Abstract Abstract
Three new species of saddled hypostomine loricariids are described. According to a recent phylogenetic analysis, these species are members of the genus Peckoltia. The species differ from all described Peckoltia except Peckoltia furcata and Peckoltia sabaji by having the dentaries meet at an angle greater than 90°. The species also have similarities to Hemiancistrus, and can be separated from all described species by having dorsal saddles. We discuss the taxonomy of Peckoltia, Hemiancistrus, and allied genera and recognize Ancistomus as valid for Peckoltia feldbergae, Hemiancistrus micrommatos, Ancistrus snethlageae, Hemiancistrus spilomma, and Hemiancistrus spinosissimus. We recommend descriptions of genera for several clades of Hemiancistrus and restriction of Hemiancistrus to the type species of the genus, Hemiancistrus medians. Chaetostomus macrops is transferred to Pseudancistrus and recognized as a junior synonym of Pseudancistrus megacephalus. The Hemiancistrus annectens group of species (Hemiancistrus annectens, Hemiancistrus argus, Hypostomus aspidolepis, Hemiancistrus fugleri, Hemiancistrus holostictus, Hemiancistrus maracaiboensis, Hemiancistrus panamensis, Hemiancistrus wilsoni) are recognized in Hypostomus. Multivariate analysis reveals that the newly described species differ from one another in shape space, but overlap broadly with other Peckoltia (Peckoltia lujani), narrowly with other Peckoltia (Peckoltia greedoi), or broadly with Etsaputu (Peckoltia ephippiata).
Keywords: Ancistrini, Hypostominae, Peckoltia, Siluriformes, Systematics, Taxonomy
Introduction
The Loricariidae, or suckermouth armored catfishes, comprise over 800 species and present numerous difficult taxonomic problems. Among the worst problems is the identity of Peckoltia Miranda Ribeiro and allied genera like Ancistomus Isbrücker and Seidel and Hemiancistrus Bleeker. The genus Peckoltia was described by Miranda Ribeiro (1912) with Chaetostomus vittatus (Steindachner, 1881) as the type. The definition has since remained unclear. Armbruster (2008) revised Peckoltia sensu stricto, and he defined the genus as having dentaries meeting at an angle less than 90°, but lacking any of the synapomorphies of other ancistrin genera with angled jaws. All other ancistrin species without characteristics that united them into other genera were lumped into Hemiancistrus by Armbruster (2008). Despite this definition, other researchers used an expanded definition of Peckoltia without any clear delineations, including such species as Ancistrus snethlageae and Peckoltia sabaji, which Armbruster (2008) considered part of the polyphyletic Hemiancistrus (Oliveira et al. 2010, 2012; Fisch-Muller et al. 2012). The result was a likely polyphyletic Peckoltia in addition to a polyphyletic Hemiancistrus. This confusion on the identity of the species is due to a lack of clear morphological groups into which species can be assigned, and attempts to constrain species into two artificial taxa. Because of the lack of a clear morphological signal, a molecular phylogeny was needed to make some sense of the taxa.
Lujan et al. (2015) provide the largest phylogenetic analysis of loricariids to date focusing on the Hypostominae. The analysis included 181 species and 91 genera of loricariids, and was based on two mitochondrial and three nuclear genes. The study offers a compelling answer to why the taxa are so complex: morphology of loricariids is incredibly plastic, with lots of convergence. Lujan et al. (2015) showed that the genera Peckoltia and Hemiancistrus are not monophyletic as previously defined, and offer a new taxonomy for species of Peckoltia and Hemiancistrus (expanded upon here in Table 1). Although morphological support for most of the clades in the molecular phylogeny is currently unknown, the phylogeny offers a framework for describing new species of loricariids, and no morphological support was present for any of the previous taxonomic hypotheses. Lujan et al. (2015) suggested that Peckoltia sabaji does belong in Peckoltia as well as the three species described herein; however, Lujan et al. (2015) also suggested that Ancistomus is a valid genus, and it includes Peckoltia feldbergae. Chaetostomus bachi was included in Peckoltia by Armbruster (2008), but the unusual species was not closely related to the remainder of Peckoltia in the molecular phylogeny, and Peckoltichthys was resurrected as monotypic. Hemiancistrus likely only contains Hemiancistrus medians, and several species groups within Hemiancistrus require new genera (Table 1). Etsaputu was found nested within Peckoltia, but Lujan et al. do not sink the genus as it has a very unusual anatomy (lacks a fully evertible cheek apparatus), and the placement of the genus needs further work.
Table 1.
Species once part of Hemiancistrus and/or Peckoltia and their current taxonomy. Current names are per this study, Isbrücker 1980, 2001; Fisch-Muller 2003; Weber 2003; Armbruster 2004, 2005, 2008; or original descriptions.
| Original name | Current name | Author | Notes |
|---|---|---|---|
| Ancistrus annectens | Hypostomus annectens | Regan, 1904 | comb. n. |
| Ancistrus brachyurus | Dekeyseria brachyura | Kner, 1854 | |
| Ancistrus medians | Hemiancistrus medians | Kner, 1854 | |
| Ancistrus multispinis | Peckoltia multispinis | Holly, 1929 | |
| Ancistrus pulcher | Dekeyseria pulchra | Steindachner, 1915 | |
| Ancistrus salgadae | Hypostomus salgadae | Fowler, 1941 | comb. n. |
| Ancistrus scaphirhynchus | Dekeyseria scaphirhyncha | Kner, 1854 | |
| Ancistrus snethlageae | Ancistomus snethlageae | Steindachner, 1911 | |
| Ancistrus yaravi | Neblinichthys yaravi | Steindachner, 1915 | |
| Chaetostomus macrops | ‘Pseudancistrus’ megacephalus | Lütken, 1874 | |
| Chaetostomus aspidolepis | Hypostomus aspidolepis | Günther, 1867 | comb. n. |
| Chaetostomus bachi | Peckoltichthys bachi | Boulenger, 1898 | |
| Chaetostomus furcatus | Peckoltia furcata | Fowler, 1940 | |
| Chaetostomus megacephalus | ‘Pseudancistrus’ megacephalus | Günther, 1868 | |
| Chaetostomus oligospilus | Peckoltia oligospila | Günther, 1864 | |
| Chaetostomus platycephalus | Cordylancistrus platycephalus | Boulenger, 1898 | |
| Chaetostomus vittatus | Peckoltia vittata | Steindachner, 1881 | |
| Hemiancistrus albocinctus | Ancistrus multispinis | Ahl, 1936 | |
| Hemiancistrus arenarius | Peckoltichthys bachi | Eigenmann & Allen, 1942 | comb. n. |
| Hemiancistrus braueri | Peckoltia braueri | Eigenmann, 1912 | |
| Hemiancistrus brevis | Peckoltia brevis | La Monte, 1935 | |
| Hemiancistrus caquetae | Lasiancistrus schomburgkii | Fowler, 1945 | |
| Hemiancistrus castelnaui | Lasiancistrus schomburgkii | Miranda Ribeiro, 1911 | |
| Hemiancistrus cerrado | ‘Hemiancistrus’ cerrado | de Souza et al., 2008 | ‘Hemiancistrus’ chlorostictus group |
| Hemiancistrus chlorostictus | ‘Hemiancistrus’ chlorostictus | Cardoso & Malabarba, 1999 | ‘Hemiancistrus’ chlorostictus group |
| Hemiancistrus daguae | Cordylancistrus daguae | Eigenmann, 1912 | |
| Hemiancistrus fugleri | Hypostomus annectens | Ovchynnyk, 1971 | syn. n. |
| Hemiancistrus fuliginosus | ‘Hemiancistrus’ fuliginosus | Cardoso & Malabarba, 1999 | ‘Hemiancistrus’ chlorostictus group |
| Hemiancistrus guahiborum | ‘Hemiancistrus’ guahiborum | Werneke et al., 2005 | ‘Hemiancistrus’ guahibroum group |
| Hemiancistrus hammarlundi | ‘Hemiancistrus’ landoni | Rendahl, 1937 | syn. n. |
| Hemiancistrus holostictus | ‘Hypostomus’ holostictus | Regan, 1913 | comb. n. |
| Hemiancistrus landoni | ‘Hemiancistrus’ landoni | Eigenmann, 1916 | ‘Hemiancistrus’ landoni group |
| Hemiancistrus longipinnis | Baryancistrus longipinnis | Kindle, 1895 | |
| Hemiancistrus maracaiboensis | Hypostomus maracaiboensis | Schultz, 1944 | comb. n. |
| Hemiancistrus mayoloi | Lasiancistrus caucanus | Eigenmann, 1912 | |
| Hemiancistrus megalopteryx | ‘Hemiancistrus’ megalopteryx | Cardoso, 2004 | ‘Hemiancistrus’ chlorostictus group |
| Hemiancistrus meizospilos | ‘Hemiancistrus’ meizospilos | Cardoso & da Silva, 2004 | ‘Hemiancistrus’ chlorostictus group |
| Hemiancistrus micrommatos | Ancistomus micrommatos | Cardoso & Lucinda, 2003 | comb. n. |
| Hemiancistrus niceforoi | Hypostomus niceforoi | Fowler, 1943 | |
| Hemiancistrus niger | Guyanancistrus niger | Norman, 1926 | |
| Hemiancistrus pankimpuju | Peckoltia pankimpuju | Lujan & Chamon, 2008 | comb. n. |
| Hemiancistrus platyrhynchus | Chaetostoma platyrhyncha | Fowler, 1943 | |
| Hemiancistrus punctulatus | ‘Hemiancistrus’ punctulatus | Cardoso & Malabarba, 1999 | ‘Hemiancistrus’ chlorostictus group |
| Hemiancistrus spilomma | Ancistomus spilomma | Cardoso & Lucinda, 2003 | comb. n. |
| Hemiancistrus spinosissimus | Ancistomus spinosissimus | Cardoso & Lucinda, 2003 | comb. n. |
| Hemiancistrus subviridis | ‘Hemiancistrus’ subviridis | Werneke et al., 2005 | ‘Hemiancistrus’ guahibroum group |
| Hemiancistrus ucayalensis | Peckoltichthys bachi | Fowler, 1940 | comb. n. |
| Hemiancistrus votouro | ‘Hemiancistrus’ votouro | Cardoso and da Silva, 2004 | ‘Hemiancistrus’ chlorostictus group |
| Hemiancistrus wilsoni | Hypostomus wilsoni | Eigenmann, 1918 | comb. n. |
| Hypostomus itacua | Hypostomus itacua incertae sedis | Valenciennes, 1836 | probably is a Hypostomus |
| Hypostomus pictus | Lasiancistrus schomburgkii | Castelnau, 1855 | |
| Peckoltia caenosa | Peckoltia caenosa | Armbruster, 2008 | |
| Peckoltia capitulata | Peckoltia capitulata | Fisch-Muller & Covain, 2012 | |
| Peckoltia cavatica | Peckoltia cavatica | Armbruster & Werneke, 2005 | |
| Peckoltia compta | Peckoltia compta | de Oliveira et al., 2010 | |
| Peckoltia feldbergae | Ancistomus feldbergae | de Oliveira et al., 2012 | comb. n. |
| Peckoltia lineola | Peckoltia lineola | Armbruster, 2008 | |
| Peckoltia otali | Peckoltia otali | Fisch-Muller & Covain, 2012 | |
| Peckoltia sabaji | Peckoltia sabaji | Armbruster, 2003 | |
| Peckoltia simulata | Peckoltia simulata | Fisch-Muller & Covain, 2012 | |
| Peckoltichthys filicaudatus | Peckoltichthys bachi | Miranda Ribeiro, 1917 | |
| Peckoltichthys kuhlmanni | Peckoltia vittata | Miranda Ribeiro, 1920 | |
| Plecostomus niveatus | Dekeyseria niveata | La Monte, 1929 | |
| Plecostomus panamensis panamensis | Hypostomus aspidolepis | Eigenmann, 1922 |
In this paper, we describe three new species of Peckoltia. These species differ from Peckoltia sensu stricto. by having their jaws meet at an angle greater than 90°, but share with most species of Peckoltia the presence of dorsal saddles (absent in Hemiancistrus sensu lato). In addition, we discuss the implications of the molecular phylogeny of Lujan et al. (2015) on the taxonomy of Peckoltia, Hemiancistrus, and allied genera, and we recognize several species groups that may represent new genera.
Methods
Methods follow Armbruster (2003) with the addition of counts of mid-dorsal and mid-ventral plates (the number of plates in these series from the head to caudal fin and excluding the last plate, which is beyond the hypural). Institutional abbreviations are as in Sabaj Pérez (2014). Names of skeletal characteristics are as in Schaefer (1987) and of plate rows as in Schaefer (1997). A principal component analysis for the morphometric data of all Peckoltia examined in Armbruster (2008) and the specimens below was performed using a covariate matrix and log-transformed measurements in JMP (Vers. 5.01a, SAS Institute, 2002). We have not examined specimens of recently described species (Peckoltia capitulata, Peckoltia compta, Peckoltia otali, and Peckoltia stimulata). Principal component one was excluded from further analysis because it represented overall size differences (vs. relative size differences). The following abbreviations are used in the text: dr. = drainage, nm. = not measured. Full morphometric dataset is presented in Suppl. material 1, locality information for species described below is presented in Suppl. material 2. Character state data for Hemiancistrus medians based on Armbruster (2004a, 2008) in Suppl. material 3.
Results
Morphometrics
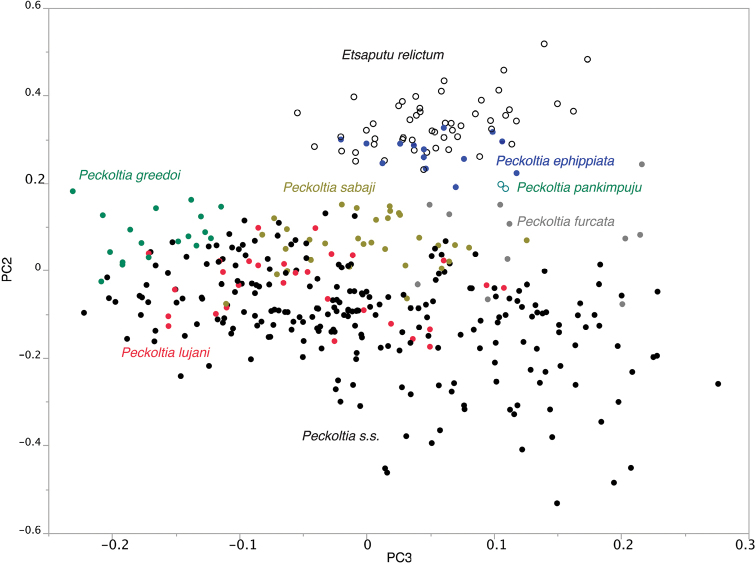
The three new species show little overlap with one another in the PCA (Fig. 1). Peckoltia lujani overlaps Peckoltia sensu stricto (Peckoltia sensu Armbruster, 2008, minus Peckoltichthys bachi), Peckoltia sabaji, and Peckoltia furcata. Peckoltia greedoi overlaps Peckoltia s.s. and Peckoltia lujani slightly. Peckoltia ephippiata overlaps only Etsaputu relictum.
Figure 1.
Principal Component Analysis of species of Peckoltia with Peckoltia s.s. being those Peckoltia with jaws forming an angle less than 90° (some Peckoltia furcata also have this low jaw angle). PC2 is most strongly influenced negatively by barbel length, internares with, and caudal-peduncle depth, and positively by dentary length, premaxillary length, and mouth width. PC3 is most strongly influenced negatively by barbel length, premaxillary length, and dorsal-adipose distance, and positively by adipose-upper caudal distance, adipose spine length, and head-dorsal distance.
Peckoltia ephippiata
Armbruster, Werneke & Tan sp. n.
http://zoobank.org/2BA0FAC3-FCA5-404D-BA5E-C2232FCD636F
Figure 2.
Dorsal, lateral, and ventral views of holotype of Peckoltia ephippiata sp. n., MCP 35627, 101.7 mm SL. Photos by J. W. Armbruster.
Table 2.
Selected morphometrics of Peckoltia ephippiata. Numbers in parentheses refer to landmark numbers in Armbruster (2003).
| Holotype | N | Mean | SD | Min | Max | |
|---|---|---|---|---|---|---|
| SL, mm (1–20) | 101.7 | 20 | 48.2 | 101.7 | ||
| %SL | ||||||
| Predorsal Length (1–10) | 40.8 | 20 | 42.1 | 1.3 | 40.4 | 45.3 |
| Head Length (1–7) | 32.1 | 20 | 33.8 | 1.4 | 31.9 | 37.3 |
| Head–dorsal Length (7–10) | 9.7 | 20 | 8.7 | 0.7 | 7.1 | 10.0 |
| Cleithral Width (8–9) | 26.9 | 20 | 28.4 | 0.9 | 26.9 | 30.2 |
| Head-pectoral Length (1–12) | 24.3 | 20 | 24.7 | 1.2 | 22.9 | 27.5 |
| Thorax Length (12–13) | 22.7 | 20 | 23.3 | 1.1 | 20.4 | 25.2 |
| Pectoral-spine Length (12–29) | 28.8 | 20 | 31.3 | 1.2 | 28.3 | 32.8 |
| Abdominal Length (13–14) | 21.7 | 20 | 22.3 | 0.8 | 21.2 | 24.0 |
| Pelvic-spine Length (13–30) | 25.3 | 19 | 25.8 | 1.8 | 23.3 | 30.4 |
| Postanal Length (14–15) | 35.8 | 20 | 36.0 | 1.0 | 34.3 | 37.8 |
| Anal-fin spine Length (14–31) | 15.9 | 18 | 14.5 | 1.4 | 11.7 | 16.9 |
| Dorsal–pectoral Distance (10–12) | 26.6 | 20 | 27.7 | 1.1 | 26.1 | 29.8 |
| Dorsal spine Length (10–11) | 32.9 | 16 | 32.9 | 1.4 | 29.8 | 34.9 |
| Dorsal-pelvic Distance (10–13) | 21.7 | 20 | 22.4 | 1.1 | 20.3 | 24.6 |
| Dorsal-fin base Length (10–16) | 28.1 | 20 | 27.3 | 1.1 | 25.2 | 30.1 |
| Dorsal-adipose Distance (16–17) | 14.9 | 20 | 14.4 | 1.4 | 11.8 | 16.9 |
| Adipose-spine Length (17–18) | 11.4 | 20 | 11.6 | 1.2 | 8.8 | 13.1 |
| Adipose-upper caudal Distance (17–19) | 16.2 | 20 | 17.5 | 1.1 | 15.7 | 19.7 |
| Caudal-peduncle Depth (15–19) | 12.2 | 20 | 13.0 | 0.9 | 11.7 | 14.8 |
| Adipose-lower caudal Distance (15–17) | 24.5 | 20 | 25.4 | 1.6 | 22.6 | 28.3 |
| Adipose-anal Distance (14–17) | 20.1 | 20 | 18.6 | 1.0 | 16.2 | 20.5 |
| Dorsal-anal Distance (14–16) | 15.1 | 20 | 14.9 | 0.4 | 14.0 | 15.7 |
| Pelvic-dorsal Distance (13–16) | 25.1 | 20 | 24.9 | 1.0 | 23.2 | 26.7 |
| % Head Length | ||||||
| Head-eye Length (5–7) | 32.7 | 20 | 34.7 | 2.6 | 30.5 | 39.0 |
| Orbit Diameter (4–5) | 20.8 | 20 | 22.6 | 1.4 | 20.4 | 25.1 |
| Snout Length (1–4) | 61.2 | 20 | 57.6 | 2.0 | 54.5 | 61.7 |
| Internares Width (2–3) | 14.8 | 20 | 15.5 | 2.0 | 11.2 | 19.4 |
| Interorbital Width (5–6) | 51.1 | 20 | 50.5 | 3.1 | 43.7 | 56.2 |
| Head Depth (7–12) | 67.8 | 20 | 69.0 | 1.7 | 65.7 | 72.2 |
| Mouth Length (1–24) | 46.2 | 20 | 45.8 | 2.0 | 41.0 | 48.4 |
| Mouth Width (21–22) | 53.1 | 20 | 50.3 | 3.0 | 43.8 | 57.4 |
| Barbel Length (22–23) | 11.0 | 20 | 11.3 | 1.8 | 7.9 | 14.0 |
| Dentary Tooth Cup Length (25–26) | 20.0 | 20 | 19.1 | 1.8 | 15.1 | 23.0 |
| Premaxillary Tooth Cup Length (27–28) | 19.2 | 20 | 19.3 | 1.7 | 16.4 | 22.6 |
Type locality.
Brazil, South America
Holotype.
MCP 35627, 1, 101.7 mm SL, BRAZIL, Rôndonia, Presidente Médici. rio Madeira dr., rio Leitão on highway BR-364, about 5 km N of Presidente Médici, -11.1328°, -061.9008°, 15 Jul 2004, R.E. Reis, P.C. Lehmann, F.C. Lima, and E.H.L. Pereira.
Paratypes.
ANSP 197614, 2, 60.4–92.5, AUM 65116, 2, 64.1–96.4, MCP 48395, 13, 48.2–97.7, MNRJ 42662, 2, 66.0–89.7, UF 237091, 2, 55.6–82.6, same locality data as holotype.
Diagnosis.
Peckoltia ephippiata can be separated from Peckoltia pankimpuju by having well developed color and eyes; from all other Peckoltia by having no spots or bands in the dorsal fin; from all except Peckoltia greedoi by having small, very faint spots on the head (vs. large spots, mottling, short lines, or thick dark areas, always much more intense than the weak spots in Peckoltia ephippiata; Peckoltia greedoi has a uniformly dark head, but the small faint spots of Peckoltia ephippiata can appear uniformly dark without closer inspection); from all Peckoltia except Peckoltia furcata, Peckoltia greedoi, Peckoltia lujani, Peckoltia pankimpuju, and Peckoltia sabaji by having the dentaries meet at an angle greater than 90°; from Peckoltia greedoi and Peckoltia lujani by lacking bands in the dorsal fin, rays light and membranes dark (vs. bands present), by having more teeth (Peckoltia ephippiata: 39–72 dentary, 41–73 premaxillary; Peckoltia greedoi: 16–39 dentary, 20–38 premaxillary; Peckoltia lujani: 20–37 dentary, 23–45 premaxillary), by having slight keels on the lateral plates, particularly the median series (vs. keels absent), and by having platelets on the central region of the abdomen posterior to the pectoral girdle present (vs. platelets maximally present below pectoral girdle and in a narrow, lateral column just posterior to pectoral fin, and below pelvic girdle); and from Peckoltia lujani by having the pectoral-fin spine relaxed position angled dorsally, pointing at insertion of dorsal fin (vs. pectoral-fin spine angled only slightly dorsally, pointing maximally to dorsal insertion of caudal fin) and by the pectoral-fin spine reaching two or more plates of the ventral series beyond the pelvic base when adpressed ventral to pelvic fin (vs. less than one plate).
Peckoltia ephippiata differs from Etsaputu by having greater than six evertible cheek odontodes, the largest of which extends posterior to the eye (vs. six or fewer, the largest not extending beyond the exposed portion of the opercle). Peckoltia ephippiata can be separated from Hemiancistrus (except ‘Hemiancistrus’ landoni) and Ancistomus by having prominent dorsal saddles (vs. dark or light spots or entirely dark); and from all Hemiancistrus and Ancistomus by having bands in the caudal fin and no free spots (vs. bands absent or present with some free spots). Peckoltia ephippiata can be separated from Peckoltichthys bachi by having small, faint spots on the head (vs. large dark spots or mottling); by having the eyes high on the head with the dorsal rim of the orbit higher than the interorbital space (vs. low on the head, dorsal rim of orbit lower than interorbital space), and by having small plates on the abdomen (vs. relatively large).
Description.
Morphometrics in Table 2. Counts and measurements based on 18 specimens. Largest specimen examined 101.7 mm SL. Body moderately elongate. Head and nape forming arc from tip of snout to insertion of dorsal fin. Dorsal slope decreasing in straight line to insertion of dorsal procurrent caudal rays then ascending to caudal fin. Body depth greatest below insertion of dorsal fin. Ventral profile flat to caudal fin. Caudal peduncle triangular in cross section with dorsal surface flattened. Body widest at insertion of pectoral fins, narrowest at insertion of caudal fin. Snout rounded.
Eye moderately sized, dorsal rim of orbit forming tall crest that continues forward to area just anterior of nares as low, rounded ridge. Iris operculum present. Interorbital space largely flat, but with slight, rounded, median hump that is contiguous with rounded ridge on snout formed from mesethmoid. Parieto-supraoccipital pointed posteriorly with the posterior point raised above nuchal region in small crest. Infraorbitals, frontal, nasal, compound pterotic, and parieto-supraoccipital supporting odontodes. Preopercle not supporting odontodes. Opercle generally covered by plates and not supporting odontodes although one to four may be present, particularly in smaller individuals.
Lips covered with short, wide papillae. Lower lip wide, upper lip narrow. Edge of lower lip with small crenulae. Maxillary barbel only barbel present, reaching about one third of distance to gill opening.
Median plates 25–26 (mode 26). Plates unkeeled, but first four or five plates of mid-ventral series bent to form slight ridge. Five caudal peduncle plate rows. Plates on all dorsolateral surfaces of body except for extreme edge of snout that only has a narrow column of platelets on either side of the snout tip. Throat mostly covered in platelets except for area right below lower lip. Abdomen covered in platelets except for broad region just anterior to level of pelvic-fin spine insertions, laterally below pelvic girdle, and small region around anus. Evertible cheek plates supporting hypertrophied odontodes that can be everted perpendicular to head. Cheek odontodes 18–40 (mode 32). Longest evertible cheek odontode reaching to about level of posterior edge of pectoral-fin spine. Hypertrophied cheek odontodes relatively weak. Odontodes slightly longer than average body odontodes present along dorsal-, adipose-, pelvic-, caudal-, and pectoral-fin spines; larger individuals with hypertrophied odontodes at tip of pectoral spine.
Dorsal fin ii,7; dorsal spinelet V-shaped, dorsal-fin locking mechanism present, last ray of dorsal fin not reaching preadipose plate when adpressed. Adipose fin with single preadipose plate and moderately long spine. Caudal fin i,14,i; caudal fin forked, ventral lobe longer than dorsal lobe; dorsal and ventral procurrent caudal rays five. Pectoral fin i,6; pectoral-fin spine reaching just posterior to pelvic fin when adpressed ventral to pelvic fin. Pelvic fin i,5; pelvic-fin spine extending to posterior end of base of anal fin when adpressed. Anal fin i,4; anal-fin spine slightly shorter than first ray.
Teeth bicuspid with lateral lobe three-quarters length of medial lobe and distal tip of lateral cusp one-half width of tip of medial cusp. 39–72 left dentary teeth (mode 56). 41–73 left premaxillary teeth (mode 64).
Color.
Base color red brown, intensity of red greater in smaller specimens. Head and nape almost completely dark brown with some extremely small spots faintly visible on posterodorsal surface of head and nape, many of the spots combining to form vermiculations. Compound pterotic slightly lighter than rest of head and small spots slightly more evident. Pectoral fin dark brown with faint, large, oblong spots along leading edge. Pelvic fin as pectoral but lighter. Dorsal fin with oblong spots along spine, rays red brown, and membranes dark. Caudal fin with three to five bands that may be regular (contiguous along height of fin) or irregular (ventral and dorsal parts offset); lighter interspaces red brown, usually slightly narrower than dark bands (the largest individual examined has the light interspaces much narrower than the bands, which are very irregular). Body with four saddles, first below middle of dorsal fin, second with anterior half below posterior end of dorsal fin and posterior half behind dorsal fin, third beginning one to two plates anterior of preadipose plate to about posterior third of adipose-fin membrane, and fourth beginning just posterior to adipose fin to end of caudal peduncle; first and second saddles and usually third connected at median plate series; saddles appear to be formed of two bars each that fuse as specimens get older, and connection between bands form because the ventral sides appear to get darker with age. Ventral surface uniformly light except for the present of blotches from anterior insertion of anal fin to caudal fin, which may or may not be extensions of the saddles onto the ventral surface.
Sexual dimorphism.
It appears that some of the larger specimens (presumably male) are slightly more hispid, suggesting that nuptial males may develop hypertrophied odontodes on the lateral plates; however, no specimens have hypertrophied odontodes. The larger specimens also have the odontodes on the pectoral-fin spines moderately hypertrophied, which may also be a nuptial male characteristic.
Distribution.
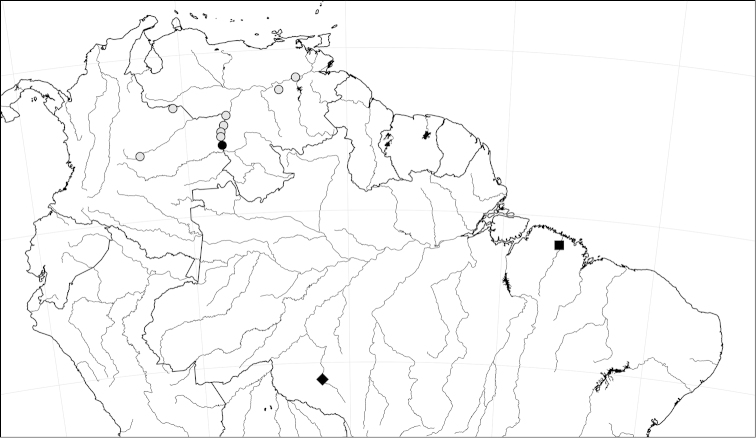
Known only from the type locality in the rio Madeira drainage of Brazil (Fig. 3).
Figure 3.
Distribution of the new species of Peckoltia: Peckoltia ephippiata (diamond), Peckoltia greedoi (square), and Peckoltia lujani (dots, black dot is type locality).
Etymology.
Ephippiata is Latin for saddled and refers to the presence of saddles in this species.
Remarks.
Many of the specimens in the type series contain a significant load of larval Neascus-type metacercariae (visible as black spots on the body and fins in Figure 2). This type of trematode burrows in the skin as larvae and the host mounts a response whereby pigment cells surround the cyst, making the cysts black (C. Sunderman and K. Hayden, pers. comm.).
Peckoltia greedoi
Armbruster, Werneke & Tan sp. n.
http://zoobank.org/F5A921BA-2746-44D2-9D01-E29EBA6A43DF
Figure 4.
Dorsal, lateral, and ventral views of holotype of Peckoltia greedoi sp. n., MCP 21972, 77.8 mm SL. Photos by J. W. Armbruster.
Table 3.
Selected morphometrics of Peckoltia greedoi. Numbers in parentheses refer to landmark numbers in Armbruster (2003).
| Holotype | N | Mean | SD | Min | Max | |
|---|---|---|---|---|---|---|
| SL, mm (1–20) | 77.8 | 30 | 45.3 | 78.0 | ||
| %SL | ||||||
| Predorsal Length (1–10) | 44.7 | 30 | 44.2 | 1.2 | 42.1 | 47.1 |
| Head Length (1–7) | 35.8 | 30 | 36.8 | 1.0 | 35.0 | 38.8 |
| Head–dorsal Length (7–10) | 8.5 | 30 | 7.5 | 0.7 | 6.0 | 8.8 |
| Cleithral Width (8–9) | 28.2 | 30 | 28.7 | 1.0 | 26.8 | 30.7 |
| Head-pectoral Length (1–12) | 27.1 | 30 | 26.8 | 1.1 | 24.5 | 29.5 |
| Thorax Length (12–13) | 20.5 | 30 | 21.9 | 1.3 | 19.7 | 25.2 |
| Pectoral-spine Length (12–29) | 32.5 | 30 | 31.9 | 1.2 | 29.7 | 33.8 |
| Abdominal Length (13–14) | 23.3 | 30 | 22.8 | 1.1 | 20.2 | 26.1 |
| Pelvic-spine Length (13–30) | 27.7 | 30 | 27.1 | 1.6 | 23.0 | 31.7 |
| Postanal Length (14–15) | 35.3 | 30 | 34.2 | 1.1 | 31.7 | 35.9 |
| Anal-fin spine Length (14–31) | 16 | 30 | 14.8 | 1.0 | 12.4 | 16.4 |
| Dorsal–pectoral Distance (10–12) | 27.9 | 30 | 28.5 | 1.1 | 26.4 | 30.6 |
| Dorsal spine Length (10–11) | broken | 25 | 31.3 | 2.5 | 26.4 | 36.4 |
| Dorsal-pelvic Distance (10–13) | 24.3 | 30 | 23.0 | 1.0 | 20.9 | 25.1 |
| Dorsal-fin base Length (10–16) | 26.3 | 30 | 25.8 | 1.3 | 24.0 | 30.4 |
| Dorsal-adipose Distance (16–17) | 16.3 | 30 | 16.0 | 1.2 | 12.4 | 18.2 |
| Adipose-spine Length (17–18) | 9.8 | 30 | 10.0 | 1.1 | 8.2 | 13.2 |
| Adipose-upper caudal Distance (17–19) | 16.5 | 30 | 16.0 | 1.5 | 13.9 | 21.0 |
| Caudal-peduncle Depth (15–19) | 11.1 | 29 | 12.3 | 0.7 | 11.1 | 14.3 |
| Adipose-lower caudal Distance (15–17) | 23.4 | 29 | 22.8 | 1.5 | 19.7 | 26.4 |
| Adipose-anal Distance (14–17) | 19 | 30 | 18.4 | 1.2 | 16.6 | 21.3 |
| Dorsal-anal Distance (14–16) | 15.9 | 30 | 15.5 | 0.5 | 14.7 | 16.5 |
| Pelvic-dorsal Distance (13–16) | 25.2 | 30 | 25.9 | 1.4 | 23.6 | 30.6 |
| % Head Length | ||||||
| Head-eye Length (5–7) | 36.7 | 30 | 35.2 | 1.4 | 32.2 | 37.7 |
| Orbit Diameter (4–5) | 22.6 | 30 | 22.9 | 1.4 | 18.0 | 25.4 |
| Snout Length (1–4) | 60.6 | 30 | 58.1 | 1.5 | 54.9 | 60.9 |
| Internares Width (2–3) | 12.9 | 30 | 13.3 | 0.7 | 11.7 | 14.3 |
| Interorbital Width (5–6) | 48.9 | 30 | 46.1 | 1.7 | 42.7 | 49.8 |
| Head Depth (7–12) | 69.6 | 30 | 67.4 | 1.5 | 64.7 | 70.4 |
| Mouth Length (1–24) | 50.3 | 30 | 46.3 | 3.1 | 37.7 | 53.4 |
| Mouth Width (21–22) | 51.9 | 27 | 47.9 | 3.6 | 41.1 | 54.5 |
| Barbel Length (22–23) | 14.9 | 29 | 15.1 | 2.4 | 12.2 | 23.7 |
| Dentary Tooth Cup Length (25–26) | 15.3 | 30 | 18.2 | 2.1 | 14.7 | 22.4 |
| Premaxillary Tooth Cup Length (27–28) | 17.5 | 30 | 17.0 | 1.2 | 14.9 | 20.6 |
Type locality.
Brazil, South America
Holotype.
MCP 21972, 78.0 mm SL, BRAZIL, Pará, río Gurupi on BR 316 at border of Pará and Maranhão, -01.8003°, -046.3167°, 23 Jul 1998, R. Reis, J. P. Silva, E. Pereira, J. Montoya.
Paratypes.
ANSP 197617, 2, 56.0–67.4, AUM 65117, 2, 57.6–71.9, MCP 48396, 23, 46.3–75.8, MNRJ 42663, 2, 55.2–71.5, same locality data as holotype.
Diagnosis.
Peckoltia greedoi can be separated from Peckoltia pankimpuju by having well developed color and eyes; from all other Peckoltia except Peckoltia ephippiata by having the head uniformly colored (vs. large spots, mottling, short lines, or thick dark areas; faint spots are present in Peckoltia ephippiata, but are not obvious); from all Peckoltia except Peckoltia braueri, Peckoltia capitulata, Peckoltia compta, Peckoltia lujani, Peckoltia oligospila, Peckoltia otali, and Peckoltia stimulata by having the abdomen largely naked posterior to the pectoral girdle (vs. only small naked patches at insertions of pelvic fins); from all Peckoltia except Peckoltia ephippiata, Peckoltia furcata, Peckoltia lujani, Peckoltia pankimpuju, and Peckoltia sabaji by having the dentaries meet at an angle greater than 90°; from Peckoltia ephippiata by having fewer teeth (Peckoltia greedoi: 16–39 dentary, 20–38 premaxillary; Peckoltia ephippiata: 39–72 dentary, 41–73 premaxilary), by having faint spots forming bands in the dorsal fin, and by having platelets maximally present below pectoral girdle and in a narrow, lateral column just posterior to pectoral fin, and below pelvic girdle (vs. platelets on the central region of the abdomen posterior to the pectoral girdle present); and by lacking slight keels on the lateral plates (vs. keels present, strongest on median series); from Peckoltia lujani by having no spots on the posterodorsal surface of head and nape (vs. large spots), and by having the pectoral-fin spine relaxed position angled dorsally, pointing at insertion of dorsal fin (vs. pectoral-fin spine angled only slightly dorsally, pointing maximally to dorsal insertion of caudal fin) and pectoral-fin spine reaching two or more plates of the ventral series beyond the pelvic base when adpressed ventral to pelvic fin (vs. less than one plate).
Peckoltia greedoi differs from Etsaputu by having greater than six evertible cheek odontodes, the largest of which extends posterior to the eye (vs. six or fewer, the largest not extending beyond the exposed portion of the opercle). Peckoltia greedoi can be separated from Hemiancistrus (except ‘Hemiancistrus’ landoni) and Ancistomus by having prominent dorsal saddles (vs. dark or light spots or entirely dark); and from all Hemiancistrus and Ancistomus by having bands in the caudal fin and no free spots (vs. bands absent or present with some free spots) and bands in the dorsal fin (vs. spots or no markings). Peckoltia greedoi can be separated from Peckoltichthys bachi by having no spots on the head (vs. large dark spots or mottling); by having the eyes high on the head with the dorsal rim of the orbit higher than the interorbital space (vs. low on the head, dorsal rim of orbit lower than interorbital space), and by having small plates on the abdomen (vs. relatively large).
Peckoltia greedoi is very similar to Peckoltia vittata. It differs from Peckoltia vittata by having the dentaries meeting at an angle >90° (vs. <90°), by having a shallower slope of the head (~30° from snout tip to orbit, vs. >45°), no change in slope of head from anterior margin of orbit to tip of parieto-supraoccipital (vs. angle becoming much shallower beyond orbits), head appearing narrower and longer when placed side-by-side with similar size specimens, abdomen without platelets between pectoral and pelvic girdles (vs. platelets present), pectoral-fin spine reaching two or more plates beyond pelvic-fin base when adpressed ventral to pelvic fin (vs. less than one plate).
Description.
Morphometrics in Table 3. Counts and measurements based on 30 specimens. Small to medium-sized loricariids, largest specimen examined 78.0 mm SL. Body moderately elongate. Head and nape forming arc from tip of snout to insertion of dorsal fin. Dorsal slope decreasing in straight line to insertion of dorsal procurrent caudal rays then ascending to caudal fin. Body depth greatest below insertion of dorsal fin. Ventral profile flat to caudal fin. Caudal peduncle triangular in cross section with dorsal surface flattened. Body widest at insertion of pectoral fins, narrowest at insertion of caudal fin. Snout rounded.
Eye moderately sized, dorsal rim of orbit forming tall crest that continues forward to area just anterior of nares as low, rounded ridge. Iris operculum present. Interorbital space flat anteriorly, but with slight, rounded, median hump posteriorly that is contiguous with ridge of parieto-supraoccipital. Parieto-supraoccipital pointed posteriorly with the posterior point raised above nuchal region in small crest. Infraorbitals, frontal, nasal, compound pterotic, and parieto-supraoccipital supporting odontodes. Preopercle not supporting odontodes. Opercle generally covered by plates and not supporting odontodes although one to four may be present, particularly in smaller individuals.
Lips covered with short, wide papillae. Lower lip wide, upper lip narrow. Edge of lower lip with small crenulae. Maxillary barbel only barbel present, reaching about one third of distance to gill opening.
Median plates 24–26 (mode 25). Plates unkeeled, but first four or five plates of mid-ventral series bent to form slight ridge. Five caudal peduncle plate rows. Plates on all dorsolateral surfaces of body except for extreme edge of snout that only has a narrow column of platelets on either side of the snout tip. Throat mostly naked with platelets confined to lateral margins. Pectoral girdle covered in platelets on ventral surface. Breast naked except for one or two platelets laterally between pectoral and pelvic fin insertions. Abdomen covered in platelets behind last pelvic-fin ray insertion except for lateral margins and small region around anus. Evertible cheek plates supporting hypertrophied odontodes that can be everted perpendicular to head. Cheek odontodes 17–40 (mode 33). Longest evertible cheek odontode reaching to about level of posterior edge of pectoral-fin spine. Hypertrophied cheek odontodes relatively weak. Odontodes slightly longer than average body odontodes present along dorsal-, adipose-, pelvic-, caudal-, and pectoral-fin spines; larger individuals with hypertrophied odontodes at tip of pectoral spine.
Dorsal fin ii,7; dorsal spinelet V-shaped, dorsal-fin locking mechanism present, last ray of dorsal fin not reaching preadipose plate when adpressed. Adipose fin with single preadipose plate and moderately long spine. Caudal fin i,14,i; caudal fin forked, ventral lobe longer than dorsal lobe; dorsal and ventral procurrent caudal rays five. Pectoral fin i,6; pectoral-fin spine reaching just posterior to pelvic fin when adpressed ventral to pelvic fin. Pelvic fin i,5; pelvic-fin spine extending to posterior end of base of anal fin when adpressed. Anal fin i,4; anal-fin spine slightly shorter than first ray.
Teeth bicuspid with lateral lobe one-half to three-quarters length of medial lobe and distal tip of lateral cusp one-half width of tip of medial cusp. 16–39 left dentary teeth (mode 28). 20–38 left premaxillary teeth (mode 27).
Color.
Base color red brown. Head and nape almost completely dark brown. Pectoral-fin spine dark brown with faint, large, oblong spots on dorsal surface forming faint bands across pectoral-fin rays. Pelvic fin as pectoral but lighter. Dorsal fin with oblong spots along spine forming bands across dorsal-fin rays. Caudal fin with three to four bands that may be regular (contiguous along height of fin) or irregular (ventral and dorsal parts offset); lighter interspaces tan, usually equal in diameter to dark. Body with three saddles, first below middle of dorsal fin, second with anterior half below posterior end of dorsal fin and posterior half behind dorsal fin, and third beginning at preadipose plate to about middle adipose-fin membrane; saddles connected at median plate series; saddles appear to be formed of two bars each that fuse as specimens get older, and connection between bands form because the ventral sides appear to get darker with age. Ventral surface uniformly light except for the present of blotches from anterior insertion of anal fin to caudal fin, which may or may not be extensions of the saddles onto the ventral surface.
Sexual dimorphism.
None observed.
Distribution.
Known only from the type locality in the rio Gurupi drainage of Brazil (Fig. 3).
Remarks.
Armbruster (2008) reported Peckoltia vittata from the rio Gurupi drainage; however, this was based on the collection identified here as Peckoltia greedoi. Characters to separate Peckoltia greedoi from Peckoltia vittata are detailed in the diagnosis.
Etymology.
Named for Greedo of Rodia, a bounty hunter killed by Han Solo in Chalmun’s Spaceport Cantina in the movie “Star Wars: Episode IV – A New Hope” (Lucasfilm, Twentieth Century Fox, 1977) with whom this species shares a remarkable resemblance.
Peckoltia lujani
Armbruster, Werneke & Tan sp. n.
http://zoobank.org/2AAE8CC5-5B6A-4A50-A972-777D4FB74EAF
Figure 5.
Dorsal, lateral, and ventral views of holotype of Peckoltia lujani sp. n., AUM 53523, 75.1 mm SL. Photos by J. W. Armbruster.
Table 4.
Selected morphometrics of Peckoltia lujani. Numbers in parentheses refer to landmark numbers in Armbruster (2003).
| Holotype | N | Mean | SD | Max | Min | |
|---|---|---|---|---|---|---|
| SL, mm (1–20) | 75.1 | 34 | 31.6 | 75.1 | ||
| %SL | ||||||
| Predorsal Length (1–10) | 41.2 | 34 | 42.3 | 1.2 | 39.4 | 44.3 |
| Head Length (1–7) | 33.5 | 34 | 35.6 | 1.5 | 32.9 | 41.2 |
| Head–dorsal Length (7–10) | 9.3 | 34 | 7.1 | 1.0 | 5.3 | 9.5 |
| Cleithral Width (8–9) | 25.5 | 34 | 27.8 | 1.4 | 23.1 | 29.6 |
| Head-pectoral Length (1–12) | 27.9 | 34 | 26.7 | 1.5 | 22.9 | 29.7 |
| Thorax Length (12–13) | 21.5 | 34 | 23.7 | 1.7 | 21.3 | 30.7 |
| Pectoral-spine Length (12–29) | 27.7 | 34 | 28.7 | 1.0 | 26.6 | 30.9 |
| Abdominal Length (13–14) | 22.7 | 34 | 22.1 | 0.9 | 19.5 | 23.7 |
| Pelvic-spine Length (13–30) | 25.4 | 34 | 25.0 | 1.4 | 22.5 | 28.6 |
| Postanal Length (14–15) | 35.9 | 34 | 33.6 | 1.5 | 29.4 | 35.9 |
| Anal-fin spine Length (14–31) | 14.3 | 34 | 12.9 | 1.0 | 10.1 | 15.3 |
| Dorsal–pectoral Distance (10–12) | 25.0 | 31 | 26.7 | 1.2 | 24.3 | 29.6 |
| Dorsal spine Length (10–11) | 26.8 | 32 | 26.4 | 2.7 | 17.6 | 31.4 |
| Dorsal-pelvic Distance (10–13) | 19.5 | 34 | 21.0 | 1.7 | 15.5 | 23.6 |
| Dorsal-fin base Length (10–16) | 28.2 | 34 | 26.7 | 1.4 | 23.2 | 29.9 |
| Dorsal-adipose Distance (16–17) | 14.4 | 34 | 14.3 | 2.0 | 9.7 | 18.6 |
| Adipose-spine Length (17–18) | 7.9 | 34 | 9.3 | 1.6 | 6.6 | 12.7 |
| Adipose-upper caudal Distance (17–19) | 15.9 | 34 | 16.9 | 2.3 | 12.3 | 21.2 |
| Caudal-peduncle Depth (15–19) | 9.2 | 34 | 9.8 | 1.0 | 8.1 | 12.1 |
| Adipose-lower caudal Distance (15–17) | 21.5 | 34 | 22.1 | 1.9 | 18.9 | 26.9 |
| Adipose-anal Distance (14–17) | 18.0 | 34 | 17.9 | 1.8 | 14.7 | 22.4 |
| Dorsal-anal Distance (14–16) | 12.6 | 34 | 13.4 | 0.9 | 11.4 | 15.0 |
| Pelvic-dorsal Distance (13–16) | 24.0 | 34 | 24.5 | 1.7 | 21.3 | 27.8 |
| % Head Length | ||||||
| Head-eye Length (5–7) | 35.6 | 34 | 37.4 | 2.9 | 29.0 | 45.8 |
| Orbit Diameter (4–5) | 20.4 | 34 | 20.7 | 2.1 | 16.6 | 24.1 |
| Snout Length (1–4) | 55.2 | 34 | 55.7 | 3.0 | 45.0 | 61.6 |
| Internares Width (2–3) | 15.0 | 33 | 14.8 | 1.9 | 11.4 | 19.5 |
| Interorbital Width (5–6) | 44.8 | 43 | 43.7 | 3.8 | 36.5 | 54.8 |
| Head Depth (7–12) | 63.3 | 43 | 64.8 | 3.4 | 51.5 | 73.2 |
| Mouth Length (1–24) | 53.1 | 44 | 49.6 | 3.4 | 42.4 | 57.1 |
| Mouth Width (21–22) | 55.6 | 44 | 50.1 | 5.1 | 41.6 | 58.8 |
| Barbel Length (22–23) | 15.8 | 42 | 13.8 | 2.3 | 10.2 | 19.7 |
| Dentary Tooth Cup Length (25–26) | 15.5 | 40 | 13.0 | 2.0 | 10.1 | 17.9 |
| Premaxillary Tooth Cup Length (27–28) | 16.5 | 42 | 13.9 | 1.5 | 10.8 | 17.0 |
Type locality.
Venezuela, South America
Holotype.
AUM 53523, 75.1 mm SL, VENEZUELA, Amazonas, río Orinoco at Paso Ganado, 38 km NNW of San Fernando de Atabapo, 04.3842°, -067.7747°, 27 Mar 2010, N.K. Lujan, D.C. Werneke, M.H. Sabaj, T. Carvalho, V. Meza, and O. León.
Paratypes.
ANSP 162174, 13, 46.0–74.3, VENEZUELA, Amazonas, río Orinoco at El Burro, 06.2°, -067.4333°, 26 Nov 1985, B. Chernoff et al.; AUM 43008, 4 nm, 19.8–32.4, VENEZUELA, Amazonas, río Orinoco dr., río Orinoco, at Puerto Venado 2 km NW of Samariapo and 56.4 km SSW of Puerto Ayacucho, 05.2106°, -067.8049°, 26 Feb 2005, N.K. Lujan, D.C. Werneke, M.H. Sabaj, M. Arce, R. Betancur, and T.E. Wesley; AUM 53474, 1, 37.4, VENEZUELA, Amazonas, rio Orinoco at Raudales Atures, 8.3 km SSW of Puerto Ayacucho, 05.5989°, -067.6139°, 23 Mar 2010, N.K. Lujan, D.C. Werneke, M.H. Sabaj, T. Carvalho, V. Meza; AUM 53979, 2, 31.6–34.3, VENEZUELA, Amazonas, rio Orinoco at Merey, 97.6 km N of San Fernando de Atabapo, 04.9178°, -067.8329°, 18 Apr 2010, J. Birindelli, N.K. Lujan, and V. Meza; MCNG 56579, 1,62.9, MCP 48401, 1, 57.8, same data as holotype; ROM 93352, 12, 38.0–64.5, VENEZUELA, Amazonas, rio Orinoco across channel from Puerto Venado (near Samariapo), 56.7 km south-southwest of Puerto Ayacucho, 05.2095°, -067.8095°, 24 Mar 2010, N.K. Lujan, M.H. Sabaj, D.C. Werneke, V. Meza, and T. Carvalho.
Other material.
ANSP 166770, 1, 61.3, VENEZUELA, Bolivar, río Orinoco dr., rio Aro, Salto El Pajaro, 18 Oct 1987, M. Rodriguez; ICNMHN 1480, 13, 35.3–76.3 (4 nm), COLOMBIA, Meta, río Meta - río Orinoco dr., río Negro on the Villavicencio - Puerto Lopez road, (4.1025°, -072.9368°), 11 Jan 1988, H. Silvergrip; ICNMH 9096, 1, 79.5, COLOMBIA, Arauca, río Meta - río Orinoco dr., Caño Ormedillo, Arauca-Caño Norte road, (06.8514°, -070.6486°) 27 Feb 1977, P. Cala; MCNG 19318, 2 nm., VENEZUELA, Bolivar, río Orinoco to the east of Ciudad Bolivar in the population of El Rosario, 08.3167°, -063.0833°, 24 Sep 1987, G. Feo, R. Morales, and H. Barbarino.
Diagnosis.
Peckoltia lujani can be separated from Peckoltia pankimpuju by having well developed color and eyes; from all Peckoltia except Peckoltia braueri, Peckoltia capitulata, Peckoltia compta, Peckoltia greedoi, Peckoltia oligospila, Peckoltia otali, and Peckoltia stimulata by having the abdomen largely naked posterior to the pectoral girdle (vs. only small naked patches at insertions of pelvic fins); from all Peckoltia except Peckoltia furcata, Peckoltia greedoi, Peckoltia lujani, Peckoltia pankimpuju, and Peckoltia sabaji by having the dentaries meet at an angle greater than 90°; from Peckoltia ephippiata and Peckoltia greedoi by having large spots or blotches on the posterolateral surface of head and nape (vs. very small, very faint spots); from Peckoltia ephippiata and by lacking slight keels on the lateral plates, particularly the median series (vs. slight keels present), by having bands in the dorsal fin (vs. dorsal fin with light rays and dark membranes), by having fewer teeth (Peckoltia ephippiata: 39–72 dentary, 41–73 premaxillary; Peckoltia lujani: 20–37 dentary, 23–45 premaxillary); from Peckoltia greedoi by having the pectoral-fin spine relaxed position only slightly dorsally, pointing maximally to dorsal insertion of caudal fin (vs. angled dorsally, pointing at insertion of dorsal fin) and pectoral-fin spine reaching less than one plate of the ventral series beyond the pelvic base when adpressed ventral to pelvic fin (vs. two or more).
Peckoltia lujani differs from Etsaputu by having greater than six evertible cheek odontodes, the largest of which extends posterior to the eye (vs. six or fewer, the largest not extending beyond the exposed portion of the opercle). Peckoltia lujani can be separated from Hemiancistrus (except ‘Hemiancistrus’ landoni) and Ancistomus by having prominent dorsal saddles (vs. dark or light spots or entirely dark); and from all Hemiancistrus and Ancistomus by having bands in the caudal fin and no free spots (vs. bands absent or present with some free spots) and bands in the dorsal fin (vs. spots or no markings). Peckoltia ephippiata can be separated from Peckoltichthys bachi by having no spots on the head (vs. large dark spots or mottling); by having the eyes high on the head with the dorsal rim of the orbit higher than the interorbital space (vs. low on the head, dorsal rim of orbit lower than interorbital space), and by having small plates on the abdomen (vs. relatively large).
Peckoltia caenosa is known from the same region as Peckoltia lujani and can be difficult to tell apart when juveniles. Peckoltia lujani differs from adult Peckoltia caenosa by lacking vermiculations on the abdomen and head, and from all Peckoltia caenosa by having the dentaries meet in a broad arc that is greater than 120° (vs. meeting at an angle less than 90°), and by having fewer teeth (all except one specimen with 24–37 dentary teeth and 22–45 premaxillary teeth [16 and 19 respectively in aberrant specimen] vs. 10–18 dentary teeth and 11–21 premaxillary teeth).
Description.
Morphometrics in Table 4, counts and measurements based on 25 specimens unless noted. Largest specimen examined 75.1 mm SL. Body moderately elongate. Head and nape forming arc from tip of snout to anterior of parieto-supraoccipital, rising more rapidly to parieto-supraoccipital crest, and then more slowly to dorsal-fin. Dorsal slope decreasing in straight line to insertion of dorsal procurrent caudal rays then ascending to caudal fin. Body depth greatest below insertion of dorsal fin. Ventral profile flat to ventral procurrent caudal-fin rays, and then sloped ventrally. Caudal peduncle oval in cross section with dorsal and ventral surfaces flattened. Body widest at insertion of pectoral fins, narrowest at insertion of caudal fin. Snout rounded.
Eye moderately sized, dorsal rim of orbit forming moderate crest that continues forward of orbit to area just anterior of nares as a low, rounded ridge. Iris operculum present. Interorbital space flat. Parieto-supraoccipital pointed posteriorly with a moderate crest formed along near entire length of parieto-supraoccipital. Infraorbitals, frontal, nasal, compound pterotic, and parieto-supraoccipital supporting odontodes. Preopercle not supporting odontodes. Opercle generally covered by plates and not supporting odontodes although one to two may be present, particularly in smaller individuals.
Lips covered with short, wide papillae. Lower lip wide, upper lip narrow. Edge of lower lip with small crenulae. Maxillary barbel only barbel present, reaching about one third of distance to gill opening.
Median plates 25–27 (mode 26). Plates unkeeled, but first four or five plates of mid-ventral series very slightly bent to form slight ridge. Five caudal peduncle plate rows. Plates on all dorsolateral surfaces of body except for oval naked area at snout tip. Throat with a few plates posterior to lip. Abdomen only with few sparse platelets below pectoral girdle and in narrow column posterolaterally to pectoral girdle in type series (some platelets below pelvic girdle in Meta specimens). Evertible cheek plates supporting hypertrophied odontodes that can be everted perpendicular to head. Cheek odontodes 5–49 (mode 25). Longest evertible cheek odontode reaching to about level of posterior edge of pectoral-fin spine. Hypertrophied cheek odontodes relatively weak. Odontodes slightly longer than average body odontodes present along dorsal-, adipose-, pelvic-, caudal-, and pectoral-fin spines; larger individuals with hypertrophied odontodes at tip of pectoral spine.
Dorsal fin ii,7; dorsal spinelet V-shaped, dorsal-fin locking mechanism present, last ray of dorsal fin not reaching preadipose plate when adpressed. Adipose fin with single preadipose plate and moderately long spine. Caudal fin i,14,i; caudal fin forked, ventral lobe longer than dorsal lobe; dorsal procurrent caudal rays five, and ventral procurrent caudal rays four to five (mode five; n=24 for dorsals). Pectoral fin i,6; pectoral-fin spine reaching just posterior to pelvic fin when adpressed ventral to pelvic fin. Pelvic fin i,5; pelvic-fin spine extending to posterior end of base of anal fin when adpressed. Anal fin i,4; anal-fin spine slightly shorter than first ray.
Teeth bicuspid with lateral lobe three-quarters length of medial lobe and distal tip of lateral cusp one-half width of tip of medial cusp. 20–37 left dentary teeth (mode 32). 23–45 left premaxillary teeth (mode 39).
Color.
Base color light tan with brown to black markings. Four dorsal saddles on the body, the first below the middle rays of the dorsal fin, the second below the posterior rays of the dorsal fin and slightly posterior, the third below the adipose fin and slightly anterior, and the fourth at the end of the caudal peduncle. Third and fourth saddles may have anterior extensions or have an anterior projection making them h-shaped or may be split nearly in half. Saddles two to four extend to median plate row, saddle one continues to insertion of pectoral fin. Very large blotches or spots present from saddle one to caudal fin. All fins except dorsal always with dark bands with dark areas from about as wide to about twice as wide as light areas. Number of bands increases with size. Dorsal fin coloration complex, ranging from a mix of dark and light spots that may or may not combine to form bands (bands always forming distally). The dark or light spots/bands in dorsal fin may or may not combine with those dorsal or ventral. Dark spots/bands typically darker on the rays distally and on the membranes proximally. Dark spot present between dorsal-fin spinelet and spine. Abdomen with medium spots. Lower surface of caudal peduncle with dark blotches. Juveniles colored as adults, but with bar two extending to insertion of pelvic fin, without anterior extensions of the third and fourth dorsal bars, third and fourth bars extending across ventral margin of caudal peduncle, without spots on the abdomen, and with the spots or blotches on the sides (if present) just between first and second bars.
Sexual dimorphism.
None observed.
Distribution.
Known from the Meta Drainage near Villavicencio and the Orinoco from the mouth of the Meta to around Ciudad Bolivar (Fig. 3).
Remarks.
The type locality was restricted to collection localities in Amazonas, Venezuela. There is variation within the species, and it is possible that other forms may be present within the species. The Colombian specimens (ICNMHN 1480 and ICNMH 9096) differed in shape from the other members of the species, and we do not have the specimens to check their identity, so they were excluded from counts and measurements.
Etymology.
Named in honor of the former graduate student of JWA, Dr. Nathan Lujan. Dr. Lujan has led expeditions to some of the most remote regions of South America and obtained some of the most important specimens for the study of loricariid systematics specifically as well as South American fish systematics and ecology in general. In the process, he has given JWA more taxonomic work in the last decade than he had thought possible, and he is very thankful. Dr. Lujan also collected the best specimens known of the species.
Discussion
We offer a taxonomy for Hemiancistrus, Peckoltia, and allied genera based mostly on the molecular phylogeny and conclusions of Lujan et al. (2015) (Table 1). We do not currently have characteristics to diagnose the various genera or species groups that arise from this phylogeny, but the molecular phylogeny offers the best case right now for handling these confusing species. The molecular phylogeny suggests that the only species that should be left in Hemiancistrus is the type species. Hemiancistrus medians is larger than most species that were left in Hemiancistrus in Armbruster (2008), has well-developed keels (only present elsewhere in ‘Hemiancistrus’ landoni), and a different body shape. The remainder of the taxa that do not have established genera that they can be placed in will be recognized as species groups in ‘Hemiancistrus’ in single quotes until they can be examined further. We recognize three species groups: ‘Hemiancistrus’ chlorostictus group, ‘Hemiancistrus’ guahiborum group, and ‘Hemiancistrus’ landoni group.
Ancistomus is recognized as valid with Peckoltia feldbergae, Hemiancistrus micrommatos, Hemiancistrus spinosissimus, and Hemiancistrus spilomma along with the type, Ancistrus snethlageae. Ancistomus micrommatos, Ancistomus spinosissimus, and Ancistomus spilomma were not examined by Lujan et al. (2015); however, they are very similar in appearance to Ancistrus snethlageae, and Ancistomus spilomma was sister to Ancistrus snethlageae in Armbruster (2008). We cannot find any characteristics to separate Ancistomus micrommatos, Ancistomus spinosissimus, and Ancistomus spilomma, and they may represent the same species.
The Hemiancistrus annectens group of Armbruster (1998) shares with Pterygoplichthys a connective tissue sheet that connects laterally to the abdominal wall and evertible cheek plates with hypertrophied odontodes. Armbruster (1998, 2004a) suggested that a new genus needed to be described for them, but the species consistently come out as sister to Hypostomus in analyses of molecular data (Evans 2002; Lujan et al. 2015), and we recognize the species in Hypostomus. Further study will be needed to determine if the group requires its own, separate genus.
The Hemiancistrus from southern Brazil and Uruguay are in a polytomy with the Hemiancistrus annectens group and the rest of Hypostomus in Lujan et al. (2015); however, the group is very different morphologically with all of the characters used to unite the Ancistrini of Armbruster (2004a, 2008). We recognize the group as the ‘Hemiancistrus’ chlorostictus group until its relationships can be examined further. We tentatively consider ‘Hemiancistrus’ cerrado as a member of the ‘Hemiancistrus’ chlorostictus group due to overall similarity in form between it and the southern species.
‘Hemiancistrus’ guahiborum and ‘Hemiancistrus’ subviridis are part of the same clade (though not sister species), are morphometrically very similar (pers. obs.), and we recognize them as the ‘Hemiancistrus’ guahiborum group (Lujan et al. 2015). The species differ greatly in color (mottled brown vs. green with small yellow spots respectively), and represent another example of upper Orinoco loricariids differing strongly in color pattern, but not shape. Pseudolithoxus and Hypancistrus from the Orinoco have a similar wide variation in color between species (Armbruster and Provenzano 2000; Armbruster et al. 2007; Lujan and Birindelli 2011). ‘Hemiancistrus’ guahiborum and ‘Hemiancistrus’ subviridis are in a clade with Baryancistrus beggini and Baryancistrus demantoides. The two Orinoco Baryancistrus are not closely related to true Baryancistrus suggesting that a new genus may need to be described for these four Orinoco species.
We recognize a monotypic ‘Hemiancistrus’ landoni group for the trans-Andean species. The type of ‘Hemiancistrus’ hammarlundi is a juvenile ‘Hemiancistrus’ landoni, and we place it into the synonymy of ‘Hemiancistrus’ landoni.
The three new species of Peckoltia differ from Peckoltia sensu stricto by having straighter jaws (dentaries meeting at an angle greater than 90°). Curiously, the sister to Peckoltia lujani in Lujan et al. (2015) is the Orinoco population of Peckoltia vittata (likely another undescribed species). The Orinoco Peckoltia vittata has the dentaries meet at an angle less than 90° and a deep, short body whereas Peckoltia lujani is much flatter and more elongate with straighter jaws.
Peckoltia sabaji does belong in Peckoltia and is sister to the morphologically similar Peckoltia furcata. Etsaputu relictum was in a clade with Peckoltia furcata + Peckoltia sabaji and another undescribed species of Peckoltia from the Madeira (Lujan et al. 2015), and was morphometrically similar to Peckoltia ephippiata. Etsaputu is unusual in that it has very few odontodes on the cheek (six or fewer), and they are not very evertible (Lujan et al. 2011). It was found to be at the base of the Ancistrini in the morphological phylogeny (Armbruster 2008). We feel that more information is needed to determine if Etsaputu should be recognized as a synonym of Peckoltia.
Chaetostomus macrops
Chaetostomus macrops Lütken is usually listed as a species of Hemiancistrus (Isbrücker 1980, 2001, Fisch-Muller 2003, Ferraris 2007); however, this appears to be incorrect. Photographs from P.R. Møller are excellent (Fig. 6), and indicate that the type is not a Hemiancistrus. The type of Chaetostomus macrops has odontodes on the opercle over a broad area, a condition that is not seen in Hemiancistrus, but which is common to most other ancistrins. In Hemiancistrus (as well as Baryancistrus, Hypancistrus, Panaque, Parancistrus, and Peckoltia) the opercle has its lateral face restricted to a narrow ridge, so if odontodes are present, they are present normally in just a narrow row just one odontode wide. It is also clear on the image that the sphenotic does not have an exterior contact with the last infraorbital; although this feature exhibits some homoplasy, it is a synapomorphy of Pseudancistrus (Armbruster 2004b). Also common to Pseudancistrus, but not Hemiancistrus, Peckoltia, or allied genera, is the presence of four to five plates prior to the dorsal fin (vs. three) and a completely naked abdomen (vs. almost always some plates in Hemiancistrus).
Figure 6.

Lateral view of holotype of Chaetostomus macrops, ZMUC P30142, 92.7 mm SL. Photos by P. Møller.
Cardoso and Lucinda (2003) suggested that Chaetostomus macrops is a synonym of Chaetostomus megacephalus Günther, which Armbruster (2004a, b) found to be a member of Pseudancistrus based on the presence of many synapomorphies (including the lack of exterior contact between the sphenotic and last infraorbital). The type of Chaetostomus macrops shares with Pseudancistrus megacephalus very large eyes, a wide body, and an unusual color pattern consisting of large white spots on the sides and dark bands on the caudal fin. This color pattern is unusual in loricariids because species with dark bands in the tail usually have dark spots or saddles on the body and those with white spots on the body generally either have spots also on the caudal fin or have the caudal fin entirely dark (Pseudancistrus brevispinnis can have a similar color pattern, but it has smaller spots, a narrower body, and smaller eyes). The types of Chaetostomus macrops and Chaetostomus megacephalus are both likely from Suriname, and the species are synonymous. Pseudancistrus sensu Armbruster (2004a,b, 2008) was found to be a polyphyletic taxon in Lujan et al. (2015), and we recognize ‘Pseudancistrus’ megacaphalus with its genus in single quotes to indicate it is a taxon that needs further work.
Hypostomus itacua
Hypostomus itacua Valenciennes (supposedly from the La Plata system) is occasionally listed in Chaetostomus or Hemiancistrus (for example Günther 1864, Isbrücker 1980, Cardoso and Lucinda 2003), but the type appears to be lost. Kner (1854) compared Ancistrus medians with Hypostomus itacua and stated that both are between his two groups of Ancistrus; however, the specimen he referred to as Ancistrus itacua is now the type of Peckoltia braueri. Weber (2003) indicates that the description in Cuvier and Valenciennes (1840) does not agree with the illustration in Valenciennes (1836), and the 1840 description was used by Günther (1864) to place the species in Chaetostomus and Isbrücker (1980) to place it in Hemiancistrus. We believe the figure in Valenciennes (1836) is of a Hypostomus; however, the species should remain incertae sedis in the Loricariidae as in Weber (2003) and Ferraris (2007).
Supplementary Material
Acknowledgements
This project was supported by NSF grants DEB-0107751, DEB-0315963, and DEB-1023403. We are indebted to numerous people for help when visiting museums and for help in collecting specimens including: Mark Sabaj, John Lundberg, Marry Anne Rogers, Barry Chernoff, Phillip Willink, Mark Westneat, Richard Vari, Susan Jewett, Jeffrey Williams, Karsten Hartel, Lawrence Page, Robert Robins, Nathan Lujan, Lesley de Souza, Paul Pera, Justin Evans, Michael Hardman, Jackie Arjoon, Christopher Chin, Calvin Bernhard, Graham Watkins, Donald Taphorn, Roberto Reis, Luiz Malabarba, Pablo Lehman, Paulo Buckup, Michael Retzer, Patrick Ceas, Christopher Laird, Kevin Cummings, Christine Mayer, Oscar León, Jeffrey Thomas, Brooks Burr, Jeffrey Stewart, Matt Thomas, Mario de Pinna, Oswaldo Oyakawa, John Friel, Hernan Ortega, and Erling Holm. Thanks to Ronald Fricke and Sonia Fisch-Muller for discussion on the potential type of Ancistrus medians, Mark Allen for taking pictures of the type of Chaetostomus macrops, and Mark Sabaj for making the pictures available via the All Catfish Species Inventory website. Thanks to Nathan Lujan for the measurements of Peckoltia pankimpuju. Thanks to Christopher Hamilton for recognizing the similarity between plecos and Greedo. This paper is contribution No. 710 of the Auburn University Musuem of Natural History.
Citation
Armbruster JW, Werneke DC, Tan M (2015) Three new species of saddled loricariid catfishes, and a review of Hemiancistrus, Peckoltia, and allied genera (Siluriformes). ZooKeys 480: 97–123. doi: 10.3897/zookeys.480.6540
Supplementary materials
Morphometrics and Meristics for species of Peckoltia and Etsaputu.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Jonathan W. Armbruster, David C. Werneke, Milton Tan
Data type: Mesurements and meristics.
Explanation note: Morphometirc and meristic data based on Armbruster (2003) for all specimens of Peckoltia and Etsaputu examined.
Locality information for all collections of the three species described in this paper.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Jonathan W. Armbruster, David C. Werneke, Milton Tan
Data type: Occurences.
Explanation note: Database containing catalog numbers, numbers of specimens, and localities for the three new species of Pecoltia descrived in this paper.
Morphological characters for Hemiancistrus medians
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Jonathan W. Armbruster, David C. Werneke, Milton Tan
Data type: character state data.
Explanation note: Character state data based on Armbruster (2004, 2008) for Hemiancistrus medians. These data were accidentally removed from Armbruster 2008.
References
- Armbruster JW. (1998) Modifications of the digestive tract for holding air in loricariid and scoloplacid catfishes. Copeia 1998: 663–675. doi: 10.2307/1447796 [Google Scholar]
- Armbruster JW. (2003) Peckoltia sabaji, a new species from the Guyana Shield (Siluriformes: Loricariidae). Zootaxa 344: 1–12 http://www.mapress.com/zootaxa/2003f/zt00344.pdf [Google Scholar]
- Armbruster JW. (2004a) Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. Zoological Journal of the Linnean Society 141: 1–80. doi: 10.1111/j.1096-3642.2004.00109.x [Google Scholar]
- Armbruster JW. (2004b) Pseudancistrus sidereus, a new species from southern Venezuela (Siluriformes: Loricariidae) with a redescription of Pseudancistrus. Zootaxa 628: 1–15 http://www.mapress.com/zootaxa/2004f/zt00628.pdf [Google Scholar]
- Armbruster JW. (2005) The loricariid catfish genus Lasiancistrus with description of two new species. Neotropical Ichthyology 3: 549–569. doi: 10.1590/S1679-62252005000400013, http://www.scielo.br/scielo.php?pid=s1679-62252005000400013&script=sci_arttext [Google Scholar]
- Armbruster JW. (2008) The Genus Peckoltia with the Description of Two New Species and a Reanalysis of the Phylogeny of the Genera of the Hypostominae (Siluriformes: Loricariidae). Zootaxa 1822: 1–76 http://www.mapress.com/zootaxa/2008/f/zt01822p076.pdf [Google Scholar]
- Armbruster JW, Lujan NK, Taphorn DC. (2007) Four new species of Hypancistrus from southern Venezuela (Siluriformes: Loricariidae). Copeia 2007: 62–79. doi: 10.1643/0045-8511(2007)7[62:FNHSLF]2.0.CO;2 [Google Scholar]
- Armbruster JW, Provenzano F. (2000) Four new species of the suckermouth armored catfish genus Lasiancistrus (Loricariidae: Ancistrinae). Ichthyological Exploration of Freshwaters, 11: 241–254. [Google Scholar]
- Cardoso AR, Lucinda PHF. (2003) Three new species of Hemiancistrus (Teleostei: Siluriformes: Loricariidae) from the rio Tocantins basin with comments on the genus. Ichthyological Exploration of Freshwaters 14: 73–84. [Google Scholar]
- Cuvier G, Valenciennes A. (1840) Histoire naturelle des poissons. Tome quatorzième. Suite du livre seizième. Labroïdes. Livre dix-septième. Des Malacoptérygiens. Ch. Pitois & Ve Levrault, Paris, Strasbourg, xxii + 2 + 464 + 4 pp., pls. 389–420. [Google Scholar]
- Evans JD. (2002) Taxonomic and phylogenetic assessment of the Hemiancistrus annectens group by morphometric and molecular methods (Teleostei: Loricariidae). Unpublished MS thesis, Auburn University, Auburn. [Google Scholar]
- Ferraris CJ Jr. (2007) Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa 1418: 1–628 http://www.mapress.com/zootaxa/2007f/zt01418p300.pdf, http://www.mapress.com/zootaxa/2007f/zt01418p628.pdf [Google Scholar]
- Fisch-Muller S. (2003) Ancistrinae. In: Reis RE, Kullander SO, Ferraris CJ Jr. (Eds) Check list of the freshwater fishes of South and Central America. Edipucrs, Porto Alegre, 373–400. [Google Scholar]
- Fisch-Muller S, Montoya-Burgos JI, Le Bail PY, Covain R. (2012) Diversity of the Ancistrini (Siluriformes: Loricariidae) from the Guianas: the Panaque group, a molecular appraisal with descriptions of new species. Cybium 36: 163–193. [Google Scholar]
- Günther A. (1864) Catalogue of the fishes in the British Museum, vol. 5. Catalogue of the Physostomi, containing the families Siluridae, Characinidae, Haplochitonidae, Sternoptychidae, Scopelidae, Stomiatidae in the collection of the British Museum. Trustees, London, xxii + 455 pp. [Google Scholar]
- Isbrücker IJH. (1980) Classification and catalogue of the mailed Loricariidae (Pisces, Siluriformes). Verslagen en Technische Gegevens, Instituut voor Taxonomische Zoölogie, Universiteit van Amsterdam 22: 1–181. [Google Scholar]
- Isbrücker IJH. (2001) Nomenklator der Gattungen und Arten der Harnischwelse, Familie Loricariidae Rafinesque, 1815 (Teleostei, Ostariophysi). In: Stawikowski R. (Ed.) Harnischwelse 2.Die Aquarien- und Terrarien Zeitschrift, Eugen Ulmer, Stuttgart, 373–400. [Google Scholar]
- Kner R. (1854) Die Hypostomiden. Zweite Hauptgruppe der Familie der Panzerfische (Loricata vel Goniodontes). Denkschriften der Mathematisch- Naturwissenschaftlichen Classe der Kaiserlichen Akademie der Wissenschaften in Wien 7: 251–286, pls. 1–5. [Google Scholar]
- Lujan NK, Armbruster JW, Lovejoy N, López-Fernández H. (2015) Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Molecular Phylogenetics and Evolution 62: 269–288. doi: 10.1016/j.ympev.2014.08.020 [DOI] [PubMed] [Google Scholar]
- Lujan NK, Armbruster JW, Rengifo B. (2011) A new basal Ancistrini genus and species from the Andes of Northern Peru (Siluriformes: Loricariidae). Copeia: 497–502. doi: 10.1643/CI-10-201
- Lujan NK, Birindelli JLO. (2011) A new distinctively banded species of Pseudolithoxus (Siluriformes: Loricariidae) from the upper Orinoco River. Zootaxa 2941: 38–46. [Google Scholar]
- de Oliveira RR, Zuanon J, Rapp Py-Daniel L, Rocha MS. (2010) Peckoltia compta, a new species of catfish from Brazilian Amazon, Rio Tapajós basin (Siluriformes: Loricariidae). Zootaxa 2534: 48–56. [Google Scholar]
- de Oliveira RR, Rapp Py-Daniel L, Zuanon J, Rocha MS. (2012) A New Species of the Ornamental Catfish Genus Peckoltia (Siluriformes: Loricariidae) from Rio Xingu Basin, Brazilian Amazon. Copeia: 547–553.
- de Miranda Ribeiro A. (1912) Loricariidae, Callichthyidae, Doradidae e Trichomycteridae. In: Commissão de LinhasTelegraphicas Estrategicas de Matto-Grosso ao Amazonas. Matto-Grosso, Annexo no. 5 (Historia Natural: Zoologia), 1–31. [Google Scholar]
- Sabaj Pérez MH. (Ed.) (2014) Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an Online Reference. Version 5.0 (22 Septemeber 2014). Electronically accessible at http://www.asih.org/, American Society of Ichthyologists and Herpetologists, Washington, D.C. [Google Scholar]
- Schaefer SA. (1987) Osteology of Hypostomus plecostomus (Linnaeus), with a phylogenetic analysis of the loricariid subfamilies (Pisces: Siluriformes). Contributions in Science, Los Angeles County Museum 394: 1–31. [Google Scholar]
- Schaefer SA. (1997) The neotropical cascudinhos: systematics and biogeography of the Otocinclus catfishes (Siluriformes: Loricariidae). Proceedings of the Academy of Natural Sciences, Philadelphia 148: 1–120. [Google Scholar]
- Valenciennes A. (1836) Poissons. In: d’Orbigny A. Voyage dans L=Amérique Méridionale (le Brésil, la République Orientale de l=Uruguay, la République Argentine, la Patagonie, la République du Chili, la République de Bolivia, la République du Pérou), exécuté pendant les années 1826, 1827, 1828, 1829, 1830, 1832 et 1833. Bertrand et Levrault, Paris, Pls. 4–7. [Google Scholar]
- Weber C. (2003) Hypostominae. In: Reis RE, Kullander SO, Ferraris CJ Jr. (Eds) Check list of the freshwater fishes of South and Central America. Edipucrs, Porto Alegre, 351–372. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Morphometrics and Meristics for species of Peckoltia and Etsaputu.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Jonathan W. Armbruster, David C. Werneke, Milton Tan
Data type: Mesurements and meristics.
Explanation note: Morphometirc and meristic data based on Armbruster (2003) for all specimens of Peckoltia and Etsaputu examined.
Locality information for all collections of the three species described in this paper.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Jonathan W. Armbruster, David C. Werneke, Milton Tan
Data type: Occurences.
Explanation note: Database containing catalog numbers, numbers of specimens, and localities for the three new species of Pecoltia descrived in this paper.
Morphological characters for Hemiancistrus medians
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Jonathan W. Armbruster, David C. Werneke, Milton Tan
Data type: character state data.
Explanation note: Character state data based on Armbruster (2004, 2008) for Hemiancistrus medians. These data were accidentally removed from Armbruster 2008.