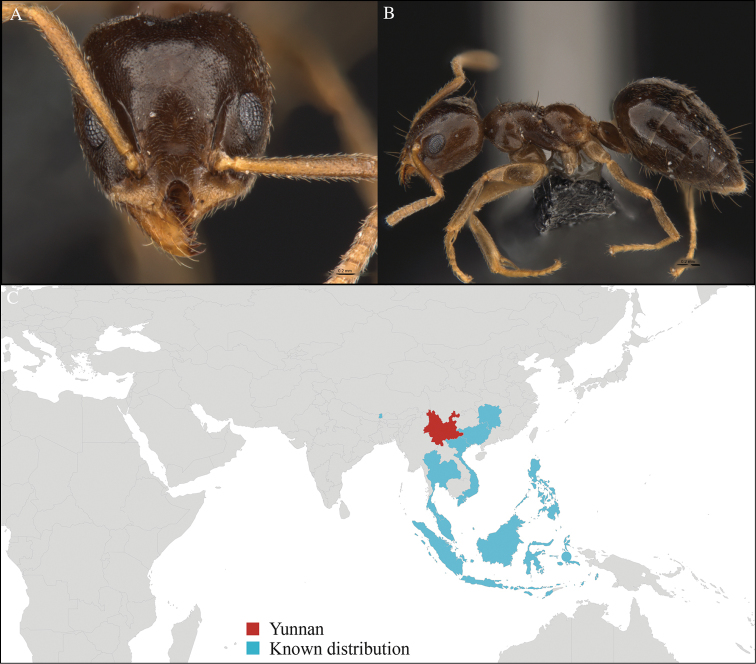
Odontoponera denticulata
(Smith, 1858)
Figure 17.
Odontoponera denticulata worker, CASENT0717236. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: “Holy Hills” (21.920°N, 101.240°E), Secondary forest, 07.vi.2013, 1 worker, 655m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; “Holy Hills” (21.920°N, 101.239°E), Secondary forest, 07.vi.2013, 2 workers, 665m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Secondary forest, 13.vi.2013, 1 worker, 825m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Secondary forest, 13.vi.2013, 1 worker, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 13.vi.2013, 3 workers, 805m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.963°N, 101.201°E), Rain forest, 13.vi.2013, 2 workers, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.964°N, 101.202°E), Rain forest, 13.vi.2013, 1 worker, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.907°N, 101.273°E), Rubber plantation, 12.vi.2013, 1 worker, 635m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.860°N, 101.278°E), Rubber plantation, 12.vi.2013, 1 worker, 710m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 1 worker, 685m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.860°N, 101.278°E), Secondary forest, 12.vi.2013, 1 worker, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.933°N, 101.269°E), Rubber plantation, 09.vi.2013, 1 worker, 655m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.271°E), Rubber plantation, 09.vi.2013, 5 workers, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.270°E), Rubber plantation, 09.vi.2013, 2 workers, 645m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.269°E), Rubber plantation, 09.vi.2013, 1 worker, 645m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.924°N, 101.268°E), Rubber plantation, 06.vi.2013, 3 workers, 571m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 1 worker, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.284°E), Limestone forest, 06.vi.2013, 3 workers, 690m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.912°N, 101.282°E), Limestone forest, 05.vi.2013, 1 worker, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.917°N, 101.274°E), Secondary forest, 08.vi.2013, 1 worker, 625m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.889°N, 101.267°E), Rubber Plantation, 14.vi.2013, 4 workers, 630m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.888°N, 101.266°E), Rubber Plantation, 14.vi.2013, 2 workers, 600m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.888°N, 101.266°E), Rubber Plantation, 14.vi.2013, 3 workers, 620m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.889°N, 101.267°E), Rubber Plantation, 14.vi.2013, 3 workers, 630m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.922°N, 101.268°E), Rubber Plantation, 14.vi.2013, 4 workers, 620m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.890°N, 101.267°E), Rubber Plantation, 14.vi.2013, 2 workers, 620m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in the Indo-Malayan subregion (Figure 17C).
Taxonomic note.
The identification of our material is based on Yamane’s (2009) redescription of Odontoponera denticulata.
Natural history.
Odontoponera denticulata has been collected from the leaf litter in various habitats such as rain forest, secondary forest, limestone forest and rubber plantation.
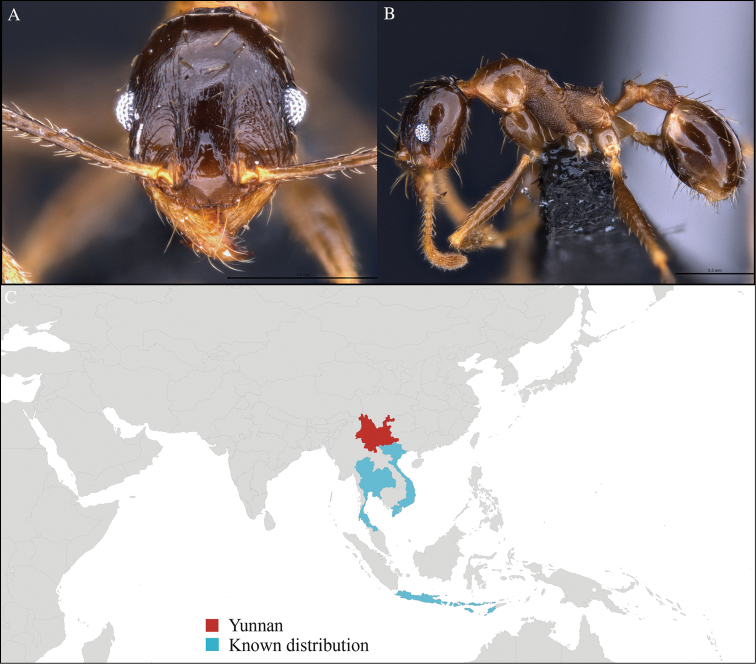
Pheidole hongkongensis
Wheeler, 1928
Figure 18.
Pheidole hongkongensis worker, CASENT0714788. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 1 worker, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.274°E), Secondary forest, 05.vi.2013, 3 workers, 1 Soldier, 552m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.924°N, 101.268°E), Secondary forest, 05.vi.2013, 2 workers, 571m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.918°N, 101.271°E), Secondary forest, 05.vi.2013, 1 worker, 581m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.912°N, 101.285°E), Limestone forest, 06.vi.2013, 49 workers, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.912°N, 101.282°E), Limestone forest, 06.vi.2013, 6 workers, 1 Soldier, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.933°N, 101.269°E), Rubber plantation, 09.vi.2013, 3 workers, 655m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 20 workers, 685m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.860°N, 101.278°E), Secondary forest, 12.vi.2013, 1 worker, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.907°N, 101.273°E), Rubber plantation, 12.vi.2013, 3 workers, 635m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 13.vi.2013, 5 workers, 1 Soldier, 805m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.922°N, 101.268°E), Rubber Plantation, 14.vi.2013, 1 worker, 629m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
South China, Vietnam and Thailand (Figure 18C).
Taxonomic note.
Pheidole hongkongensis can be identified with the identification key to northern Vietnamese Pheidole published by Eguchi (2008).
Natural history.
Pheidole hongkongensis has been collected from leaf litter in secondary forest, limestone forest and rubber plantations. It has also been reported inhabiting the soil of woody gardens, forest edges and open areas (Eguchi 2008).
Pheidole plagiaria
Smith, 1860
Figure 19.
Pheidole plagiaria worker, CASENT0713421. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.912°N, 101.282°E), Limestone forest, 06.vi.2013, 2 workers, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.933°N, 101.269°E), Rubber Plantation, 09.vi.2013, 1 worker, 655m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.960°N, 101.199°E), Rain forest, 10.vi.2013, 13 workers, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 10.vi.2013, 2 workers, 830m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in the Australasian and Indo-Malayan subregions (Figure 19C).
Taxonomic note.
Pheidole plagiaria can be identified with the identification key to Northern Vietnamese Pheidole provided by Eguchi (2008).
Natural history.
Pheidole plagiaria has been collected from leaf litter from rain forest, limestone forest and rubber plantation. It has also been reported inhabiting in the soil of forest edge and open land (Eguchi 2008).
Pheidole planifrons
Santschi, 1920
Figure 20.
Pheidole planifrons worker, CASENT0713099. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.919°N, 101.274°E), Secondary forest, 05.vi.2013, 2 workers, 552m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.924°N, 101.268°E), Rubber plantation, 05.vi.2013, 3 workers, 571m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.283°E), Limestone forest, 06.vi.2013, 2 workers, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.912°N, 101.282°E), Limestone forest, 06.vi.2013, 4 workers, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.281°E), Limestone forest, 06.vi.2013, 2 workers, 650m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.916°N, 101.274°E), Limestone forest, 08.vi.2013, 2 workers, 615m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.271°E), Rubber plantation, 09.vi.2013, 1 worker, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.277°E), Rubber plantation, 12.vi.2013, 4 workers, 705m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.857°N, 101.277°E), Rubber plantation, 12.vi.2013, 1 worker, 710m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.907°N, 101.273°E), Rubber plantation, 12.vi.2013, 2 workers, 635m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.277°E), Secondary forest, 12.vi.2013, 2 workers, 685m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 9 workers, 690m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.860°N, 101.273°E), Secondary forest, 12.vi.2013, 1 worker, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Rain forest, 13.vi.2013, 3 workers, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.889°N, 101.267°E), Rubber Plantation, 14.vi.2013, 33 workers, 630m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.922°N, 101.268°E), Rubber Plantation, 14.vi.2013, 2 workers, 620m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record), Vietnam, Thailand and Java (Figure 20C). This new record represents the northern-most occurrence in the known distribution of Pheidole planifrons.
Taxonomic note.
Pheidole planifrons can be identified with the identification key to Northern Vietnamese Pheidole provided by Eguchi (2008).
Natural history.
Pheidole planifrons has been collected from leaf litter in rain forest, limestone forest and rubber plantations. It has also been reported inhabiting in the soil of forest edge and woody habitats (Eguchi 2008).
Pheidole rugithorax
Eguchi, 2008
Figure 21.
Pheidole rugithorax worker, CASENT0717083. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 3 workers, 1 Soldier, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.283°E), Limestone forest, 06.vi.2013, 1 worker, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.281°E), Limestone forest, 06.vi.2013, 3 workers, 650m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; “Holy Hills” (21.920°N, 101.239°E), Rain forest, 07.vi.2013, 2 workers, 665m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Yunnan (new record), Vietnam, Myanmar and Thailand (Figure 21C).
Taxonomic note.
Pheidole rugithorax can be identified with the identification key to Northern Vietnamese Pheidole provided by Eguchi (2008).
Natural history.
Pheidole rugithorax has been collected from leaf litter in rain forest, secondary forest and limestone forest. Otherwise there is no available information on its biology.
Pheidole smythiesii
Forel, 1902
Figure 22.
Pheidole smythiesii worker, CASENT0713851. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
China, Yunnan, Xishuangbanna: XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 9 workers, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.918°N, 101.271°E), Secondary forest, 05.vi.2013, 2 workers, 581m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.283°E), Limestone forest, 05.vi.2013, 1 worker, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 5 workers, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in South China, Vietnam, Thailand and India (Figure 22C).
Taxonomic note.
Pheidole smythiesii can be identified with the identification key to Northern Vietnamese Pheidole provided by Eguchi (2008).
Natural history.
Pheidole smythiesii has been collected from leaf litter from secondary forest. Eguchi (2008) reported the species to usually inhabit woody habitats and sometimes open areas where it nests in the soil. Pheidole smythiesii is also known to tend aphid colonies (Alfred and Agarwal 1990).
Pheidole tumida
Eguchi, 2008
Figure 23.
Pheidole tumida worker, CASENT0713125. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: “Holy Hills” (21.920°N, 101.239°E), Rain forest, 07.vi.2013, 2 workers, 665m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Rain forest, 13.vi.2013, 2 workers, 825m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Rain forest, 13.vi.2013, 3 workers, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.277°E), Rubber plantation, 12.vi.2013, 4 workers, 705m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.907°N, 101.273°E), Rubber plantation, 12.vi.2013, 2 workers, 635m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.857°N, 101.277°E), Rubber plantation, 12.vi.2013, 3 workers, 710m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.277°E), Secondary forest, 12.vi.2013, 15 workers, 685m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 2 workers, 690m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.860°N, 101.278°E), Secondary forest, 12.vi.2013, 2 workers, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 4 workers, 2 Soldiers, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.934°N, 101.269°E), Rubber plantation, 09.vi.2013, 5 workers, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.933°N, 101.269°E), Rubber plantation, 09.vi.2013, 34 workers, 655m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.270°E), Rubber plantation, 09.vi.2013, 2 workers, 645m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.271°E), Rubber plantation, 09.vi.2013, 3 workers, 645m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.931°N, 101.269°E), Rubber plantation, 09.vi.2013, 1 worker, 645m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.933°N, 101.269°E), Rubber plantation, 09.vi.2013, 2 workers, 655m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.274°E), Secondary forest, 05.vi.2013, 1 worker, 552m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.283°E), Secondary forest, 06.vi.2013, 1 worker, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.924°N, 101.268°E), Secondary forest, 05.vi.2013, 1 worker, 571m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.888°N, 101.266°E), Rubber Plantation, 14.vi.2013, 1 worker, 600m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.888°N, 101.266°E), Rubber Plantation, 14.vi.2013, 1 worker, 620m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.922°N, 101.268°E), Rubber Plantation, 14.vi.2013, 6 workers, 620m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in the Australasian and Indo-Malayan subregions (Figure 23C).
Taxonomic note.
Pheidole tumida can be identified with the identification key to Northern Vietnamese Pheidole provided by Eguchi (2008).
Natural history.
Pheidole tumida has been collected from leaf litter in rain forest, secondary forest and rubber plantation. It has also been reported nesting in the soil and rotting logs of forest edges (Eguchi 2008).
Pheidole vieti
Eguchi, 2008
Figure 24.
Pheidole vieti worker, CASENT0713428. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.912°N, 101.285°E), Limestone forest, 06.vi.2013, 3 workers, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.917°N, 101.274°E), Secondary forest, 06.vi.2013, 3 workers, 625m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.961°N, 101.200°E), Rain forest, 10.vi.2013, 3 workers, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Known from Yunnan (new record) and Vietnam (Figure 24C). This new record represents the northern-most occurrence in the distribution of Pheidole vieti.
Taxonomic note.
Pheidole vieti can be identified with the key given provided by Eguchi (2008).
Natural history.
Pheidole vieti has been collected from leaf litter from rain forest, secondary forest and limestone forest.
Pheidole zoceana
Santschi, 1925
Figure 25.
Pheidole zoceana worker, CASENT0714742. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: “Holy Hills” (21.920°N, 101.240°E), Secondary forest, 07.vi.2013, 44 workers, 644m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; “Holy Hills” (21.920°N, 101.239°E), Secondary forest, 07.vi.2013, 5 workers, 665m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; “Holy Hills” (21.919°N, 101.239°E), Secondary forest, 07.vi.2013, 11 workers, 670m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Secondary forest, 13.vi.2013, 15 workers, 825m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Secondary forest, 13.vi.2013, 1 worker, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 13.vi.2013, 7 workers, 3 Soldiers, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.960°N, 101.199°E), Rain forest, 13.vi.2013, 25 workers, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 13.vi.2013, 5 workers, 805m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.963°N, 101.201°E), Rain forest, 13.vi.2013, 14 workers, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.964°N, 101.202°E), Rain forest, 13.vi.2013, 2 workers, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 2 workers, 690m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 1 worker, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.271°E), Rubber Plantation, 09.vi.2013, 1 worker, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 77 workers, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.912°N, 101.285°E), Limestone forest, 05.vi.2013, 22 workers, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.274°E), Secondary forest, 06.vi.2013, 2 workers, 552m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.924°N, 101.268°E), Rubber plantation, 05.vi.2013, 3 workers, 571m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.918°N, 101.271°E), Secondary forest, 05.vi.2013, 1 worker, 581m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.916°N, 101.274°E), Secondary forest, 05.vi.2013, 12 workers, 615m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Known from a few localities in China, Vietnam and Thailand (Figure 25C).
Taxonomic note.
Pheidole zoceana can be identified with the identification key to Northern Vietnamese Pheidole provided by Eguchi (2008).
Natural history.
Pheidole zoceana has been collected from leaf litter in rain forest, secondary forest and rubber plantations. It has also been reported nesting in the soil of forest edges and mountainous area (Eguchi 2008).
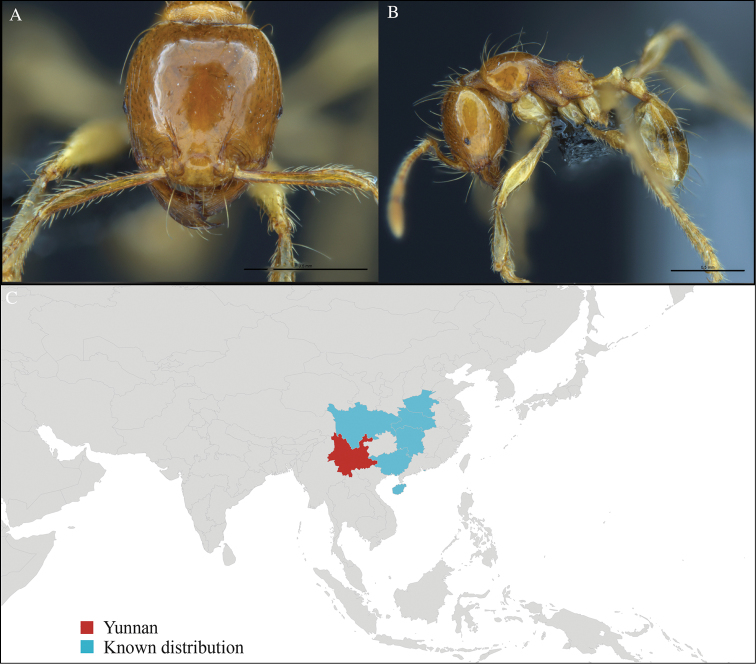
Prenolepis sphingthoraxa
Zhou & Zheng, 1998
Figure 26.
Prenolepis sphingthoraxa worker, CASENT0715549. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: Kilometer 55 station (21.960°N, 101.199°E), Rain forest, 10.vi.2013, 1 worker, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Known from Middle and South China (Figure 26C). This new record represents the western-most record in the distribution of Prenolepis sphingthoraxa.
Taxonomic note.
The identification is based on the original description (Zhou and Zheng 1998).
Natural history.
Prenolepis sphingthoraxa has been collected from leaf litter in rain forest and little is known about its bionomics.
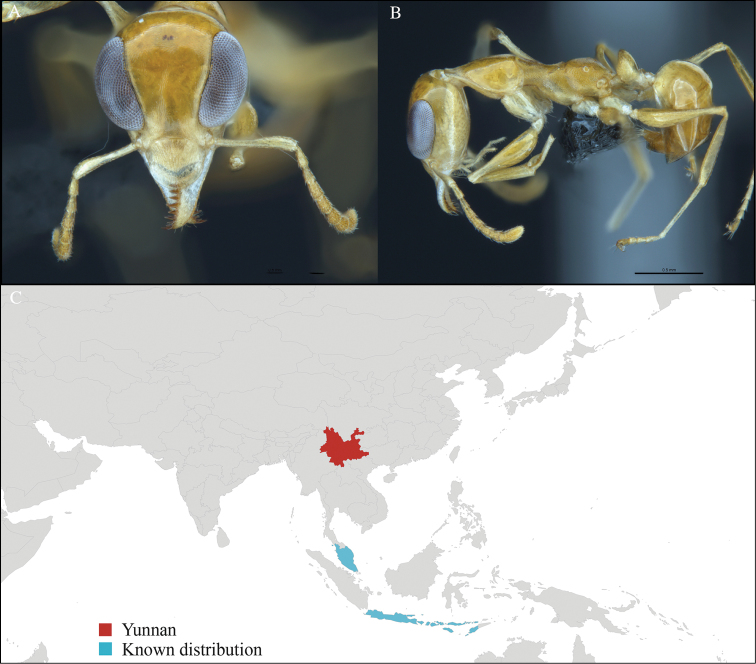
Proceratium deelemani
Perrault, 1981
Figure 27.
Proceratium deelemani worker, CASENT0717686. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: Kilometer 55 station (21.964°N, 101.202°E), Rain forest, 13.vi.2013, 1 worker, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Known from Yunnan (new record), Singapore, Thailand and Borneo (Figure 27C). This new record represents the northern-most record in the distribution of Proceratium deelemani.
Taxonomic note.
The identification of Proceratium deelemani is relatively straightforward with the key provided by Baroni Urbani and De Andrade (2003).
Natural history.
Proceratium deelemani has been collected from leaf litter in rain forest, and little is known about its bionomics.
Recurvidris kemneri
(Wheeler & Wheeler, 1954)
Figure 28.
Recurvidris kemneri worker, CASENT0715218. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: “Holy Hills” (21.919°N, 101.239°E), Secondary forest, 07.vi.2013, 1 worker, 670m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.857°N, 101.277°E), Rubber plantation, 12.vi.2013, 3 workers, 710m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 1 worker, 685m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.860°N, 101.278°E), Secondary forest, 12.vi.2013, 1 worker, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.963°N, 101.201°E), Rain forest, 10.vi.2013, 7 workers, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in the Austral-Asian and Indo-Malayan subregions (Figure 28C). This new northern-most record represents an important extension in the distribution of Recurvidris kemneri.
Taxonomic note.
The identification is based on Bolton’s (1992) key. Our material from Yunnan fits the re-description in the latter publication very well, even though the propodeal spines seem somewhat longer than in the material from Borneo. However, we consider this as a minor geographic variation.
Natural history.
Recurvidris kemneri has been collected from leaf litter from rain forest, secondary forest and rubber plantation, and little is known about its bionomics.
Strumigenys dyschima
(Bolton, 2000)
Figure 29.
Strumigenys dyschima worker, CASENT0717009. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.911°N, 101.283°E), Limestone forest, 06.vi.2013, 2 workers, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Known from Yunnan (new record) and Borneo (Figure 29C). This new record represents an important extension in the northern range of the distribution of Strumigenys dyschima.
Taxonomic note.
Strumigenys dyschima can be identified with the identification key given by Bolton (2000; treated as Pyramica dyschima).
Natural history.
Strumigenys dyschima has been collected from leaf litter in limestone forest, and little is known about its bionomics.
Strumigenys kichijo
(Terayama, Lin & Wu, 1996)
Figure 30.
Strumigenys kichijo worker, CASENT0713674. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.924°N, 101.268°E), Rubber Plantation, 05.vi.2013, 1 worker, 571m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.934°N, 101.269°E), Rubber Plantation, 09.vi.2013, 1 worker, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in Indo-Malayan subregions (Figure 30C).
Taxonomic note.
Strumigenys kichijo can be identified with the identification key given by Bolton (2000; treated as Pyramica kichijo).
Natural history.
Strumigenys kichijo has been collected from leaf litter in rubber plantations, and little is known about its bionomics.
Strumigenys mitis
(Brown, 2000)
Figure 31.
Strumigenys mitis worker, CASENT0713676. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: “Holy Hills” (21.920°N, 101.239°E), Secondary forest, 07.vi.2013, 9 workers, 655m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; “Holy Hills” (21.919°N, 101.239°E), Rain forest, 07.vi.2013, 7 workers, 670m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Secondary forest, 13.vi.2013, 40 workers, 825m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.966°N, 101.203°E), Secondary forest, 13.vi.2013, 1 worker, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 10.vi.2013, 19 workers, 830m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.961°N, 101.200°E), Rain forest, 10.vi.2013, 8 workers, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.960°N, 101.199°E), Rain forest, 13.vi.2013, 1 worker, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 13.vi.2013, 111 worker, 805m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.963°N, 101.201°E), Rain forest, 13.vi.2013, 122 workers, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.964°N, 101.202°E), Rain forest, 13.vi.2013, 50 workers, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.277°E), Secondary forest, 12.vi.2013, 1 worker, 685m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 12 workers, 690m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.271°E), Rubber plantation, 09.vi.2013, 8 workers, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.932°N, 101.270°E), Rubber plantation, 09.vi.2013, 1 worker, 645m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.931°N, 101.269°E), Rubber plantation, 09.vi.2013, 1 worker, 645m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.924°N, 101.268°E), Rubber Plantation, 05.vi.2013, 1 worker, 571m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 82 workers, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.274°E), Secondary forest, 05.vi.2013, 48 workers, 552m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.918°N, 101.271°E), Secondary forest, 05.vi.2013, 71 workers, 581m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.916°N, 101.274°E), Secondary forest, 08.vi.2013, 2 workers, 615m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.917°N, 101.274°E), Secondary forest, 08.vi.2013, 25 workers, 625m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in Austral-Asian and Indo-Malayan subregions (Figure 31C). This new record represents the northern-most known occurrence in the distribution of Strumigenys mitis.
Taxonomic note.
Strumigenys mitis can be identified with the identification key given by Bolton (2000; treated as Pyramica mitis) and Bharti (2013, treated as Pyramica mitis).
Natural history.
Strumigenys mitis has been collected from leaf litter in rain forest, secondary forest and rubber plantations, and little is known about its bionomics.
Strumigenys nepalensis
Baroni Urbani & De Andrade, 1994
Figure 32.
Strumigenys nepalensis worker, CASENT0715046. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: “Holy Hills” (21.920°N, 101.240°E), Secondary forest, 07.vi.2013, 5 workers, 655m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; “Holy Hills” (21.920°N, 101.239°E), Secondary forest, 07.vi.2013, 12 workers, 665m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; “Holy Hills” (21.919°N, 101.239°E), Rain forest, 07.vi.2013, 1 worker, 670m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.963°N, 101.201°E), Rain forest, 13.vi.2013, 2 workers, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.277°E), Rubber Plantation, 12.vi.2013, 2 workers, 705m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.857°N, 101.277°E), Rubber Plantation, 12.vi.2013, 2 workers, 710m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.277°E), Secondary forest, 12.vi.2013, 2 workers, 685m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 3 workers, 690m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 4 workers, 685m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.860°N, 101.278°E), Secondary forest, 12.vi.2013, 6 workers, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.858°N, 101.276°E), Secondary forest, 12.vi.2013, 1 worker, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 57 workers, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.912°N, 101.285°E), Limestone forest, 06.vi.2013, 1 worker, 680m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.274°E), Secondary forest, 05.vi.2013, 13 workers, 552m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.283°E), Limestone forest, 06.vi.2013, 7 workers, 675m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.912°N, 101.282°E), Limestone forest, 06.vi.2013, 21 workers, 640m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.281°E), Limestone forest, 06.vi.2013, 21 workers, 650m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.888°N, 101.266°E), Rubber plantation, 14.vi.2013, 3 workers, 600m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.888°N, 101.266°E), Rubber plantation, 14.vi.2013, 6 workers, 620m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Banna University construction site (21.922°N, 101.268°E), Rubber plantation, 14.vi.2013, 1 worker, 620m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Known from Yunnan (new record), North Indian, Vietnam and Thailand (Figure 32C).
Taxonomic note.
Strumigenys nepalensis can be identified with the identification key given by Bolton (2000; treated as Pyramica nepalensis).
Natural history.
Strumigenys nepalensis has been collected from leaf litter in rain forest, secondary forest, limestone forest and rubber plantations, and little is known about its bionomics.
Strumigenys rallarhina
Bolton, 2000
Figure 33.
Strumigenys rallarhina worker, CASENT0715395. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.919°N, 101.272°E), Secondary forest, 05.vi.2013, 121 workers, 550m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.274°E), Secondary forest, 05.vi.2013, 34 workers, 552m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.918°N, 101.271°E), Secondary forest, 05.vi.2013, 35 workers, 581m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.916°N, 101.274°E), Secondary forest, 08.vi.2013, 7 workers, 615m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.917°N, 101.274°E), Secondary forest, 08.vi.2013, 44 workers, 625m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 10.vi.2013, 22 workers, 830m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.961°N, 101.200°E), Rain forest, 10.vi.2013, 15 workers, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.960°N, 101.199°E), Rain forest, 10.vi.2013, 26 workers, 840m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 13.vi.2013, 9 workers, 805m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.964°N, 101.202°E), Rain forest, 13.vi.2013, 16 workers, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Known from Yunnan (new record), Guangxi and Vietnam (Figure 33C).
Taxonomic note.
Strumigenys rallarhina can be identified with the identification key provided by Bolton (2000).
Natural history.
Strumigenys rallarhina has been collected from leaf litter in rain forest and secondary forest, and little is known about its bionomics.
Strumigenys sauteri
(Forel, 1912)
Figure 34.
Strumigenys sauteri worker, CASENT0717023. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.918°N, 101.271°E), Secondary forest, 05.vi.2013, 10 workers, 581m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.917°N, 101.274°E), Secondary forest, 08.vi.2013, 3 workers, 625m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.962°N, 101.200°E), Rain forest, 13.vi.2013, 9 workers, 805m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.963°N, 101.201°E), Rain forest, 13.vi.2013, 3 workers, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in Indo-Malayan subregions (Figure 34C).
Taxonomic note.
Strumigenys sauteri can be identified with the identification key given by Bolton (2000; treated as Pyramica sauteri).
Natural history.
Strumigenys sauteri has been collected from leaf litter in rain forest and secondary forest, and little is known about its bionomics.
Technomyrmex pratensis
(Smith, 1860)
Figure 35.
Technomyrmex pratensis worker, CASENT0715863. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.918°N, 101.271°E), Secondary forest, 05.vi.2013, 4 workers, 581 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.274°E), Secondary forest, 11.vi.2013, 4 workers, 590 m, Hand collection, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in the Austral-Asian and Indo-Malayan subregions (Figure 35C).
Taxonomic note.
Technomyrmex pratensis is the only member of the Technomyrmex pratensis species group. It is a very conspicuous species within the genus, and its identification is very easy with the key provided by Bolton (2007).
Natural history.
Technomyrmex pratensis has been collected from leaf litter in secondary forest, and little is known about its bionomics.
Tetramorium difficile
Bolton, 1977
Figure 36.
Tetramorium difficile worker, CASENT0713193. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.918°N, 101.271°E), Secondary forest, 05.vi.2013, 2 workers, 552 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Known form Yunnan (new record), northern India, and Vietnam (Figure 36C).
Taxonomic note.
Tetramorium difficile is a member of the Tetramorium tonganum group and can be identified with the key provided by Bolton (1977). However, Tetramorium difficile under its current definition is morphologically very close to Tetramorium tonganum. It is likely that both are conspecific and the material listed as Tetramorium difficile represents intraspecific forms of the very widespread Tetramorium tonganum.
Natural history.
Tetramorium difficile has been collected from leaf litter in secondary forest, and little is known about its bionomics.
Tetramorium flavipes
Emery, 1893
Figure 37.
Tetramorium flavipes worker, CASENT0713761. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.918°N, 101.271°E), Secondary forest, 05.vi.2013, 35 workers, 552 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.917°N, 101.274°E), Secondary forest, 08.vi.2013, 33 workers, 625 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.961°N, 101.201°E), Rain forest, 10.vi.2013, 8 workers, 820m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Kilometer 55 station (21.963°N, 101.200°E), Rain forest, 13.vi.2013, 5 workers, 815m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Known from Yunnan (new record), Vietnam, Thailand and Sri Lanka (Figure 37C). This new record represents the northern-most record in the distribution of this species.
Taxonomic note.
Tetramorium flavipes is a member of the Tetramorium tortuosum group. Its identification is relatively straightforward with the key given by Bolton (1977). However, Tetramorium flavipes, originally described from Thailand, is very close to Tetramorium eleates Forel, 1913 from Borneo and the Philippines, and as already pointed out by Bolton (1977), both could represent geographic variants of the same species.
Natural history.
Tetramorium flavipes has been collected from leaf litter in secondary forest, and very little is known about its bionomics.
Tetramorium parvispinum
(Emery, 1893)
Figure 38.
Tetramorium parvispinum worker, CASENT0735806. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.919°N, 101.274°E), Secondary forest, 05.vi.2013, 155 workers, 550 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.924°N, 101.268°E), Rubber plantation, 05.vi.2013, 6 workers, 571 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.917°N, 101.270°E), Secondary forest, 05.vi.2013, 7 workers, 580 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.911°N, 101.281°E), Limestone rain forest, 06.vi.2013, 155 workers, 650 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.916°N, 101.274°E), Secondary forest, 08.vi.2013, 58 workers, 615 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.930°N, 101.269°E), Rubber plantation, 09.vi.2013, 2 workers, 640 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.890°N, 101.267°E), Rubber plantation, 14.vi.2013, 9 workers, 620 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Widely distributed in the Indo-Malayan subregion (Figure 38C).
Taxonomic note.
Tetramorium parvispinum is a member of the Tetramorium walshi species group. It can be identified with the key presented by Bolton (1976; as Triglyphothrix parvispina)
Natural history.
Tetramorium parvispinum has been collected from leaf litter in secondary forest, limestone forest and rubber plantations, and little is known about its bionomics.
Tetramorium polymorphum
Yamane & Jaitrong, 2011
Figure 39.
Tetramorium polymorphum worker, CASENT0713055. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.917°N, 101.274°E), Rain forest, 05.vi.2013, 1 major worker, 552 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.918°N, 101.270°E), Rain forest, 05.vi.2013, 3 workers, 581 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.919°N, 101.272°E), Rain forest, 05.vi.2013, 10 workers, 550 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; “Holy Hills” (21.920°N, 101.239°E), Rain forest, 07.vi.2013, 10 worker, 665m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; XTBG (21.928°N, 101.256°E), Rain forest, 07.vi.2013, 10 workers, 565 m, Hand collection, B. Guénard, B. Blanchard and C. Liu; Man Sai village (21.860°N, 101.278°E), Rain forest, 12.vi.2013, 1 worker, 680 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Known from Yunnan (new record), Laos and Thailand (Figure 39C). This new record represents the northern-most record in the distribution of Tetramorium polymorphum.
Taxonomic note.
Tetramorium polymorphum is a member of the Tetramorium walshi species group. Its identification is not easy since the species was not known when Bolton (1976) published his revision of the genus Triglyphothrix (now Tetramorium), in which he provided keys to the Indo-Malayan and Austral-Asian Tetramorium walshi and Tetramorium obesum species groups. However, by combining Bolton’s (1976) work with the recent species description of Yamane and Jaitrong (2011) the identification is relatively straightforward. It is very similar to the closely related and sympatric Tetramorium kheperra Bolton, 1976, and the identification key of Bolton (1976) will lead the user to that species. The recent addition to Bolton’s key provided by Yamane and Jaitrong (2011) clearly separates both species.
Natural history.
Tetramorium polymorphum is a very special member of the genus Tetramorium since it is the only known species that possesses a polymorphic worker caste divisible into distinctive minor, media and major workers (Yamane and Jaitrong 2011). Yamane and Jaitrong (2011) also report that this species is comparatively aggressive and hypothesize that the major worker could have a defensive function. In addition, they emphasize that Tetramorium polymorphum is only found in undisturbed rain forest habitats in Thailand and Laos. Our data from Yunnan supports this since it was predominantly sampled from rain forest.
Tetramorium tonganum
Mayr, 1870
Figure 40.
Tetramorium tonganum worker, CASENT0713454. A Head in front view B Mesosoma in profile view C Global distribution map.
Material examined.
CHINA, Yunnan, Xishuangbanna: XTBG (21.919°N, 101.274°E), Secondary forest, 05.vi.2013, 9 workers, 552 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu; Menglun town (21.934°N, 101.269°E), Secondary forest, 09.vi.2013, 2 workers, 640 m, Winkler sifting, B. Guénard, B. Blanchard and C. Liu.
Distribution.
Tetramorium tonganum is widely distributed in the Austral-Asian and Indo-Malayan subregions where it ranges from western Oceania to South East Asia (Figure 40C). Bolton (1977) has noted already that the species is widespread in its native range and has the characteristics of tramp species. It is very likely that future collections will reveal its presence in more Chinese provinces Southeast Asian countries.
Taxonomic note.
Tetramorium polymorphum belongs to the Tetramorium tonganum group and can be easily identified with the key provided by Bolton (1977).
Natural history.
Tetramorium polymorphum has been collected from leaf litter in secondary forest, and is known to be an exotic species in China (Guenard and Dunn 2012). Despite its wide distribution and tramping ability, there is very little information about its biology. In addition to Bolton (1977), Sarnat and Economo (2012) also confirm that Tetramorium tonganum is able to establish populations outside its native range without damaging or significantly altering ecological or agricultural systems in its introduced habitats. They also report that Tetramorium tonganum is mostly found on vegetation in disturbed or edge habitats.
Discussion
The total number of named ant species in China is 939, but the true species richness is expected to be significantly higher, perhaps as high as 1200 to 1600 species (Guénard and Dunn 2012). The collection of these 40 new ant records for Yunnan and 17 for China through Winkler extraction, combined with the discovery of the extremely rare ant species Bannapone scrobiceps (Guenard et al. 2013), should encourage myrmecologists to consider leaf litter extraction as one of the primary methods to collect leaf litter ants, especially for places where this method has not previously been used. Nevertheless, further sampling methods that specifically target different strata will very likely yield additional species, which is especially true for hypogaeic and arboreal ant communities.
Based on our collections, many newly recorded species, such as Discothyrea clavicornis, Myrmecina curvispina, and Odontoponera denticulata are relatively common. The reason why those species were never reported from Yunnan before may be due to different collection techniques and/or misidentifications. For example, Odontoponera denticulata has long been misidentified as Odontoponera transversa (Yamane 2009). Another reason may be that some of the newly recorded species have been described only recently outside of Yunnan and/or China, such as Myrmecina curvispina and Pheidole tumida (Zhou et al. 2008, Eguchi 2008).
Many new species records in our collection such as Aenictus artipus, Aenictus maneerati, Aenictus paradentatus, Discothyrea clavicornis, Dolichoderus laotius, Gesomyrmex kalshoveni, Gnamptogenys treta, Pheidole plagiaria, Pheidole planifrons, Pheidole rugithorax, Pheidole tumida, Pheidole vieti, Recurvidris kemneri, Strumigenys dyschima, Strumigenys mitis, Tetramorium difficile, Tetramorium flavipes, Tetramorium parvispinum, and Tetramorium tonganum, are at the northern limit of their known distribution in Yunnan. Interestingly, the occurrence of several species in Yunnan, such as Discothyrea clavicornis, Gesomyrmex kalshoveni, Gnamptogenys treta, Recurvidris kemneri, and Strumigenys dyschima constitutes a disjunction from the rest of their known distribution in the Malay Peninsula. At present, it is unclear if these represent sampling artifacts and the ranges are actually continuous in the region, if these species ranges represent true biogeographic disjunctions, or if they are actually different species. Only future diversity inventories and taxonomic treatments, of which this paper represents one modest contribution, can answer these questions and further resolve the biodiversity map for ants and other organisms.
Despite the comparatively short collecting time we invested in the inventory of the myrmecofauna, we were able to identify 145 species, of which over 30% represent new records. This increases the list of known species for Yunnan by 10%, and there are still more than 60 species that we tentatively consider undescribed. This shows how little was previously known about the ant fauna of the region, and we are convinced that more intensive sampling in different habitats and microhabitats will likely reveal the presence of even more species or help improve the current taxonomic resolution. In this context, we think that Yunnan should be considered an area of high biodiversity value and deserving of attention of both biologists and conservationists. Regrettably, this interesting biota is being degraded at an alarming speed, particularly due to the rapid expansion of rubber plantations in the area (Li et al. 2007).
Supplementary Material
Acknowledgments
We would like to thank Yan-Qiong Peng, and Jia-jia Liu for their assistance and advice on conducting this ant diversity survey. We also thank B. Bolton, P.S. Ward, G. Fischer, and M. Yoshimura for their help in ant identification. The authors acknowledge the support of OIST and an NSF grant to EPE (NSF DEB-1145989).
Citation
Liu C, Guénard B, Garcia FH, Yamane S, Blanchard B, Yang D-R, Economo E (2015) New records of ant species from Yunnan, China. ZooKeys 477: 17–78. doi: 10.3897/zookeys.477.8775
References
- Agosti D, Majer J, Alonso E, Schultz T. (Eds) (2000) Sampling Ground-dwelling Ants: Case Studies from the Worlds’ Rain Forests. School of Environmental Biology Bulletin 18, 118 pp. [Google Scholar]
- Alfred JRB, Agarwal J. (1991) Aphid (Micromyzus kalimponginsis) - ant (Pheidole smythiesii) interrelationship - A preliminary study. Records of the Zoological Survey of India 87: 109–119. [Google Scholar]
- AntWeb (2002–2014) AntWeb v5.18.6. http://www.antweb.org [accessed 13 October 2014]
- Baroni Urbani C, De Andrade ML. (2003) The ant genus Proceratium in the extant and fossil record (Hymenoptera: Formicidae). Museo Regionale di Scienze Naturali Monografie (Turin) 36: 1–492. [Google Scholar]
- Bharti H, Gul I. (2012) Echinopla cherapunjiensis sp. n. (Hymenoptera, Formicidae) from India. Vestnik Zoologii 46: 371–373. doi: 10.2478/v10058-012-0031-z [Google Scholar]
- Bharti H, Ali S. (2013) Taxonomic studies on ant genus Strumigenys Smith, 1860 (Hymenoptera, Formicidae) with report of two new species and five new records including a tramp species from India. Sociobiology 60: 387–396. doi: 10.13102/sociobiology.v60i4.387-396 [Google Scholar]
- Bolton B. (1976) The ant tribe Tetramoriini (Hymenoptera: Formicidae). Constituent genera, review of smaller genera and revision of Triglyphothrix Forel. Bulletin of the British Museum (Natural History). Bulletin of the British Museum (Natural History). Entomology 34: 281–379. [Google Scholar]
- Bolton B. (1977) The ant tribe Tetramoriini (Hymenoptera: Formicidae). The genus Tetramorium Mayr in the Oriental and Indo-Australian regions, and in Australia. Bulletin of the British Museum (Natural History). Entomology 36: 67–151. [Google Scholar]
- Bolton B. (1992) A review of the ant genus Recurvidris (Hym.: Formicidae), a new name for Trigonogaster Forel. Psyche (Cambridge) 99: 35–48. doi: 10.1155/1992/58186 [Google Scholar]
- Bolton B. (1994) Identification Guide to the Ant Genera of the World. Harvard University Press, Cambridge, Massachusetts, 232 pp. [Google Scholar]
- Bolton B. (2000) The ant tribe Dacetini. Memoirs of the American Entomological Institute 65: 1–1028. [Google Scholar]
- Bolton B. (2007) Taxonomy of the dolichoderine ant genus Technomyrmex Mayr (Hymenoptera: Formicidae) based on the worker caste. Contributions of the American Entomological Institute 35: 1–150. [Google Scholar]
- Bolton B. (2014) An online catalog of the ants of the world. http://antcat.org [accessed 13 October 2014]
- Dill M, Williams DJ, Maschwitz U. (2002) Herdsman ants and their mealybug partners. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 557: 1–373. [Google Scholar]
- Eguchi K. (2008) A revision of Northern Vietnamese species of the ant genus Pheidole (Insecta: Hymenoptera: Formicidae: Myrmicinae). Zootaxa 1902: 1–118. [Google Scholar]
- Emery C. (1897) Formicidarum species novae vel minus cognitae in collectione Musaei Nationalis Hungarici quas in Nova-Guinea, colonia germanica, collegit L. Biró. Természetrajzi Füzetek 20: 571–599. [Google Scholar]
- Fellowes JR. (1996) Community composition of Hong Kong ants: spatial and seasonal patterns. PhD thesis, University of Hong Kong, Hong Kong, PRC. [Google Scholar]
- Fisher BL. (1999) Improving inventory efficiency: A case study of leaf-litter ant diversity in Madagascar. Ecological Applications 9: 714–731. doi: 10.1890/1051-0761(1999)009[0714:IIEACS]2.0.CO;2 [Google Scholar]
- Forel A. (1913) Wissenschaftliche Ergebnisse einer Forschungsreise nach Ostindien ausgeführt im Auftrage der Kgl. Preuss. Akademie der Wissenschaften zu Berlin von H. v. Buttel-Reepen. II. Ameisen aus Sumatra, Java, Malacca und Ceylon. Gesammelt von Herrn Prof. Dr. v. Buttel-Reepen in den Jahren 1911–1912. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 36: 1–148. [Google Scholar]
- Guénard B, Blanchard B, Liu C, Yang DR, Economo EP. (2013) Rediscovery of the rare ant genus Bannapone (Hymenoptera: Formicidae: Amblyoponinae) and description of the worker caste. Zootaxa 3734: 371–379. doi: 10.11646/zootaxa.3734.3.6 [DOI] [PubMed] [Google Scholar]
- Guénard B, Weiser MD, Dunn RR. (2012) Global models of ant diversity suggest regions where new discoveries are most likely are under disproportionate deforestation threat. PNAS 109(19): 7368–7373. doi: 10.1073/pnas.1113867109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guénard B, Dunn RR. (2012) A checklist of the ants of China. Zootaxa 3558: 1–77. [Google Scholar]
- Guénard B, Lucky A. (2011) Shuffling leaf litter samples produces more accurate and precise snapshots of terrestrial arthropod community composition. Environmental Entomology 40: 1523–1529. doi: 10.1603/EN11104 [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. (1990) The ants. Harvard University Press, Cambridge, Massachusetts, 732 pp. [Google Scholar]
- Ivanov K, Keiper J. (2009) Effectiveness and biases of winkler litter extraction and pitfall trapping for collecting ground-dwelling ants in northern temperate forests. Environmental Entomology 38: 1724–1736. doi: 10.1603/022.038.0626 [DOI] [PubMed] [Google Scholar]
- Jaitrong W, Yamane SK. (2011) Synopsis of Aenictus species groups and revision of the A. currax and A. laeviceps groups in the eastern Oriental, Indo-Australian, and Australasiann regions (Hymenoptera: Formicidae: Aenictinae). Zootaxa 3128: 1–46. [Google Scholar]
- Jaitrong W, Yamane SK. (2012) Review of the Southeast Asian species of the Aenictus javanus and Aenictus philippinensis species groups (Hymenoptera, Formicidae, Aenictinae). Zookeys 193: 49–78. doi: 10.3897/zookeys.193.2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitrong W, Yamane SK. (2013) The Aenictus ceylonicus species group (Hymenoptera, Formicidae, Aenictinae) from Southeast Asia. Journal of Hymenoptera Research 31: 165–233. doi: 10.3897/jhr.31.4274 [Google Scholar]
- Jaitrong W, Yamane SK, Wiwatwitaya D. (2010) The army ant Aenictus wroughtonii (Hymenoptera, Formicidae, Aenictinae) and related species in the oriental region, with descriptions of two new species. Japanese Journal of Systematic Entomology 16: 33–46. [Google Scholar]
- Kubota M, Terayama M. (1999) A description of a new species of the genus Discothyrea Roger from the Ryukyus, Japan (Hymenoptera: Formicidae). Memoirs of the Myrmecological Society of Japan 1: 1–5. [Google Scholar]
- Lattke JE. (2004) A taxonomic revision and phylogenetic analysis of the ant genus Gnamptogenys Roger in Southeast Asia and Australasia (Hymenoptera: Formicidae: Ponerinae). University of California Publications in Entomology 122: 1–266. [Google Scholar]
- Li H, Aide TM, Ma Y, Liu W, Cao M. (2007) Demand for rubber is causing the loss of high diversity rain forest in SW China. Biodiversity and Conservation 16: 1731–1745. doi: 10.1007/s10531-006-9052-7 [Google Scholar]
- Long CL. (1995) On biodiversity of Xishuangbanna ant its conservation. Biodiversity Science 3: 55–62. [Google Scholar]
- Martelli M, Ward M, Fraser A. (2004) Ant diversity sampling on the Southern Cumberland Plateau: a comparison of litter sifting and pitfall trapping. Southeastern Naturalist 3: 113–126. doi: 10.1656/1528-7092(2004)003[0113:ADSOTS]2.0.CO;2 [Google Scholar]
- Maschwitz U. (2002) Herdsmen ants and their mealybug partners. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 557: 1–373. [Google Scholar]
- Olson D. (1991) A comparison of the efficacy of litter sifting and pitfall traps for sampling leaf litter ants (Hymenoptera, Formicidae) in a tropical wet forest, Costa Rica. Biotropica 23: 166–172. doi: 10.2307/2388302 [Google Scholar]
- Sarnat EM, Economo EP. (2012) The ants of Fiji. University of California Publications in Entomology 132: 1–384. [Google Scholar]
- Terayama M, Lin CC, Wu WJ. (1996) The Taiwanese species of the ant genus Smithistruma (Hymenoptera, Formicidae). Japanese Journal of Entomology 64: 327–339. [Google Scholar]
- Vasconcelos H, Lopes C. (2008) Evaluation of three methods for sampling ground-dwelling Ants in the Brazilian Cerrado. Neotropical Entomology 37: 399–405. doi: 10.1590/S1519-566X2008000400007 [DOI] [PubMed] [Google Scholar]
- Xu ZH. (1998) A report of fourty-one ant species newly recorded in China from Xishuangbanna District of Yunnan Province (Hymenoptera: Formicidae). Abstract of Chinese Academic Periodicals 4: 1119–1121. [Google Scholar]
- Xu ZH. (1999) An analysis on the ant fauna of the tropical rain forest in Xishuangbanna of China. Zoological Research 20: 379–384. [Google Scholar]
- Xu ZH. (2002) A study on the biodiversity of Formicidae ants of Xishuangbanna Nature Reserve. Yunnan Science and Technology Press, Yunnan, 181 pp. [Google Scholar]
- Xu ZH, Burwell CJ, Nakamura A. (2014a) Two new species of the proceratiine ant genus Discothyrea Roger from Yunnan, China, with a key to the known Oriental species. Asian Myrmecology 6: 33–41. [Google Scholar]
- Xu ZH, Burwell CJ, Nakamura A. (2014b) A new species of the ponerine ant genus Myopias Roger from Yunnan, China, with a key to the known Oriental species. Sociobiology 61(2): 164–170. doi: 10.13102/sociobiology.v61i2.164-170 [Google Scholar]
- Xu ZH, Zhou XG. (2004) Systematic study on the ant genus Pyramica Roger (Hymenoptera, Formicidae) of China. Acta Zootaxonomica Sinica 29: 440–450. [Google Scholar]
- Yamane SK. (2009) Odontoponera denticulata (F. Smith) (Formicidae: Ponerinae), a distinct species inhabiting disturbed areas. Ari 32: 1–8. [Google Scholar]
- Yamane S, Jaitrong W. (2011) A first species of Tetramorium (Hymenoptera, Formicidae, Myrmicinae) with a polymorphic worker caste. Insecta Matsumurana 67: 61–74. [Google Scholar]
- Wheeler WM. (1929) The identity of the ant genera Gesomyrmex Mayr and Dimorphomyrmex Ernest André. Psyche (Cambridge) 36: 1–12. doi: 10.1155/1929/71489 [Google Scholar]
- Zhou SY, Huang J, Ma L. (2008) Two new species of the ant genus Myrmecina (Hymenoptera: Formicidae), with a key to Chinese species. Sociobiology 52: 283–291. [Google Scholar]
- Zhou SY, Zheng Z. (1997) A taxonomic study on the ant genus Pheidologeton Mayr in Guangxi (Hymenoptera: Formicidae). Zoological Research 18: 163–170. [Google Scholar]
- Zhou SY, Zheng Z. (1998) Three new species and a new record species of tribe Prenolepidini (Hymenoptera: Formicidae) from Guangxi, China. Entomologia Sinica 5: 42–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.