Abstract
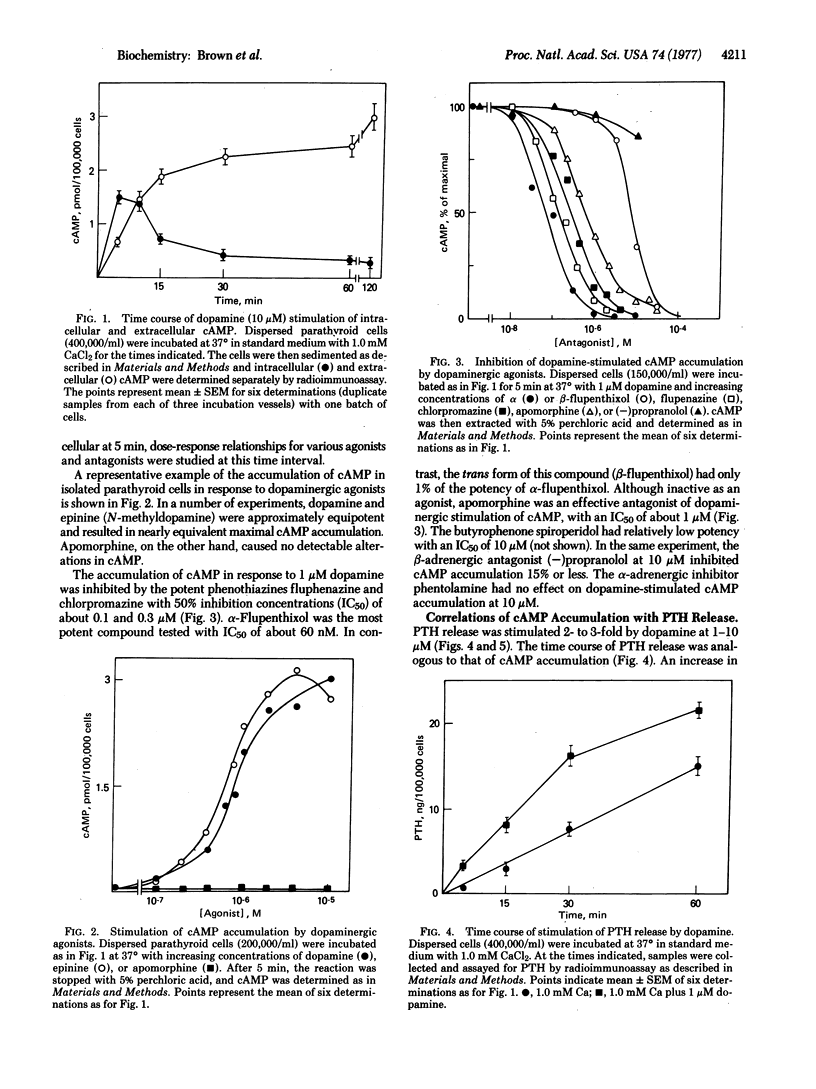
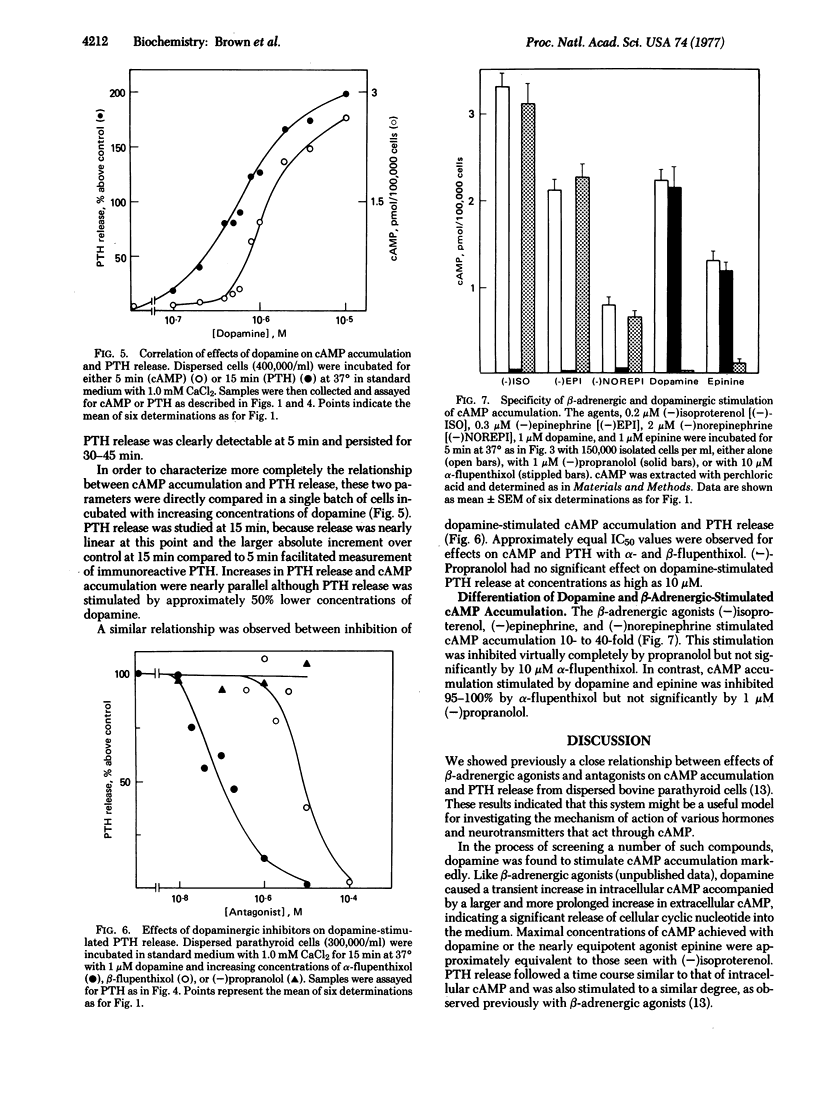
The effects of dopaminergic agonists and antagonists have been studied in dispersed bovine parathyroid cells. Dopaminergic agonists caused a transient 20- to 40-fold increase in cellular cyclic AMP and a 2- to 3-fold increase in parathyroid hormone release. Dose-response relationships were similar for cyclic AMP accumulation and hormone release, whether studied by increasing agonist concentration or by increasing concentration of antagonist with constant agonist. The effects on the dopamine receptor could be differentiated from those of the previously characterized β-adrenergic receptor by specific inhibitors. These results appear to represent proof with a homogeneous cell population that dopaminergic receptors linked to adenylate cyclase can regulate a secretory process mediated by cyclic AMP. This system should be useful in further studies on dopamine receptors and should provide a valid tool for determining interactions of radiolabeled ligands with such receptors.
Keywords: dopamine, dispersed cells
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Sherwood L. M. Regulation of parathyroid hormone secretion by adrenyl cyclase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):396–401. doi: 10.1016/s0006-291x(72)80064-0. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Hurwitz S., Aurbach G. D. Beta-adrenergic stimulation of cyclic AMP content and parathyroid hormone release from isolated bovine parathyroid cells. Endocrinology. 1977 Jun;100(6):1696–1702. doi: 10.1210/endo-100-6-1696. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Hurwitz S., Aurbach G. D. Preparation of viable isolated bovine parathyroid cells. Endocrinology. 1976 Dec;99(6):1582–1588. doi: 10.1210/endo-99-6-1582. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Hurwitz S., Woodard C. J., Aurbach G. D. Direct identification of beta-adrenergic receptors on isolated bovine parathyroid cells. Endocrinology. 1977 Jun;100(6):1703–1709. doi: 10.1210/endo-100-6-1703. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Makman M. H. Stimulation by dopamine of adenylate cyclase in retinal homogenates and of adenosine-3':5'-cyclic monophosphate formation in intact retina. Proc Natl Acad Sci U S A. 1972 Mar;69(3):539–543. doi: 10.1073/pnas.69.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt D. R., Enna S. J., Creese I., Snyder S. H. Dopamine receptor binding in the corpus striatum of mammalian brain. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4655–4659. doi: 10.1073/pnas.72.11.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg L. I. Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacol Rev. 1972 Mar;24(1):1–29. [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- Iversen L. L. Dopamine receptors in the brain. Science. 1975 Jun 13;188(4193):1084–1089. doi: 10.1126/science.2976. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science. 1971 Dec 24;174(4016):1346–1349. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the "dopamine receptor". Proc Natl Acad Sci U S A. 1972 Aug;69(8):2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N., Gilmer K. N. Demonstration of dopamine-sensitive adenylate cyclase in malignant neuroblastoma cells and change in sensitivity of adenylate cyclase to catecholamines in "differentiated" cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2525–2529. doi: 10.1073/pnas.71.6.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pénit J., Cantau B., Huot J., Jard S. Adenylate cyclase from synchronized neuroblastoma cells: responsiveness to prostaglandin E1, adenosine, and dopamine during the cell cycle. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1575–1579. doi: 10.1073/pnas.74.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P., Chau-Wong M., Tedesco J., Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. A., Hargis G. K., Bowser E. N., Henderson W. J., Martinez N. J. Evidence for a role of adenosine 3',5'-monophosphate in parathyroid hormone release. Endocrinology. 1973 Mar;92(3):687–691. doi: 10.1210/endo-92-3-687. [DOI] [PubMed] [Google Scholar]



