Abstract
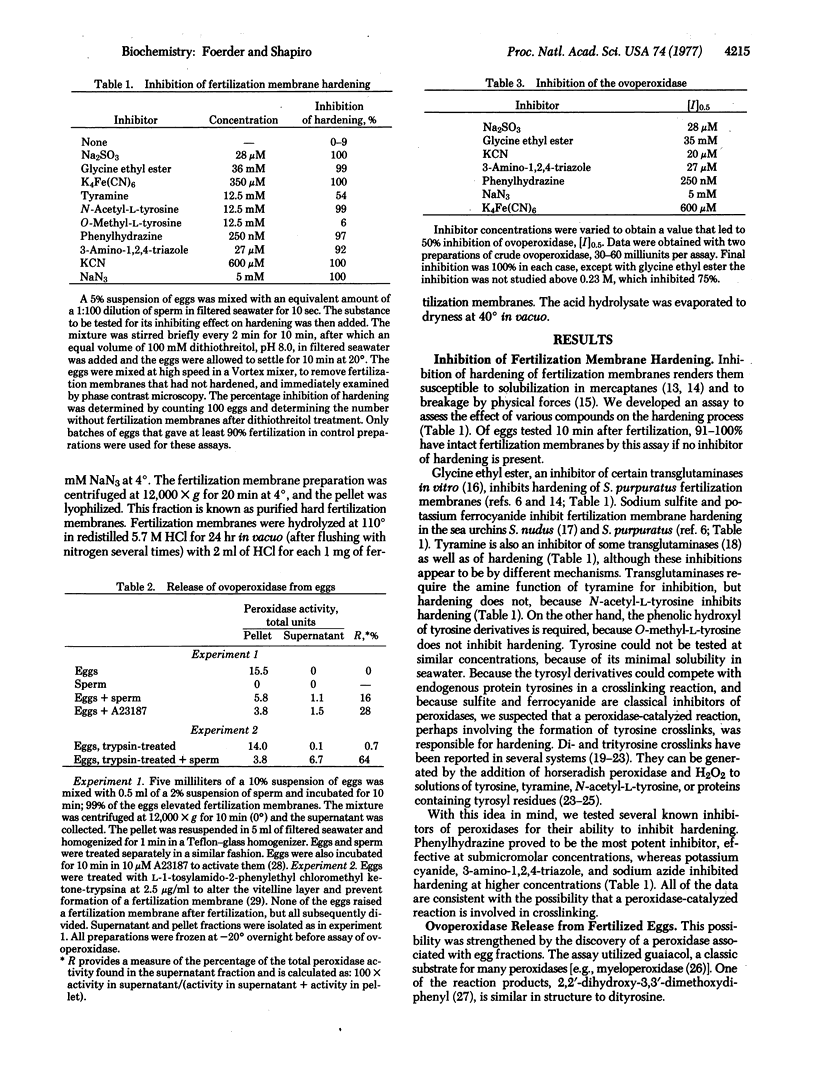
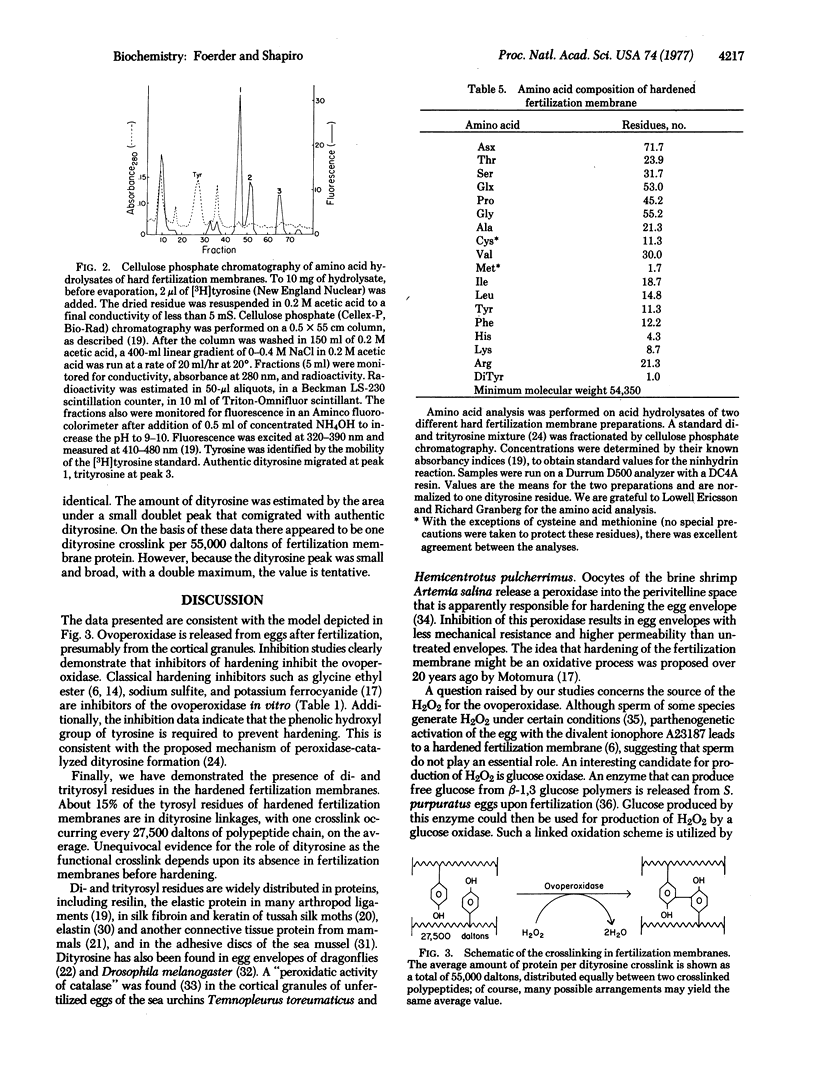
One feature of fertilization is the alteration of the vitelline layer, by components released from the egg, to produce an elevated, covalently crosslinked, hard, insoluble, fertilization membrane. The following evidence indicates that crosslinking and hardening are caused by the production of diand trityrosyl residues, by oxidation of protein-bound tyrosyl residues in the presence of a peroxidase. Hardening of the fertilization membrane, as evidenced by its loss of solubility in 50 mM dithiothreitol, is inhibited by compounds known to inhibit many peroxidases. A peroxidase, here called the ovoperoxidase, is released from eggs at fertilization. This enzyme is inhibited by the same compounds that inhibit hardening and at similar concentrations. Inhibitors of the ovoperoxidase and the hardening reaction include KCN, 3-amino-1,2,4-triazole, NaN3, phenylhydrazine, K4Fe(CN)6, sodium sulfite, and glycine ethyl ester. In addition, tyramine and N-acetyltyrosine both inhibit hardening, but O-methyltyrosine does not. Dityrosyl and trityrosyl residues are found in acid hydrolysates of isolated, hardened fertilization membranes. These residues have been identified by cellulose phosphate column chromatography, thin-layer chromatography, and amino acid analysis. The amino acid data have been used to estimate that there is one dityrosine crosslink per 55,000 daltons of protein.
We suggest that, by catalyzing the crosslinking of tyrosyl residues, the ovoperoxidase leads to the production of a hard fertilization membrane that blocks the entry of additional sperm. Because peroxidases are spermicidal, a secondary function of the enzyme could be to kill sperm in the vicinity of the fertilized egg.
Keywords: protein crosslinking, tyrosine, egg shell, peroxidase, polyspermy
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeschbach R., Amadò R., Neukom H. Formation of dityrosine cross-links in proteins by oxidation of tyrosine residues. Biochim Biophys Acta. 1976 Aug 9;439(2):292–301. doi: 10.1016/0005-2795(76)90064-7. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structural aspects of the fibrinogen to fibrin conversion. Adv Protein Chem. 1973;27:1–109. doi: 10.1016/s0065-3233(08)60446-5. [DOI] [PubMed] [Google Scholar]
- Dutton A., Singer S. J. Crosslinking and labeling of membrane proteins by transglutaminase-catalyzed reactions. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2568–2571. doi: 10.1073/pnas.72.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel D. Methods for removal of the vitelline membrane of sea urchin eggs. II. Controlled exposure to trypsin to eliminate post-fertilization clumping of embryos. Exp Cell Res. 1970 Jul;61(1):69–70. doi: 10.1016/0014-4827(70)90258-2. [DOI] [PubMed] [Google Scholar]
- Epel D., Weaver A. M., Muchmore A. V., Schimke R. T. Beta-1,3-glucanase of sea urchin eggs: release from particles at fertilization. Science. 1969 Jan 17;163(3864):294–296. doi: 10.1126/science.163.3864.294. [DOI] [PubMed] [Google Scholar]
- GROSS A. J., SIZER I. W. The oxidation of tyramine, tyrosine, and related compounds by peroxidase. J Biol Chem. 1959 Jun;234(6):1611–1614. [PubMed] [Google Scholar]
- Himmelhoch S. R., Evans W. H., Mage M. G., Peterson E. A. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969 Mar;8(3):914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- Inoue S., Hardy J. P., Cousineau G. H., Bal A. K. Fertilization membranes structure analysis with the surface replica method. Exp Cell Res. 1967 Oct;48(1):248–251. doi: 10.1016/0014-4827(67)90316-3. [DOI] [PubMed] [Google Scholar]
- Jaffe L. A. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature. 1976 May 6;261(5555):68–71. doi: 10.1038/261068a0. [DOI] [PubMed] [Google Scholar]
- Katsura S., Tominaga A. Peroxidatic activity of catalase in the cortical granules of sea urchin eggs. Dev Biol. 1974 Oct;40(2):292–297. doi: 10.1016/0012-1606(74)90131-6. [DOI] [PubMed] [Google Scholar]
- Keeley F. W., Labella F. S. Isolation of dityrosine from an alkali-soluble connective tissue protein. Biochim Biophys Acta. 1972 Mar 15;263(1):52–59. doi: 10.1016/0005-2795(72)90158-4. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Smith D. C. The source of H2O2 for the uterine fluid-mediated sperm-inhibitory system. Biol Reprod. 1970 Oct;3(2):236–242. doi: 10.1093/biolreprod/3.2.236. [DOI] [PubMed] [Google Scholar]
- LaBella F., Keeley F., Vivian S., Thornhill D. Evidence for dityrosine in elastin. Biochem Biophys Res Commun. 1967 Mar 21;26(6):748–753. doi: 10.1016/s0006-291x(67)80137-2. [DOI] [PubMed] [Google Scholar]
- LaBella F., Waykole P., Queen G. Formation of insoluble gels and dityrosine by the action of peroxidase on soluble collagens. Biochem Biophys Res Commun. 1968 Feb 26;30(4):333–338. doi: 10.1016/0006-291x(68)90746-8. [DOI] [PubMed] [Google Scholar]
- Lallier R. A. Formation of fertilization membrane in sea urchin eggs. Exp Cell Res. 1970 Dec;63(2):460–462. doi: 10.1016/0014-4827(70)90239-9. [DOI] [PubMed] [Google Scholar]
- Lallier R. Effects of various inhibitors of protein cross-linking on the formation of fertilization membrane in sea urchin egg. Experientia. 1971;27(11):1323–1324. doi: 10.1007/BF02136716. [DOI] [PubMed] [Google Scholar]
- Petri W. H., Wyman A. R., Kafatos F. C. Specific protein synthesis in cellular differentiation. III. The eggshell proteins of Drosophila melanogaster and their program of synthesis. Dev Biol. 1976 Mar;49(1):185–199. doi: 10.1016/0012-1606(76)90266-9. [DOI] [PubMed] [Google Scholar]
- Raven D. J., Earland C., Little M. Occurrence of dityrosine in Tussah silk fibroin and keratin. Biochim Biophys Acta. 1971 Oct;251(1):96–99. doi: 10.1016/0005-2795(71)90065-1. [DOI] [PubMed] [Google Scholar]
- Roels F. Peroxidatic activity in Artemia salina oocytes: role in egg-envelope hardening. Exp Cell Res. 1971 Dec;69(2):452–456. doi: 10.1016/0014-4827(71)90252-7. [DOI] [PubMed] [Google Scholar]
- Runnström J. The vitelline membrane and cortical particles in sea urchin eggs and their function in maturation and fertilization. Adv Morphog. 1966;5:221–325. doi: 10.1016/b978-1-4831-9952-8.50010-9. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M. Limited proteolysis of some egg surface components is an early event following fertilization of the sea urchin, Strongylocentrotus purpuratus. Dev Biol. 1975 Sep;46(1):88–102. doi: 10.1016/0012-1606(75)90089-5. [DOI] [PubMed] [Google Scholar]
- Smith D. C., Klebanoff S. J. A uterine fluid-mediated sperm-inhibitory system. Biol Reprod. 1970 Oct;3(2):229–235. doi: 10.1093/biolreprod/3.2.229. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D., Carroll E. J., Jr, Yanagimachi R. Is calcium ionophore a universal activator for unfertilised eggs? Nature. 1974 Nov 1;252(5478):41–43. doi: 10.1038/252041a0. [DOI] [PubMed] [Google Scholar]
- Vacquier V. D., Tegner M. J., Epel D. Protease activity establishes the block against polyspermy in sea urchin eggs. Nature. 1972 Dec 8;240(5380):352–353. doi: 10.1038/240352a0. [DOI] [PubMed] [Google Scholar]
- Veron M., Foerder C., Eddy E. M., Shapiro Sequential biochemical and morphological events during assembly of the fertilization membrane of the sea urchin. Cell. 1977 Feb;10(2):321–328. doi: 10.1016/0092-8674(77)90226-4. [DOI] [PubMed] [Google Scholar]




