Summary
We all experience a host of common life stressors such as the death of a family member, medical illness, and financial uncertainty. While most of us are resilient to such stressors, continuing to function normally, for a subset of individuals, experiencing these stressors increases the likelihood of developing treatment-resistant, chronic psychological problems, including depression and anxiety. It is thus paramount to identify predictive markers of risk, particularly those reflecting fundamental biological processes that can be targets for intervention and prevention. Using data from a longitudinal study of 340 healthy young adults, we demonstrate that individual differences in threat-related amygdala reactivity predict psychological vulnerability to life stress occurring as much as 1 to 4 years later. These results highlight a readily assayed biomarker, threat-related amygdala reactivity, which predicts psychological vulnerability to commonly experienced stressors and represents a discrete target for intervention and prevention.
Introduction
Exposure to stressful life events is a robust risk factor for the development of treatment-resistant, chronic psychological problems including major depression and anxiety disorders (Faravelli, 1985; Galea et al., 2002; Kendler et al., 1999). However, whereas most individuals experience stressful life events at some point, the lifetime prevalence for major depression and anxiety disorders is 17% and 29%, respectively (Kessler et al., 2005), indicating that only a subset of individuals experiencing life events will ultimately develop psychopathology. The ability to prospectively identify which individuals are at greatest risk represents a critical gap in our ability to effectively intervene and prevent the emergence of disabling psychological problems. Focusing such efforts on biological processes involved in stress reactivity and regulation is particularly important as they represent not only predictive markers of risk but also discrete targets for intervention and prevention.
Amygdala reactivity to threat is a prime candidate biomarker for psychological risk to common stressors given its critical roles in threat detection, stress reactivity, and memory for negative information (Herman and Cullinan, 1997; Kim et al., 2003; Murty et al., 2010; Pessoa and Ungerleider, 2004). Thus, relatively greater amygdala reactivity to common stressors could lead to an altered stress response and biased appraisal and memory of stressful events, all of which are core symptoms and features of depression and anxiety disorders (Burke et al., 2005; Espejo et al., 2012; Fales et al., 2008; Hamilton and Gotlib, 2008). Not surprisingly, heightened threat-related amygdala reactivity is consistently observed in patients with depression and anxiety (Etkin and Wager, 2007; Groenewold et al., 2013). However, cross-sectional research in patients cannot determine whether heightened amygdala reactivity is a premorbid vulnerability present before the development of symptoms, or whether this neural phenotype is a secondary correlate that emerges as a downstream consequence of the onset of symptoms. Prospective research is required to address this limitation and test whether amygdala reactivity predicts internalizing symptoms at a future point in time, controlling for baseline symptom levels.
Adolescents at heightened risk for the development of depression and anxiety through a positive family history for these disorders evidence heightened amygdala reactivity to threat (Joormann et al., 2012; Monk et al., 2008; Swartz et al., in press), suggesting that this neural biomarker can be observed before the onset of disorder. It remains to be determined, however, whether such heightened amygdala reactivity predicts the development of internalizing symptoms following the experience of stress in the future. Indeed, only two studies with small samples have examined such a prospective association, with both finding that relatively increased amygdala reactivity measured before the experience of a major traumatic event (i.e., warzone combat (Admon et al., 2009) or a terrorist attack (McLaughlin et al., 2014)) predicted greater subsequent posttraumatic stress disorder symptoms. While these findings suggest that threat-related amygdala reactivity may represent a predictive biomarker of psychological vulnerability to extreme and rare forms of trauma, we do not know if threat-related amygdala reactivity has similar predictive utility in the broader population who experience milder forms of common stressful life events.
To examine whether relatively increased threat-related amygdala reactivity prospectively predicts psychological vulnerability to common life stressors, we used functional magnetic resonance imaging (fMRI) to assess baseline threat-related amygdala reactivity in 753 participants aged 18-22 years old, all of whom were free of current depression or anxiety disorders. We chose a sample of young adults as this developmental stage marks the beginning of a peak period of risk for the emergence of a number of internalizing disorders, including major depression, panic disorder, generalized anxiety disorder, and posttraumatic stress disorder (Kessler et al., 2005). A widely utilized and well-established face matching paradigm was used to robustly elicit threat-related amygdala reactivity (Nikolova et al., 2014; Prather et al., 2013). At the time of scanning, participants reported the number of stressful life events they had experienced in the prior year, as well as their experience of childhood trauma and their current levels of depression and anxiety symptoms. We used these baseline measures as covariates in all analyses to test whether amygdala reactivity prospectively predicts future psychological problems as a function of stress above and beyond participants’ reported symptoms and stress levels at baseline.
After successful completion of the baseline protocol including fMRI, all participants were subsequently contacted by e-mail every 3 months and invited to complete a short online assessment of their current mood and experience of stressful life events since their last assessment. At baseline and each follow-up, participants were given a checklist (Clements and Turpin, 1996) of stressful life events commonly experienced by students (e.g., death of a very good friend, major car accident, parent losing a job) and were asked to indicate which events had occurred since the last assessment as well as the impact of that event. We calculated the sum of all impact scores for each event reported; thus, higher scores can reflect both a greater number of events as well as more severe events. Symptoms of depression and anxiety were reported at baseline and each follow-up assessment (Watson et al., 1995).
Results
Amygdala reactivity to threat
Functional MRI results were first examined in SPM8 to ensure that the task elicited predicted activation in the amygdala (Figure 1A). As expected, the contrast of fearful and angry faces > shapes was associated with bilateral amygdala reactivity: left amygdala, t(810)=28.7, p<.001 FWE-corrected, peak coordinates (x,y,z): (−22, −6, −18), and right amygdala, t(810)=32.5, p<.001 corrected, (28, −4, 20). A mean parameter estimate reflecting amygdala reactivity as a function of our task (i.e., fearful and angry facial expressions vs. shapes) was extracted for each participant and entered into regression models in MPlus v7.
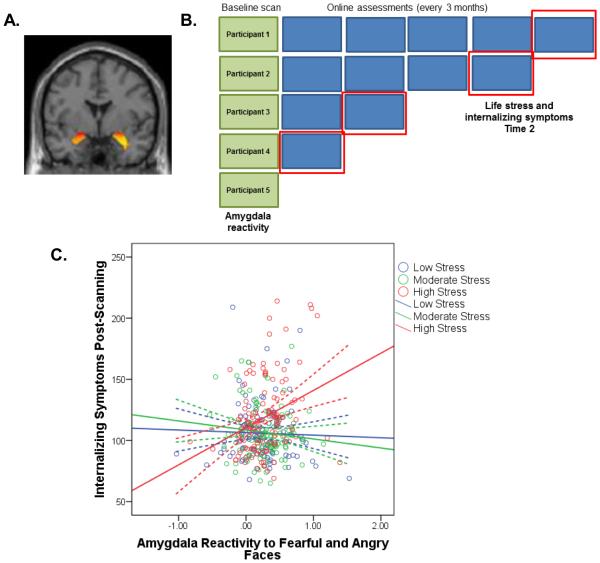
Fig. 1. Model A: All participants completing a post-scanning assessment (n=340).
A) Participants underwent a baseline fMRI scan to measure threat-related amygdala reactivity. The main effect of task (fearful and angry faces>shapes) elicited bilateral amygdala reactivity (thresholded at p<.05 corrected). (B) Participants were invited to complete an online assessment every 3 months post-scanning. The green boxes indicate the baseline scanning assessment and the blue boxes indicate online assessments. For Model A, life stress at Time 2 (as measured by the Life Events Scale for Students) and internalizing symptoms at Time 2 (as measured by the Mood and Anxiety Symptom Questionnaire) were taken from the most recent assessment completed by each participant, as indicated by the red boxes. (C) Depressive and anxiety symptoms at Time 2 are plotted as a function of the parameter estimates of threat-related amygdala reactivity and life stress post-scanning (groups divided into terciles). Dotted lines indicate 95% confidence bands. Internalizing symptoms were predicted by a significant interaction between amygdala reactivity and life stress experienced post-scanning, B=2.01, SE=.7, t(339)=3.08, p=.002. See also Table S1 and Figure S1.
Model A: Using amygdala reactivity to predict internalizing symptoms as a function of recent stress
To test our hypothesis that baseline threat-related amygdala reactivity predicts psychological vulnerability to the subsequent experience of common life stressors at any point in the future, we first created a model (Model A; Figure 1B) using the largest sample of participants that completed an online assessment at any time post-scanning. For participants that completed multiple assessments, we selected data from the most recent assessment available. Follow-up assessments were available from a total of 340 participants, and were collected approximately 1 year post-scanning (M=468 days, Min-Max=90-1402). Model A was significant (Table S1; p<.001), with the interaction between amygdala reactivity and life stress reported post-scanning predicting the severity of symptoms (B=2.01, SE=.7, t(339)=3.08, p=.002). Specifically, individuals with relatively heightened amygdala reactivity at baseline who also reported experiencing greater life stress subsequent to scanning had significantly greater symptoms at follow-up (Figure 1C and Figure S1). Exploratory whole-brain results are reported in Table S2.
Model B: Long-term predictive utility of amygdala reactivity
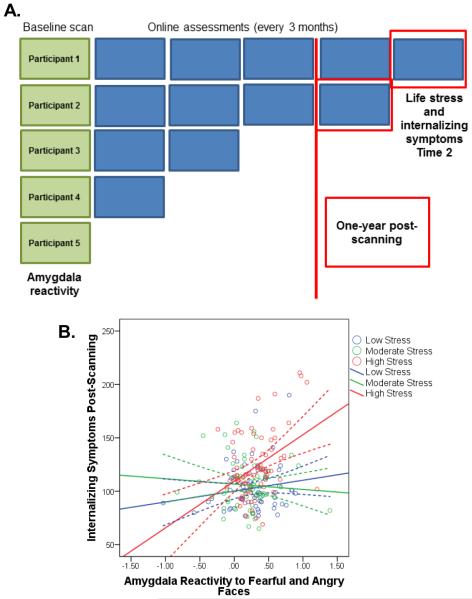
While Model A confirmed our hypothesis that amygdala reactivity represents a predictive biomarker of psychological vulnerability to common life stressors, this model included participants who completed post-scanning assessments in relatively close proximity to the scan (e.g., 3 months). To determine the long-term predictive utility of threat-related amygdala reactivity, we next analyzed data from only those participants who completed an online assessment at least one year post-scanning (Model B; Figure 2A). For this model, 192 participants were included who completed their assessment on average 2 years post-baseline (M=683 days, Min-Max=365-1402). Model B was significant (Table S3; p<.001), with the interaction between amygdala reactivity and life stress predicting symptom severity following stress occurring approximately 2 years later (B=1.75, SE=.8, t(191)=2.33, p=.02). Again, participants with relatively heightened amygdala reactivity at baseline who experienced relatively high life stress post-scanning reported the greatest symptoms (Figure 2B and Figure S2).
Fig. 2. Model B: All participants completing an assessment at least 1-year post-scanning (n=192).
(A) For Model B, we selected data from all participants who completed a follow-up assessment at least 1 year post-scanning. (B) Internalizing symptoms at Time 2 as a function of amygdala reactivity and life stress experienced post-scanning. Internalizing symptoms were predicted by a significant interaction between amygdala reactivity and life stress experienced post-scanning, B=1.75, SE=.8, t(191)=2.33, p=.02. See also Table S3 and Figure S2.
Model C: Prospective assessment of stressful life events
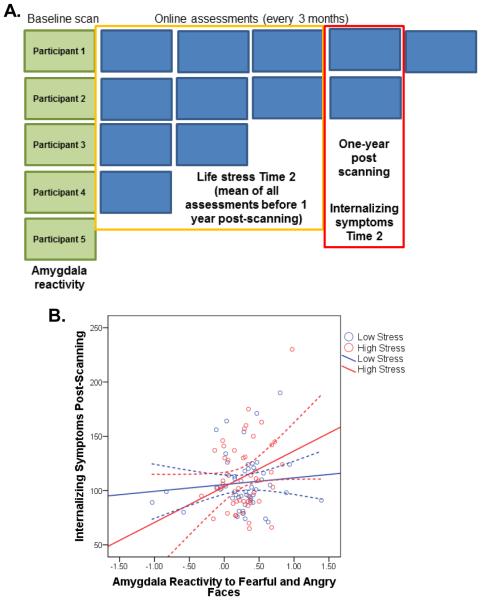
A limitation of our previous models is that we used concurrent reports of life stress and internalizing symptoms from the most recent assessment available. Participants experiencing greater negative affect at the time of assessment may be biased toward indicating greater severity of recent life stress. Therefore, we created a final model (Model C, Figure 3A) in which life stress was assessed prospective to the reporting of symptoms. To equate participants in terms of when stressful life events and symptoms were measured, we obtained symptoms from assessments completed approximately 1 year post-scanning. We then computed the mean life stress score for all assessments completed prior to this, yielding a prospective set of variables including threat-related amygdala reactivity at baseline, life stress that occurred post-scanning, and symptoms approximately 1 year post-scanning. Data from 99 participants were available for this analysis. The moderation Model C was significant (Table S3; p<.001), indicating a significant interaction between threat-related amygdala reactivity and subsequent life stress in predicting internalizing symptoms 1 year later (B=5.56, SE=1.9, t(98)=2.96, p=.003). As in our previous models, individuals with relatively heightened amygdala reactivity who experienced greater life stress post-scanning reported greater internalizing symptoms 1 year later (Figure 3B and Figure S3). The interaction between amygdala reactivity and life stress explained an additional 5% of the variance in symptoms, above and beyond all covariates, including symptoms reported at baseline.
Fig. 3. Model C: Predicting symptoms reported approximately 1 year post-scanning from amygdala reactivity at baseline and life stress reported between the scanning session and 1 year post-scanning (n=99).
(A) For Model C, to obtain a prospective assessment of life stress, we selected depression and anxiety symptoms from the assessment completed approximately 1 year post-scanning (range: 365-455 days) and a mean stress score from all assessments completed before then. (B) Internalizing symptoms at Time 2 as a function of amygdala reactivity and life stress experienced post-scanning (groups created by median split). Internalizing symptoms were predicted by a significant interaction between amygdala reactivity and life stress experienced post-scanning, B=5.56, SE=1.9, t(98)=2.96, p=.003. See also Table S3 and Figure S3.
Discussion
Using data from a large longitudinal study of healthy young adults, we provide novel evidence for the utility of threat-related amygdala reactivity - assessed with fMRI - as a predictive biomarker of risk for broad psychological vulnerability to commonly experienced stressful life events. Our neural risk biomarker predicted vulnerability consistent with a diathesis-stress model in both the short- and long-term as well as independently of negative reporting biases. Critically, we did not find a main effect of amygdala reactivity in predicting future internalizing symptoms, indicating that this neural biomarker predicts greater symptoms only within the context of experiencing relatively high life stress.
Remarkably, amygdala reactivity measured at one time point significantly predicted internalizing symptoms, above and beyond baseline symptoms, as much as 1 to 4 years into the future, indicating possible utility of this neural phenotype for prediction of long-term internalizing outcomes. However, it is important to note that the interaction between amygdala reactivity and stress only explained an additional 1-5% of the variance in symptoms, with the long-term model (Model B) evidencing the weakest effect. There are several methodological limitations of the current study that may have led us to underestimate the size of this effect. First, participants were not guaranteed payment for completing follow-up online assessments but entered into a larger gift card raffle. Thus, missing data may have biased our results. Second, life stress was assessed through a self-report checklist rather than through a more objective approach such as a calendar interview. Third, our non-clinical sample of undergraduate students was generally low-risk and the associated range and variability in internalizing symptoms truncated in comparison to clinical or high-risk samples. We anticipate that future research addressing these limitations may find larger effect sizes than those reported in the present study. Nevertheless, these results underscore the need to identify additional biomarkers, whether neural or genetic, that can explain additional variance in addition to or in interaction with that accounted for by threat-related amygdala reactivity.
Notably, a range of previously identified risk factors for depression and anxiety disorders are all associated with relatively increased amygdala reactivity to threat (Bogdan et al., 2012; Swartz et al., in press; Nikolova et al., 2014; White et al., 2012), and our biomarker is consistent with specific pathways of increased risk, including exaggerated hypothalamic-pituitary-adrenal axis stress responsiveness and cognitive biases. Although our follow-up assessments did not allow for direct mapping of threat-related amygdala reactivity onto formal clinical diagnosis, the increased stress-related symptoms of depression and anxiety predicted by amygdala reactivity have been directly associated with dysfunction (Bredemeier et al., 2010; Buckby et al., 2007), and relatively increased amygdala reactivity is consistently observed in patients with clinical depression and anxiety disorders (Etkin and Wager, 2007; Groenewold et al., 2013). Thus, threat-related amygdala reactivity represents a predictive neural biomarker through which a range of risk factors may create a common diathesis for psychological vulnerability to the experience of common life stressors in the general population. As such, relatively increased threat-related amygdala reactivity further represents a discrete biological mechanism that can be targeted in the development of novel strategies for more effective prevention of otherwise chronic and treatment-resistant psychiatric disorders. The amygdala, of course, represents only one node of an extended corticolimbic circuit supporting emotion processing and stress responsiveness. Other circuit nodes include the hypothalamus, brainstem, insula, hippocampal formation, and prefrontal cortex. Future research using paradigms designed to target these other circuit nodes as well as their dynamic interactions may further illuminate biological pathways through which individual differences in stress responsiveness may eventually manifest as disorder.
Experimental Procedures
Participants
Young adult college students were recruited as part of the Duke Neurogenetics Study. All procedures were approved by the Duke University Medical Center and participants provided informed consent before participating in the study. Participants were included in the present sample if they met the following criteria: 1) free of medical diagnoses of cancer, stroke, diabetes, chronic kidney or liver disease, or lifetime history of psychotic symptoms; 2) no use of psychotropic, glucocorticoid, or hypolipidemic medication; 3) no conditions affecting cerebral blood flow and metabolism (e.g., hypertension); and 4) met quality control criteria for functional MRI scanning. Moreover, due to our interest in predicting internalizing symptoms post-scanning, we excluded any participants with a current mood, anxiety, or eating disorder diagnosis at the time of scanning, based on the electronic Mini International Neuropsychiatric Interview (Sheehan et al., 1998). A covariate was included in all analyses to control for participants with a non-internalizing diagnosis (e.g., substance abuse) or with a past internalizing diagnosis. A total of 811 participants met inclusion criteria for the imaging data (see Supplemental Experimental Procedures for quality control criteria), 57 were excluded for current psychopathology, and 1 participant was missing data, leaving 753 participants (57% female) ranging in age from 18 to 22 available for analyses (Table 1).
Table 1.
Participant Characteristics
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Baseline (n=753) | ||||
|
| ||||
| Age (years) | 19.6 | 1.2 | 18 | 22 |
| Childhood trauma | 33.2 | 7.9 | 25 | 75 |
| Stressful life events Time 1 | 10.1 | 8.2 | 0 | 66 |
| Internalizing symptoms Time 1 | 110.7 | 25.4 | 61 | 230 |
|
| ||||
| Time 2 Scores: Model A (n=340) | ||||
|
| ||||
| Age (years) | 20.8 | 1.5 | 18 | 26 |
| Stressful life events Time 2 | 4.3 | 5.3 | 0 | 34 |
| Internalizing symptoms Time 2 | 110.7 | 26.4 | 65 | 214 |
| Days between imaging and assessment | 467.6 | 326.7 | 90 | 1402 |
|
| ||||
| Time 2 Scores: Model B (n=192) | ||||
|
| ||||
| Age (years) | 21.4 | 1.4 | 19 | 26 |
| Stressful life events Time 2 | 5.4 | 6.2 | 0 | 34 |
| Internalizing symptoms Time 2 | 111.6 | 27.2 | 65 | 211 |
| Days between imaging and assessment | 683.0 | 278.6 | 365 | 1402 |
|
| ||||
| Time 2 Scores: Model C (n=99) | ||||
|
| ||||
| Age (years) | 20.5 | 1.1 | 19 | 23 |
| Stressful life events Time 2 | 3.3 | 4.2 | 0 | 27.7 |
| Internalizing symptoms Time 2 | 109.9 | 29.2 | 65 | 230 |
| Days between imaging and assessment | 397.3 | 39.2 | 365 | 455 |
Childhood trauma=Total of all subscales of the Childhood Trauma Questionnaire; Stressful life events=Total impact score for all life events reported on the Life Events Scale for Students; Internalizing symptoms=Total of all subscales on the Mood and Anxiety Symptoms Questionnaire; SD=standard deviation.
Measures: Baseline Assessments
Functional MRI
Participants performed a face matching task that has been to shown to elicit robust amygdala reactivity across a range of studies and samples, including the present sample (Nikolova et al., 2014; Prather et al., 2013). The current paradigm consisted of four blocks of face matching interleaved with five blocks of a shape-matching sensorimotor control task. During face-matching blocks, participants viewed a trio of faces and selected one of two faces (on bottom) matching a target face (on top). Each face block contained one of the following expressions: fearful, angry, surprised, and neutral. Each trial in the face-matching blocks lasted for 4 seconds with a variable interstimulus interval (ISI) of 2 to 6 seconds (M=4 seconds), for a total block length of 48 seconds. In the control blocks, each of the six shape trios was presented for 4 seconds with a fixed ISI of 2 seconds, for a total block length of 36 seconds. Total task time was 390 seconds. Details regarding fMRI acquisition are reported in the Supplemental Experimental Procedures.
Covariates
Because we were interested in predicting the future development of internalizing symptoms, we controlled for internalizing symptoms at baseline using the Mood and Anxiety Symptoms Questionnaire (MASQ) Short Form (Watson et al., 1995). Scores across all subscales (general distress/depression, general distress/anxiety, anxious arousal, and anhedonia) were summed to create a measure of total internalizing symptoms. Likewise, because we were interested in the effect of life stress occurring post-scanning, we controlled for life stress reported at baseline. To assess life stress, participants were administered the Life Events Scale for Students (LESS; Clements and Turpin, 1996), to measure the number of life events that occurred in the past 12 months. Participants also rated the impact that the life event had on them on a 1 to 4 scale (4=severe impact). The impact score for each event reported was summed to yield a LESS total impact score; higher values indicate both greater number and severity of life events. Additional covariates are described in the Supplement.
Measures: Post-scanning assessments
Participants were re-contacted post-scanning to complete follow-up assessments online every 3 months. Successful completers were entered into a raffle for one $50 Amazon gift card for each round of follow-up assessments. The same questions from the LESS were used to assess stressful life events in the post-scanning questionnaires; however, for these assessments participants were asked to report if any life events had occurred since their last assessment. Participants also completed the MASQ Short Form during these post-scanning assessments. In accordance with our first model (Model A), we selected the most recent assessment available for all participants, and obtained the life stress total impact score from the LESS and total internalizing symptoms from the MASQ at this most recent assessment. For Model B, we took a similar approach, except that we limited this model to those participants who had completed an assessment at least 1 year post-scanning. In accordance with our final model (Model C), we selected MASQ symptom scores from questionnaires completed approximately 1 year post-scanning (the inclusion range was set to 365-455 days post-scanning, to take into account the fact that participants may not have completed the assessment exactly 365 days post-scanning). We then computed the mean of the LESS impact score from each assessment completed before that (Figure 3A). Extra care was taken in quality control procedures for these assessments, given that they were administered online. Specifically, individual item responses were examined for any obvious patterns of false reporting (e.g., a participant indicates a yes for every stressful life event on the LESS). Attrition analyses are reported in the Supplemental Experimental Procedures.
Analyses
Functional MRI
Functional MRI data were processed in SPM8 using the standard pre-processing stream used in previously published research from the Duke Neurogenetics Study. Further details on the procedure and quality control criteria are reported in the Supplemental Experimental Procedures. We hypothesized that results would be specific to threatening facial expressions (fearful and angry), thus the main contrast analyzed was fearful and angry faces > shapes. To examine the specificity of effects to negative expressions, we also ran analyses with parameter estimates for amygdala reactivity to neutral faces > shapes as a control condition, reported in the Supplemental Data. To obtain estimates of amygdala reactivity for each condition, we first identified functional clusters within the amygdala (defined structurally with the Automated Anatomical Labeling atlas) activated at p<.05 family-wise error (FWE) corrected within the region of interest for each condition in SPM8. Then, we extracted parameter estimates for the left and right amygdala for each condition. This procedure has been used in prior published research from the Duke Neurogenetics Study (Nikolova et al., 2012; Nikolova et al., 2014). Because left and right amygdala reactivity for our contrast of interest (fearful and angry faces>shapes) was highly correlated (r=.78, p<.001), we averaged across hemispheres to obtain one mean parameter estimate of amygdala reactivity and reduce the number of comparisons performed.
Moderation model
Our hypothesis was that the association between amygdala reactivity and internalizing symptoms would be moderated by the amount of life stress experienced post-scanning. After parameter estimates of amygdala reactivity were extracted in SPM8, all subsequent analyses to test this proposed moderation model were performed in MPlus version 7. Thus, for Models A-C, MASQ total scores from the follow-up assessment were entered as the dependent variable, and extracted parameter estimates of amygdala reactivity, the total life stress impact score from follow-up, and the interaction between these were entered as predictors. The following were included as covariates: age at the most recent assessment, gender, childhood trauma total scores, LESS total impact scores from the baseline assessment, MASQ total scores at baseline, the psychopathology covariate, days between scanning and completing the follow-up questionnaire, and (for Model C only) the number of assessments contributing to the mean LESS total impact score. Predictors were mean-centered. Because symptoms at Time 2 were moderately skewed, MLR estimation was specified to estimate standard errors robust to non-normality.
Supplementary Material
Highlights.
Amygdala reactivity interacts with stress to predict internalizing symptoms.
Amygdala reactivity predicted symptoms as much as 1 to 4 years after scanning.
Acknowledgments
The Duke Neurogenetics Study is supported by Duke University and NIH grant DA033369. ARH is supported by NIH grants DA033369 and DA031579. JRS is supported by a Postdoctoral Fellowship provided by the National Institute of Child Health and Human Development through the Center for Developmental Science grant T32-HD07376 and by NIH grant P30DA023026.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No authors have a conflict of interest to report.
References
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, Hendler T. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. PNAS. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor iso/val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am. J. Psychiatry. 2012;169 doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredemeier K, Spielberg JM, Silton RL, Berenabum H, Heller W, Miller GA. Screening for depressive disorders using the Mood and Anxiety Symptoms Questionnaire Anhedonic Depression scale: A receiver-operating characteristic analysis. Psychol. Assess. 2010;22:702–710. doi: 10.1037/a0019915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckby JA, Yung AR, Cosgrave EM, Cotton SM. Distinguishing between anxiety and depression using the Mood and Anxiety Symptoms Questionnaire (MASQ) Br. J. Clin. Psychol. 2007;46:235–239. doi: 10.1348/014466506X132912. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Clements K, Turpin G. The life events scale for students: Validation for use with British samples. Pers. Individ. Dif. 1996;20:747–751. [Google Scholar]
- Espejo EP, Hammen C, Brennan PA. Elevated appraisals of the negative impact of naturally occurring life events: A risk factor for depressive and anxiety disorders. J. Abnorm. Child Psychol. 2012;40:303–315. doi: 10.1007/s10802-011-9552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Matthews J, Sheline YI. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol. Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli C. Life events preceding the onset of panic disorder. Journal of affective disorders. 1985;9:103–105. doi: 10.1016/0165-0327(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, Vlahov D. Psychological sequelae of the September 11 terrorist attacks in New York City. N. Engl. J. Med. 2002;346:982–987. doi: 10.1056/NEJMsa013404. [DOI] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: Evidence from a meta-analysis of fMRI studies. Neurosci. and Biobehav. Rev. 2013;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol. Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. J. Abnorm. Psychol. 2012;121:61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to suprised faces. NeuroReport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress. Anxiety. 2014 doi: 10.1002/da.22284. Advance online publication. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, III, Guardino M, Masten CL, McClure-Tone EB, Fromm SJ, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am. J. Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Murty VP, Ritchey M, Adcock RA, LaBar KS. FMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia. 2010;48:3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol. Psychiatry. 2012;72:157–163. doi: 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Koenen KC, Galea S, Wang C, Seney ML, Sibille E, Williamson DE, Hariri AR. Beyond genotype: Serotonin transporter epigenetic modification predicts human brain function. Nat. Neurosci. 2014;17:1153–5. doi: 10.1038/nn.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Neuroimaging studies of attention and the processing of emotion-laden stimuli. Prog. Brain Res. 2004;144:171–182. doi: 10.1016/S0079-6123(03)14412-3. [DOI] [PubMed] [Google Scholar]
- Prather AA, Bogdan R, Hariri AR. Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosom. Med. 2013;75:350–8. doi: 10.1097/PSY.0b013e31828ef15b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, Hariri AR. Developmental change in amygdala reactivity during adolescence: Effects of family history for depression and stressful life events. Am. J. Psychiatry. doi: 10.1176/appi.ajp.2014.14020195. in press. doi: 10.1176/appi.ajp.2014.14020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J. Abnorm. Psychol. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- White MG, Bogdan R, Fisher PM, Munoz KE, Williamson DE, Hariri AR. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes, Brain and Behav. 2012;11:869–878. doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.