Summary
Whether driving a car, shopping for food, or paying attention in a classroom of boisterous teenagers, it’s often hard to maintain focus on goals in the face of distraction. Brain imaging studies in humans implicate the dorsal anterior cingulate cortex (dACC) in regulating the conflict between goals and distractors. Here we show for the first time that single dACC neurons signal conflict between task goals and distractors in the rhesus macaque, particularly for biologically-relevant social stimuli. For some neurons, task conflict signals predicted subsequent changes in pupil size—a peripheral index of arousal linked to noradrenergic tone—associated with reduced distractor interference. dACC neurons also responded to errors and these signals predicted adjustments in pupil size. These findings provide the first neurophysiological endorsement of the hypothesis that dACC regulates conflict, in part, via modulation of pupil-linked processes such as arousal.
Humans and other animals preferentially process information that has predicted biologically relevant events, either in personal or evolutionary history. For example, both sudden onset stimuli [1] and social stimuli like faces [2, 3] supersede goal-relevant targets for gaze in primates. Thus, pursuing important goals like foraging in complex, dynamic environments may require regulation of conflicting demands on attention and action. Understanding how this conflict between prepotent processing of salient distractors and goal pursuit is regulated may help develop new treatments for disorders, such as attention deficit hyperactivity disorder or schizophrenia, in which these regulatory mechanisms are disrupted, as well as devise new strategies for improving performance in school or attention-demanding jobs like air-traffic control.
The dorsal anterior cingulate cortex (dACC) appears to contribute to managing conflict and regulating focus in humans. Functional and anatomical differences in dACC accompany disorders of distractibility [4, 5] and dACC activity is correlated with trial-by-trial variation in distractor interference on task performance [6]. In humans, dACC responds to conflict between a prepotent task response and alternative responses [7-13], and conflict signals evolve over multiple trials, with dACC BOLD activity on one trial predicting decreased interference of conflicting information on later trials [10, 11]. In humans, conflict signals are apparent in the firing rates of single dACC neurons [11], but surprisingly there is no evidence for conflict signaling by dACC neurons in monkeys [14-19]. This disconnect may reflect methodological differences in studies in monkeys and humans. Conflict paradigms used in humans typically evoke conflict at both the level of the task set (“task conflict”) and the physical action (“action conflict”), while studies in monkeys focus on action conflict [16, 17, 19]. Alternatively, conflict signaling may be a unique feature of human dACC [14].
It also remains unclear how conflict signals in dACC translate into subsequent adjustments in behavioral regulation. One hint is that conflict is not the only task condition that elicits dACC activation. Error signals are commonly reported in dACC in both humans [9, 20, 21] and monkeys [16], linking dACC to performance monitoring [22-25]. Moreover, dACC is required for behavioral adjustment following changes in task rules in macaques [26, 27] and errors in humans [28], suggesting this area may integrate multiple sources of information about task conditions and performance to regulate behavior [22].
One pathway by which dACC could shape behavioral control is via subcortical projections to regions implicated in arousal, a state of physiological activation, characterized by pupil dilation and increased heart rate, blood pressure, and perspiration [29]. Arousal is associated with increased reactivity to goal-irrelevant stimuli [30, 31], and thus poorer performance in many tasks. dACC targets implicated in arousal include amygdala [32], hypothalamus [33, 34], and locus coeruleus (LC) [35], a major source of cortical norepinerphrine (NE). The LC broadcasts NE signals that shape learning rate [36, 37] and distractibility [38, 39]. Pupil size under constant luminance, in parallel, also predicts learning rate [40, 41] and distractibility [30]. Pupil size is commonly used as an index of NE signaling [40-43] and NE tone is positively correlated with pupil size under constant luminance [35, 42]. Pupil size thus provide a potentially useful measure to test the hypothesis that dACC adjusts cognitive control, in part, by regulating processes like autonomic arousal and/or NE tone.
We tested these ideas in an animal model in which the precise temporal dynamics of dACC neuronal activity can be linked to behavioral performance and pupil dynamics. To do this, we recorded from single neurons in dACC and tracked pupil size in monkeys making goal-directed saccades for juice rewards while periodically confronting them with biologically salient distractors. We previously showed that large pupil size at fixation predicts increased distractor interference in this task [30], suggesting a modulatory role for pupil-linked processes in conflict regulation. We used faces as distractors because they supersede other stimuli for attention in primates [2, 3], require no training to acquire salience, and continue to intrude on task performance over tens of thousands of trials. Single neuron recordings allowed us to determine the distribution of distractor and pupil size signals within the smallest functional subunits of dACC, which constrains the computations the region could perform. We also examined the relationship between error signals and pupil size signals within single neurons in order to determine how they are linked.
Distractors could be in one of three locations relative to the rewarded target. Specific contrasts across these locations allowed us to differentiate between signals related to different forms of conflict. There is no single accepted operational definition of conflict and definitions have not always been consistent between studies in humans and monkeys. Our task evokes two types of conflict. First, as is typically done in studies in monkeys [16, 17, 19], we examined conflict evoked when opposing saccade plans are simultaneously active, by manipulating the relative physical locations of a rewarded target and an irrelevant distractor. This “action conflict” was operationalized as slowing of saccade initiation when a distractor appeared in a location incongruent with the location of the saccade target. Second, we examined the intrusion of prepotent, task-irrelevant information on goal pursuit, a second form of conflict that may also be induced in Stroop or flanker tasks used in studies of conflict in humans [7-13]. Here we define “task conflict” as any change in task performance induced by distractors, irrespective of their spatial location or saccade congruence.
We found that firing rates of single neurons in dACC differentiated between distractors that impacted task performance and those that did not, demonstrating for the first time that dACC neurons signal conflict in the macaque. Importantly, the primary conflict signal we observed was task conflict. By contrast, action conflict signals were absent in the initial time-locked distractor response and heterogeneously signed across the dACC population, consistent with previous reports in monkeys [16, 17, 19].
We also addressed the functional significance of task conflict signals in dACC for changes in pupil size. We found a decrease in pupil diameter on trials following both distractors and errors, consistent with long-term and potentially homeostatic down-regulation of arousal. Across the dACC population, some neurons responded to distractors and/or errors, some scaled their responses with pupil size, and others signaled information about task events on the current trial and predicted subsequent adjustments in pupil diameter on the next trial. Thus, the dACC population signals information about multiple aspects of task performance, including task conflict, errors, and current pupil size, and predicts subsequent adjustments in pupil size associated with reduced distraction. These findings endorse the hypothesis that dACC contributes to cognitive control, in part, through pupil-linked changes in arousal.
Results
Distractors interfere with task performance
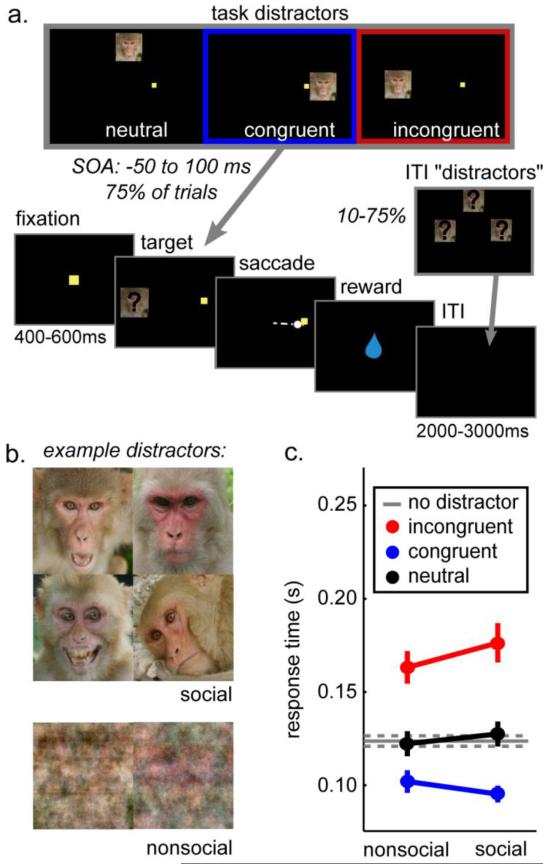
In the social interference task (figure 1a), distractors (intact and phase-scrambled faces; figure 1b) were briefly flashed (67ms) during visually-guided saccades. On a subset of trials, distractors were also flashed during the ITI (“ITI distractors”) to dissociate neural responses to distraction during task performance from responses to a flashed image [16]. Task distractors were spatially congruent, incongruent, or in a neutral position relative to the target. Interference of distractors on task performance was affected by their location (p < 0.0001, F(1,2) = 204.79) and social content (figure 1c; interaction with location p < 0.02, F(1,2) = 3.99). Neutral distractors did not influence saccade response time (< 1.5 ms different from absent response times, +/− 4 ms across session STE; p > 0.5). Incongruent distractors slowed response times (p < 0.0001; average slowing = 47 ms +/− 7 ms) but congruent distractors speeded response times (p < 0.0001, post-hoc Tukey LSD compared to distractor absent trials; average facilitation = 25 ms +/− 3 ms). Distractors also evoked errant saccades not directed towards the target (14.5% following distractors +/− 2% STE vs 8% +/− 2% without distractors; p < 0.0001, paired Wilcoxon rank sum, z(55) = 6.31). Errant saccades were more frequent after both congruent (11% +/− 2%; p < 0.002, z(55) = 3.20) and incongruent distractors (24.6% +/− 2%; p < 0.0001, z(55) = 6.48) compared to neutral distractors (9.5% +/− 2%; paired Wilcoxon rank sum tests).
Figure 1.
Social interference task. A) Distractors were briefly flashed (67 ms) during performance of a simple visually-guided saccade task. Distractors could be in 3 spatial locations relative to the target-congruent, incongruent, or a neutral position outside the plane of possible targets. In addition, distractors could be flashed in 1 of these 3 locations during the ITI. B) Distractor images could either be rhesus monkey faces or phase-scrambled versions of the same images. C) Distractors interfered with response times according to both target congruence and social content.
Social distractors (intact faces) evoked greater response time effects than nonsocial distractors (phase-scrambled faces). Incongruent social distractors slowed response times more than incongruent non-social distractors (13 ms slower, +/− 5 ms STE; p < 0.05, Tukey LSD), and there was a trend towards congruent social distractors speeding response times relative to congruent nonsocial distractors (7 ms faster, +/− 3 ms STE; p = 0.06, Tukey LSD). Across all distractor locations, errant saccades were more common for social distractors (15.5% +/− 2%) than nonsocial distractors (13.5% +/− 2%; paired Wilcoxon rank sum, p < 0.0001, z(55) = 3.90).
Both congruent and incongruent distractors affected response time and errant saccade likelihood, relative to both neutral distractors and the distractor absent baseline. Although congruent distractors sped target responses, they did so by capturing oculomotor resources, not by enhancing target detection or processing (supplement). Therefore both of these distractor types intruded on task performance and evoked task conflict. By contrast, action conflict arises from simultaneous preparation of different saccades and is manifest by slowed response times following incongruent distractors. Thus, in this task, congruent and incongruent distractors together evoke task conflict, but only incongruent distractors evoke action conflict. Social distractors increased both action conflict and task conflict, relative to nonsocial distractors.
Pupil size at fixation predicts distractor interference
Pupil size during fixation (figure 2a) predicted the magnitude of distractor effects on errant saccade likelihood and response times. As baseline pupil size increased, the proportion of trials with errant saccades also increased, regardless of distractor location (figure 2b; GLM, interaction term, p < 0.05, β3 = 0.001, see equation 1 in methods). Baseline pupil size did not predict errant saccade frequency in absence of distractors (p > 0.66), suggesting an increase in distractibility rather than a lower threshold for saccade initiation with increasing pupil size. Increasing pupil size also magnified the response time effects of distractors by slowing response times for incongruent distractors (p < 0.002, β3 = 0.036, see equation 2 in methods) and speeding response times following congruent distractors relative to baseline (p < 0.01, β3 = −0.043). Thus, larger initial pupil size predicted increases in the impact of distractors on performance.
Figure 2.
Baseline pupil size is modulated by last-trial distractor type and predicts task performance. A) Example traces of pupil size measurements during trials. Baseline pupil size was determined by taking the mean pupil size over the first 350 ms following fixation acquisition (gray shaded region). The transient change in pupil size at fixation acquisition is due to the saccade toward the fixation spot and the depression in pupil size after fixation is due to the pupil light response to distractors (see supplemental information). B) The presence and location of distractors predicts subsequent adjustments in pupil size, normalized to the no-distractor (absent) baseline for each session. C) Baseline pupil size predicted increased frequency of errant saccades. As baseline pupil size increased, so did the frequency of errant saccades following distractors. Bars ± SEM.
Baseline pupil size was smaller following trials with distractors than following trials without distractors (figure 2c; p < 0.0001, F(1,3) = 20.47), regardless of distractor location (paired post-hoc t-test, p < 0.0001, t(55) = 8.16), but this effect was larger following incongruent and congruent distractors compared to neutral distractors (p < 0.0001, t(55) = 4.69). There were no effects of distractor congruency on pupil size on the next trial (p > 0.8) nor effects of social vs. nonsocial distractors for any single location on pupil size on the next trials (incongruent and congruent, p = 0.95, t(55) = 0.06; neutral, p = 0.67, t(55) = 0.43). Thus, on trials following distractors, down-regulation in baseline pupil size predicted reduced distractibility.
Conflict signaling by dACC neurons
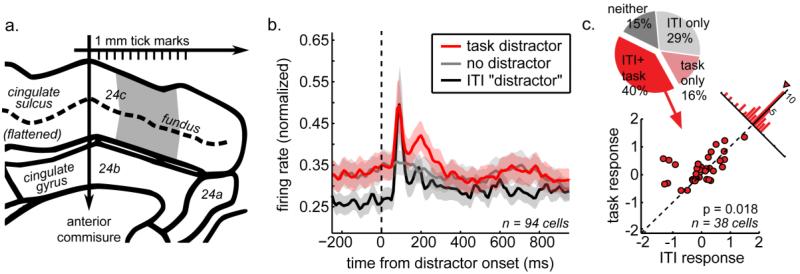
A majority of dACC neurons (recording sites in figure 3a) responded to distractors (84%; 79 out of 94 cells, figure 3b). Significant fractions of this population only responded to distractors presented within (task only, 15%, 14 cells) or outside (ITI only, 29%, 27 cells) the task. The largest population of distractor-responsive cells, however, signaled distractor presence during both the task and ITI (40%, 38 cells). Within this population, responses to task and ITI distractors differed (figure 3c), indicating these cells did not simply signal onset of a flashed stimulus. Instead, the majority of distractor-responsive neurons were sensitive to behavioral context, firing at higher rates when a distractor was presented during the task, rather than outside it (figure 3b and 4a).
Figure 3.
The dACC population signals task conflict. A) Recording sites (in gray) on a flattened, midline view of the cingulate sulcus. Neurons were recorded on the dorsal and ventral banks of the cingulate sulcus, dorsal to the genu of the corpus callosum. B) Grand average population PSTH, aligned to task, ITI, or sham distractor onset. Shading ± SEM. C) Proportion of cells that responded to only task distractors (light red), only ITI distractors (dark red), both task and ITI distractors (bright red), or did not respond to any distractors (dark gray). Among the large minority of cells that responded to both task and ITI distractors (38/94 cells, 40%), different effect sizes were observed for task and ITI distractors (scatter plot of Cohen’s d’ for the difference between distractor present and distractor absent responses in each condition). Inset is the distribution of effect size differences.
Figure 4.
dACC neurons signal task conflict. A) Population average PSTH shows differences in the population response to distractors in different locations. B) Distractor location effect sizes. Top panel: Several individual neurons encoded distractor congruency (different responses to congruent and incongruent distractors, 18 cells, in red). However, the sign of these effects was heterogeneous within the congruency-selective population (mean, red arrow) and across the whole population (mean, gray arrow), indicating that action conflict did not increase dACC firing rate. Bottom panel: Task conflict signals. Same as in top panel, but for congruent and incongruent versus neutral. Significant neurons selectively increased firing rate for both incongruent and congruent distractors, which induced task conflict, compared to neutral distractors, which did not affect task performance (Wilcoxon sign-rank, p < 0.05). C) Histogram of response latencies to task distractors. Latencies were heterogeneous across the population of responsive cells, apart from one population of early-responsive cells in green. D) Early-responding cells encoded task conflict. Left: social distractors, which had a greater impact on response time than nonsocial distractors, elicited more activity in these neurons than did nonsocial distractors (p < 0.04, z(15) = 2. 12). Right: Activity was enhanced following both incongruent and congruent distractors compared to neutral distractors (p < 0.001, Wilcoxon signed rank, z(15) = 3.31), indicating that early-responding neurons signaled task conflict. No effect of distractor congruence (action conflict) was observed in these cells (p > 0.6, z(15) = 0.46).
Incongruent and congruent distractors, which interfered with task performance and evoked task conflict, elicited greater dACC activity than did neutral distractors, which did not interfere with task performance and did not evoke task conflict (figure 4a and 4b, top panel). Several cells (18/94, 19%) showed significantly different responses to congruent and incongruent distractors, as determined by permutation tests, consistent with action conflict. However, the sign of these effects was heterogeneous across the population (figure 4b, top panel). Although fewer neurons signaled task conflict by differentiating between neutral distractors and both incongruent and congruent distractors (15/94, 16%), this signal was consistent across the population. These neurons tended to increase firing rate for both incongruent and congruent distractors, compared to neutral distractors (figure 4b, bottom panel). Thus, while task conflict signals were apparent in the peri-stimulus time histogram (PSTH) and consistently signed across the dACC population, we observed inconsistent action conflict signaling in the population.
The population average neuronal response appears biphasic, with two distinct peaks in the PSTH. However, only a small minority of individual cells (10%, 9 cells) showed biphasic distractor responses, based upon visual inspection. Biphasic responses at the population level may reflect heterogeneous response latencies of individual neurons across the population (figure 4c). The largest single subpopulation of neurons first began responding to the distractors within 50-150 ms of presentation (17%, 16 cells), and we call these the “early-responding population” (figure 4c and d).
Firing rates in the early-responding population scaled with both the social content and location of distractors. Specifically, firing rates of these neurons were modulated by whether distractors were social or nonsocial (figure 4d, first panel, p < 0.05, F(1,1) = 4.08) in the 800 ms following distractor onset. Within early-responding cells, firing rates were also enhanced for both incongruent and congruent distractors compared with neutral distractors (figure 4d panel, p < 0.0001, F(1,2) = 15.32). Post-hoc analyses revealed no significant effect of distractor congruence in these cells (p > 0.6, z(15) = 0.46). Thus, early-responding neurons signaled the same distractor properties that determined degree of task conflict. By contrast, no action conflict signals were observed in the early-responding population.
dACC neuronal responses predict future adjustments in pupil size
Other neurons began responding to distractors throughout the 1000 ms after distractor onset (figure 4c). Many neurons only signaled distractor presence after trial conclusion, suggesting these neurons did not contribute to resolving distraction on the present trial but might contribute to behavioral regulation on subsequent trials. Therefore, we next asked whether neuronal activity predicted task-facilitating adjustments in pupil size on subsequent trials. Firing rates of 31 of the distractor-responsive neurons predicted adjustments in pupil size on the next trial, and 26 of these cells showed significant interactions between distractor presence and adjustments in pupil size (corrected for multiple comparisons). Example neurons illustrating the heterogeneity of distractor and future pupil size signals are shown in figures 5a-d. Phasic responses of neurons 1 and 2 predicted pupil size on the subsequent trial. For neuron 2, the slope of the relationship between firing rate and future pupil size depended on distractor presence. Neurons 3 and 4 showed tonic modulations in firing rate. Firing rates of neuron 4, for example, predicted future pupil size before distractor onset, but nevertheless a significant interaction between distractor presence and pupil adjustments emerged after distractor onset.
Figure 5.
Relationship between distractor responses and pupil size. A-D) Example neurons that signaled distractor presence and predicted future pupil size. Traces are sorted by pupil size quantile bin on the next trial, separately for distractor present (shades of red) and distractor absent (shades of gray) trials. Lighter shades reflect smaller pupil sizes on subsequent trials, while darker shades reflect larger pupils. E) Distribution of distractor and pupil signaling in the dACC population F) For neurons that both responded to distractors and tracked pupil size, responses to distractors and adjustment in future pupil size were correlated. Line indicates least squares fit. G) Population tuning for reductions in pupil size. Across all recorded neurons, whole trial activity tended to be increased in advance of decreasing adjustments in pupil size (Pearson’s r is illustrated). Individual cells that had significant correlations between mean firing rate and adjustment in pupil size in either the Spearman (19 cells) or Pearson (14 cells) correlations are in blue. Overlay: nonparametric kernel density estimate.
One concern is that neuronal signals may only predict future pupil size due to autocorrelations in baseline pupil size over trials. To address this issue, we used a generalized linear model (GLM) to estimate effects of distractors, current pupil size, and future adjustments in pupil size on current-trial firing rate (equation 3). In this analysis, a small number of cells responded to distractors but did not scale with either current pupil size or subsequent adjustments in pupil size (11/94 cells, 12%). Half of these cells (6/11) were previously classified as early-responding cells. Moreover, this effect was temporally specific—dACC activity only predicted pupil adjustments made 1 or 2 trials into the future, and had no relationship with past adjustments (Supplemental material).
Many single neurons that responded to task distractors according to this analysis also tracked current pupil size or predicted subsequent adjustments in pupil size (figure 5). Across the population, firing rates of 31% (29/94 cells) of neurons that responded to distractors also scaled with baseline pupil size on the current trial. Moreover, some neurons signaled both presence of distractors on the current trial and subsequent adjustments in pupil size (18/94 cells, 19%). Another subset of neurons did not respond to distractors, but did signal future adjustments in pupil size (22/94 cells, 23%) and almost all of these predictive cells tracked pupil size on the current trial (20/22, 91%). Finally, firing rates of a modest fraction of neurons scaled only with baseline pupil size on the current trial, but did not distractor presence or future pupil size (15/94 cells, 16%). Thus, the activity of some neurons encoded distractor presence independently from tracking pupil size and the activity of some neurons integrated information about task conflict with pupil size. Thus dACC does not merely inherit information about distractor presence from another region that has already combined it with information about pupil size. Rather, dACC contains the necessary distribution of neuronal signals to integrate information about distractors and current pupil-linked processes to generate future adjustments in pupil-linked processes like arousal.
The co-occurrence of these signals within cells suggests that the dACC populations that encode distractors and pupil size are not separate. Moreover, there was a strong positive correlation between distractor signals and pupil adjustment signals both within neurons that responded to distractors and tracked pupil size (Pearson’s r = 0.60, p < 0.008, Spearman’s rho = 0.42, p < 0.09, figure 5b) and across the whole population (Pearson’s r = 0.28, p < 0.007, Spearman’s rho = 0.28, p < 0.006). This correlation suggests that distractor signals and pupil regulatory signals are linked within dACC.
Finally, we asked whether the sign of pupil adjustment signals in dACC was consistent across the population (figure 5c; see Methods). For this analysis, we examined a 2000ms epoch beginning at fixation acquisition, a timescale useful for comparison with fMRI studies in the literature (e.g. [7-10, 12]). We found that the dACC population response showed a significant negative correlation between firing rate and pupil size adjustments (figure 5g). Increasing firing rate correlated with decreases in pupil size on subsequent trials at the population level (Pearson’s r, mean = −0.013, p < 0.01; Spearman’s rho, mean = −0.015, p < 0.02). This observation suggests that down-regulation of baseline pupil size, or events that predict such down-regulation, may yield an increased BOLD signal in macaque dACC, a hypothesis remaining to be tested.
dACC signals mediate pupil adjustment to conflict
One goal of the present study was to evaluate the hypothesis that dACC contributes causally to behavioral control via changes in pupil-linked arousal following distracting events. Though the present study was observational rather than interventional, we could determine whether the basic tenets of this hypothesis were supported by our data. First, we observed a confluence of signals in dACC consistent with this hypothesis. Second, the time course of the signals was appropriate; dACC activity on one trial predicted adjustments in pupil size in the future but not the past. Third, the consistently signed relationship between distractor and pupil-adjustment signals suggested that their co-occurrence within neurons was not coincidental, but rather indicated a lawful relationship. Nevertheless, the number of neurons that significantly encoded both distractors and future adjustments in pupil size was small (19%, 18/94) and may have simply occurred by chance, given the independent probabilities of observing pupil adjustment signals (42.6%, 40/94 neurons) and distractor signals (42.6%, 40/94; joint probability = 18%).
To overcome these limitations, we used structural equation modeling to determine whether our data were better explained by a model in which dACC neurons predicted adjustments in pupil size or by a model in which the correlations between dACC activity and adjustments in pupil size were a coincidental byproduct of shared influences of current arousal and distractor presence. This approach allowed us to simultaneously model effects on both adjustments in pupil size and dACC activity. We fit two models to the activity of the population of neurons that both tracked distractors and predicted adjustments in pupil size (18/94 neurons). These models differed only in whether dACC activity was allowed to mediate the relationship between distractors and future adjustments in pupil size.
The first model assumed that there was no causal link between dACC and future adjustments in pupil size (equation 4, with the b1 and b2 terms fixed at 0), and distractor signals could only be independently inherited from their shared input. Fit quality of the inherited signal model was reasonable by several standard metrics (df = 72; χ2 = 817.77; CFI = 0.968; NFI = 0.921; IFI = 0.968; goodness-of-fit index: 0.999; AIC: −46741.23). Nevertheless, model fit was substantially improved by allowing a causal link between dACC activity and future adjustments in pupil size.
This second model (equation 4 with all terms fitted; illustrated graphically in figure 6) assumed that dACC mediates the relationship between distractor occurrence and future adjustments in pupil size. The model includes an interaction (or “moderation”) effect, wherein dACC activity predicts different adjustments in future pupil size, depending on the presence or absence of a distractor. Fit quality for the mediation model was better than the inherited signal model (df = 36; χ2 = 616.72; CFI = 0.975; NFI = 0.974; IFI = 0.976 goodness-of-fit index: 0.999; AIC: −46870.28). The Akaike weight [44] of the inherited signals model was less than 1 × 10−28, indicating that the mediation model was 1028 more likely to minimize information loss. Thus, it is extremely unlikely that the co-occurrence of these signals was epiphenomenonal. Rather, activity of single neurons in dACC predicts trial-by-trial fluctuations in pupil adjustment beyond what can be explained by distractor presence alone.
Figure 6.
Causal modeling of the relationship between dACC activity and adjustments in pupil size. The depicted model is the moderated mediation model. The inherited signal model fixed the regression coefficients b1 and b2 to 0. Large arrows are regressions, double-headed arrows are covariances. Paths are labeled with estimated coefficients, +/− robust standard errors. Additional coefficients for current pupil size, random effects of cell identity, and disturbance terms for each measured variable were included in the model, but not shown here for clarity.
dACC error signals and pupil dynamics
Our findings suggest dACC combines information about task conflict with information about current pupil size, into signals that predict down-regulation in pupil size. However, it remains unclear whether dACC neurons tracked other aspects of task performance in a similar, pupil-linked manner. To address this issue, we asked how error signals in dACC interact with current and future pupil size signals. Error responses are commonly found in both human [9, 20, 21] and monkey dACC [16] and inform many unifying hypotheses about dACC function [22-25]. Moreover, errors provoke changes in pupil diameter in humans and both errors and the pupil response to errors are encoded in an overlapping region of human dACC [20]. Therefore, we hypothesized that dACC error signals may be related to pupil size signals within single neurons in macaque dACC.
Monkeys showed smaller pupils on trials following errors (figure 7a; paired t-test across sessions, p < 0.0001, t(55) = −5.6), much as they did on trials following distractors. These pupil size adjustments were not better explained by the increased likelihood of distractors on trials when an error was committed. Even on distractor-absent trials, error commission on one trial predicted reduced pupil size on the subsequent trial (p < 0.0001, t(51) = −4.49; 4 sessions omitted because no errors were committed in the absence of distractors). Thus, error commission provided an additional event type, decoupled from distractor presentation, to which the monkeys exhibited down-regulated arousal on subsequent trials.
Figure 7.
An overlapping population of single neurons responds to errors and signals pupil size. A) On trials immediately following error commission, baseline pupil size was reduced, mirroring the effects found on trials following distractor presentation. B) The majority of single neurons responded to errors (69%, 65/94, in red and purple), however only a minority of this group had pure error responses (26%, 24/94, in red). Instead, 44% (41/94) of all recorded cells signaled both errors and either current or future pupil size (purple). Other populations of cells were not classified in this analysis (11% 10/94, in gray), or signaled only current or future pupil size (20%, 19/94 in blue). Significance thresholds were corrected for multiple comparisons. C) Error responses and pupil size adjustment signals were correlated within the neurons that both responded to errors and scaled with pupil size (Pearson’s r = 0.32, p < 0.05). Line reflects least squares fit.
A large number of neurons showed significant error responses (79%, 74/94 cells), by the same bootstrapping criterion used to initially identify distractor sensitive cells. Within error responsive cells, 58% were also sensitive to distractors (43/74 error responsive cells; 46% of the total population of recorded cells responded to both). Thus, the populations of distractor and error responsive cells overlapped, but were not identical.
We asked whether error responses in dACC were linked to current and future arousal state as indexed by pupil size (figure 7b). The activity of most error responsive cells scaled with current pupil size (39/74, 53%) and the activity of many also scaled with subsequent adjustment in pupil size (28/74, 38%) when all three terms were included in a GLM (significance threshold corrected for multiple comparisons). The activity of only 24/74 (32%) of all error-responsive neurons did not have any relationship with pupil size. Like distractor responses, error responses were significantly correlated with pupil size signals within cells that responded to both (figure 7c; Pearson’s r = 0.32, p < 0.05, Spearmans’ rho = 0.39, p < 0.02; n.sig across the whole population: Pearson’s r = 0.14, p > 0.1, Spearmans’ rho = 0.17, p > 0.05). Approximately 70% of the cells that responded to errors and also tracked pupil size (19/27) were sensitive to distractors, suggesting these neurons integrated multiple types of task information with subsequent adjustments in pupil size.
Discussion
We found that neurons in macaque dACC respond to salient, goal-irrelevant distractors, and do so largely by increasing firing rates. These signals are not mere visual responses, but instead reflect the conjunction of task demands and distractor presence. We found consistently signed signals related to task conflict—the contrast between distractors that intruded on task performance and those that did not. Conversely, signals related to action conflict—the contrast between physically incongruent and congruent distractors—were inconsistent across neurons and the overall direction of the trend (higher firing rate for congruent distractors) was inconsistent with a global increase in dACC firing rate with action conflict. The population distractor response includes an early-responding subpopulation of neurons that tracks social information content, a factor that systematically shaped the magnitude of both task and action conflict. However, this early responsive population only signaled information about task conflict, and carried no information about action conflict. Other neurons respond to distractors too late to contribute to performance on the current trial, but may contribute to subsequent adjustments in behavioral state.
Pupil size under constant luminance is a peripheral index of arousal [29] that is correlated with other autonomic measures [45, 46], has been linked to NE signaling [35, 42], and predicts behavioral performance in many tasks [30]. Several studies have examined phasic pupil responses during task performance, and found that the pupil transiently dilates in response to salient stimuli [47], conflict [42], and errors [20]. Here we examined tonic changes in baseline pupil size across trials, rather than within trials. We found that larger pupils predicted increases in both error likelihood and impact of distractors on response times. Surprisingly, pupil size decreased, rather than increased, on trials following either distractors or errors, consistent with an adaptive or homeostatic regulation of distraction via pupil-linked mechanisms.
Many distractor-responsive dACC neurons signaled information about current pupil size and/or predicted adjustments in future pupil size. Similarly, error-responsive neurons also signaled pupil size. Moreover, there was a consistently signed relationship between error and distractor responses on one trial and subsequent adjustments in pupil size on the next trial. We found that a model in which dACC activity mediates trial-by-trial changes in pupil size better explained our results than a model that assumes these signals are independent and inherited from a common source. Together, our findings suggest dACC combines information about current arousal state, as indexed by pupil size, errors and/or task conflict. These signals predict adjustments in pupil size, which are associated with enhanced cognitive control and improved task performance.
We found that predictive pupil-change signals are linked to the distractor responses of single dACC neurons. Distractor features that determine distractor interference but do not predict adjustments in pupil size are only weakly signaled in dACC, compared to features that predict adjustments in pupil size. The social information content of distractors, for example, influences the response time interference of distractors but does not predict pupil adjustments and is only weakly signaled in dACC. Similarly, congruent and incongruent distractors differentially impact task performance and action conflict, but these two classes of distractors have similar effects on pupil size and are not well-differentiated by dACC neurons.
In humans, conflict signals have been reported in the activity of single dACC neurons [11], however such signals have, until now, proven elusive in macaque dACC [14-19]. This dearth of evidence for conflict signals in nonhuman primate dACC has fueled speculation that this area may serve a different, potentially unique, function in humans [14]. One study often cited in support of this hypothesis found that lesions of macaque dACC had no effect on post-conflict behavioral adjustments [48]. Unlike our study, that report did not operationalize conflict in terms of interference with task performance, linked post-conflict behavioral adjustment to rule-learning, and may have induced a form of conflict that did not result in adjustments in pupil-linked processes. Moreover, dACC lesions may not affect post-conflict adjustments in control state in humans [28, 49]. To our knowledge, only one study has reported conflict-like signals in any part of the macaque cingulate cortex, albeit in pregenual ACC, not dACC [50]. Nevertheless, conflict was operationally defined in that experiment as decision difficulty, rather than suppression of a prepotent, task-irrelevant process competing with task goals. By contrast with these studies, we found clear evidence that firing rates of dACC neurons are selectively enhanced by task conflict.
There are several possible explanations for the apparent discrepancy between the results we report here and previous studies in monkeys. One possibility is that previous studies of conflict in monkeys manipulated conflict at the level of the action, but did not examine task conflict, as we do here. In those prior studies, the command to shift gaze to a particular target in space was either opposed (high-conflict) or facilitated (low conflict) by additional information, such as a color cue instructing an opposing saccade [19], a stop signal [16, 19], the presence of alternatives [17], or the discrepancy in reward value of alternatives [15]. By contrast, in standard human task conflict paradigms a prepotent task rule (e.g. read the word, look at the biologically salient distractor) must also be suppressed to perform a goal-oriented task (e.g. name the color, saccade to the rewarded target). Task conflict emerges from the intrusion of irrelevant information on performing the current task. Critically, both forms of conflict are induced in human conflict paradigms such as the Stroop and Flanker tasks, but the present study dissociated action and task conflict. We observed little evidence of action conflict at the level of the dACC population, compared to the task conflict signals apparent in the population PSTH, and no evidence of action conflict in the early distractor response. Another, not mutually exclusive, explanation is that conflict signals in dACC may be inextricably linked to arousal. In this view, previous studies in monkeys may not have provoked conflict sufficient to trigger adjustments in pupil size or other measures of arousal (indeed some argued that they did not [19]).
Critically, it remains unclear whether the signals we report here were specific to pupil size or reflect more general adjustments in autonomic arousal. In humans, dACC activity varies with non-pupil measures of autonomic arousal. For example, human dACC activity is positively correlated with autonomic responses to errors [20], and dACC microstimulation evokes increases in autonomic arousal in patients, and these changes are accompanied by a subjective sense of preparation to overcome a challenge [51]. In parallel, the dACC BOLD signal increases during self-generated down-regulation of arousal in humans [52]. These findings resonate with observations that microstimulation in feline dACC causes both pupil dilation and constriction at intermingled sites [53]. Thus, dACC may both signal arousing events and trigger down-regulation of arousal in animals and arousal regulation may be an evolutionarily conserved aspect of dACC function.
The correlation between dACC activity and baseline pupil size that we observed in a majority of neurons resonates with prior studies showing dACC responds to a broad range of task events that are correlated with baseline pupil size. For example, in humans, classic dACC-activating factors such as task conflict [42] and errors [20] also evoke changes in pupil diameter. Human dACC activity also increases with task difficulty [54], increases linearly with response time [55], and predicts the likelihood of committing errors [9, 24] (firing rates of dACC neurons also predicted error likelihood in our study [figure S1]). Pupil size under constant luminance also scales positively with task difficulty [42, 56], scales positively with response time [30], and, at least in the present task, predicts error likelihood. dACC neurons also differentiate between habitual behavioral states and flexible, exploratory modes of behavior in macaques [57, 58] and rats [59], and larger baseline pupil size predicts exploratory decisions in humans [43]. dACC activity is heightened following movement switching or task switching [60, 61] and is modulated over the course of a series of actions that must be performed to receive a reward [62, 63]. In parallel, pupil size tracks the execution of movements and scales positively with movement complexity [64], in addition to scaling positively with reward expectancy over time [65]. Given these many parallels, and the breadth of putatively cognitive signals previously reported in dACC, it may be more parsimonious to consider that dACC responds to all of these disparate factors for the common reason that each is associated with baseline pupil size—and by extension arousal. Additional work will be necessary to determine to what extent dACC signals related to each of these factors is independent of the pupil size tracking signals we report here.
Previous studies suggest dACC contributes to cognitive control via connections to other cortical regions [7, 10, 11, 22], although the necessity of dACC for adjustments in post-conflict control is debated [28, 49]. We did not find evidence of distractor effects on executive control that were independent of pupil size, but there were several differences between our study and previous studies. First, we did not have a trial-by-trial index of control state, so our measures of executive control required averaging over multiple trials, with different initial control states. Heterogeneity in control states may have masked real behavioral effects by introducing additional variability that was unrelated to the effects of distractors. Second, executive control may have been countered by other processes, like arousal, resulting in a null effect on behavior. Regardless, the cortico-cortico mechanisms by which dACC could influence control state are clear [10, 12, 60].
Regulation of processes associated with pupil size such as LC activity or autonomic arousal would be a simple, complimentary mechanism by which dACC could globally alter behavioral state. Although future manipulation studies will be needed to determine what causal role dACC plays in down-regulating arousal, a wealth of anatomical data [32-35] and limited microstimulation results in both humans [51] and cats [53] suggest dACC activity may be sufficient to initiate adjustments in autonomic arousal, as indexed by baseline pupil size. Larger baseline pupil size predicted increased distraction and poorer performance in our study, and, in other tasks, larger baseline pupil size also predicts increased likelihood of non-reward maximizing decisions [43], increased variability in evidence accumulation during perceptual decision-making [66], and reduced BOLD responses to task-relevant stimuli during learning [67]. Additional work will be needed to 1) determine to what extent baseline pupil size and/or other measures of arousal are linked to cognitive control and 2) to determine the relative contributions of cortico-cortical and pupil-linked mechanisms to mediating the relationship between task conflict or errors, dACC activity, and adjustments in control on subsequent trials.
Our findings raise many questions for future study. It remains unclear whether cognitive control is linked to pupil size in other circumstances. It remains unclear whether dACC activity predicts adjustments in other measures of autonomic arousal or if the signals we report are specific to pupil size. It remains unknown to what extent dACC is causally involved in regulating pupil-linked processes. And it remains unclear whether other signals previously reported in dACC (such as reward or exploration) are related to autonomic arousal. Given these open questions, future studies of cognitive control and/or dACC activity would benefit from including arousal-linked measures such as pupil size in their experimental design.
Methods
Behavioral techniques
Details of the social interference task (figure 1) were reported previously [3, 30]. Briefly, monkeys performed simple, visually-guided saccades while distractors were briefly flashed. Eye position was monitored by video at 1000 Hz (Eyelink). Monkeys first fixated a 1° spot (+/− 6° of error) for 450-650ms and then shifted gaze to an eccentric target (1° square, 14 degree offset) appearing left or right of fixation. Fixation on the eccentric target (+/−6° of error) for 150ms-450ms resulted in a juice reward. Pupil size was measured during the first 350 ms of fixation, to ensure constant luminance (example traces in figure 2a).
On a randomly chosen 75% of trials, a distractor image was briefly flashed (67 ms) at one of 3 locations relative to the target—congruent (same hemifield, eccentric to the target), incongruent (opposite hemifield), or neutral (directly above fixation)—selected randomly, and with a variable onset asynchrony relative to the target. Distractors were large (7° width) images of rhesus macaque faces or phase-scrambled versions of the same faces. On a variable subset of trials (10% to 75%), distractors were also flashed during the ITI, to allow us to compare responses to distractors within and outside of the context of the task.
Electrophysiological Recording
We recorded from single neurons with sharp tungsten electrodes (Frederick Haer) from the dorsal bank, ventral bank, and fundus of the cinculate sulcus, dorsal to the genu of the corpus collosum (area 24c; figure 3a). Neurons were selected based on quality of isolation only. Additional details of the recording procedures have been reported previously [68] and are included in the supplemental methods.
Data Analysis
In order to determine whether baseline pupil size predicted a distractor-dependent change in errant saccade frequency or the response time effects of distractors, we fit generalized linear models (GLMs). The model for errant saccade frequency was:
| (equation 1) |
Baseline pupil size was z-scored within session and included in the model as the “pupil” term, γ is a logical vector reflecting the presence (1) or absence (0) of a distractor. Main effects of each session were included with one term for each session minus one. β3 thus captured the interaction of distractor presence and pupil size in predicting errant saccade likelihood. β1 described any effect of baseline pupil size in the absence of distractors and β2 captured the offset between the two conditions. Fits from this model are show in figure 2B.
The model for the response time effects of distractors was:
| (equation 2) |
Here, α reflected whether a distractor was incongruent (1) or congruent (0). In this model β3 captured the interaction of distractor congruence with pupil size in predicting response time, β1 described any effect of baseline pupil size on response times following congruent distractors, and β2 captured the offset between the two conditions. Main effects of each session were included as additional terms, with one term for each session minus one.
Initial identification of distractor and error sensitive neurons was done via bootstrapping (Supplemental Experimental Procedures). To examine the relationship between pupil size signals and distractor or error signals, the following generalized linear model was run independently on the response of each cell:
| (equation 3) |
Where “fr” was the spike count in the 800 ms following event occurrence and was modeled as Poisson distributed. The term γ was a binary vector expressing the presence or absence of the event of interest (distractor presence or error comission). Error trials were excluded from the distractor response analysis. β1 thus captured any offset in firing rate due to event occurrence, β2 described the relationship between firing rate and pupil size on the current trial, and β3 described the relationship between firing rate and pupil size on the next trial.
To evaluate the hypothesis that dACC played a mediating role in the relationship between distractor presence and pupil size, we took a structural equation modeling (SEM) approach. This approach allows us to determine whether the data can be explained by the relationships we hypothesize between the multiple dependent variables, or, alternatively, if our causal hypotheses are a poor fit to the data. Because we were interested in interactions between distractors and pupil size adjustment that depended on dACC activity and we observed interactions in these signals at the level of single neurons, we developed a multilevel model based on standard modulated-mediation path analysis (see Supplemental Experimental Procedures).
| (equation 4) |
Here, γ is a binary vector describing the presence (1) or absence (0) of distractors. “fr” is a vector of mean firing rates observed over the 800 ms following distractor presentation. Delta is the difference between pupil size on the next trial (t+1) and pupil size on the current trial (t). In the inherited signals version of the model, the b1 and b2 coefficients of the model (the links between dACC activity and pupil adjustments) were fixed to 0. The inherited signals model thus explicitly assumed that any correlations between firing rate and adjustments in pupil size were due to parallel inheritance of information about current pupil size and distractor presence in the two dependant variables, without any causal linkage between the two. Figure 6 shows a graphic depiction of the full model, with fitted coefficients.
Supplementary Material
Acknowledgements
The authors would like to thank Ben Hayden, Vince McGinty, and Adrienne Mueller for helpful comments on the manuscript, Sarah Heilbronner, Karli Watson, John Pearson, Vince McGinty, and Monica Carlson for technical assistance. This work was supported by grants from the National Institutes of Health (R01-MH-086712 and R01-MH-089484) and the Department of Defense (W81XWH-11-1-0584).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: R. B. Ebitz and M. L. Platt designed the experiment and wrote the manuscript. R. B. Ebitz collected and analyzed the data.
References
- 1.Remington RW, Johnston JC, Yantis S. Involuntary attentional capture by abrupt onsets. Perception & Psychophysics. 1992;51(3):279–290. doi: 10.3758/bf03212254. [DOI] [PubMed] [Google Scholar]
- 2.Cerf M, Frady EP, Koch C. Faces and text attract gaze independent of the task: Experimental data and computer model. Journal of vision. 2009;9:10, 1–15. doi: 10.1167/9.12.10. [DOI] [PubMed] [Google Scholar]
- 3.Ebitz RB, Watson KK, Platt ML. Oxytocin blunts social vigilance in the rhesus macaque. Proceedings of the National Academy of Sciences. 2013;110(28):11630–11635. doi: 10.1073/pnas.1305230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush G, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biological psychiatry. 1999;45:1542–52. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 5.Seidman LJ, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological psychiatry. 2006;60(10):1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Weissman D, et al. The neural bases of momentary lapses in attention. Nature neuroscience. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 7.Botvinick M, et al. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 8.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Carter CS, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 10.Kerns JG, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 11.Sheth SA, et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488(7410):218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald AW, et al. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 13.Pardo JV, et al. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Sciences. 1990;87(1):256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole MW, et al. Cingulate cortex: diverging data from humans and monkeys. Trends in neurosciences. 2009;32(11):566–574. doi: 10.1016/j.tins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden BY, et al. Surprise Signals in Anterior Cingulate Cortex: Neuronal Encoding of Unsigned Reward Prediction Errors Driving Adjustment in Behavior. The Journal of Neuroscience. 2011;31:4178–4187. doi: 10.1523/JNEUROSCI.4652-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito S, et al. Performance Monitoring by the Anterior Cingulate Cortex During Saccade Countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 17.Amiez C, Joseph J.p., Procyk E. Reward Encoding in the Monkey Anterior Cingulate Cortex. Cerebral Cortex. 2006;16:1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rushworth M, Kennerley S, Walton M. Cognitive neuroscience: resolving conflict in and over the medial frontal cortex. Current Biology. 2005;15(2):R54–R56. doi: 10.1016/j.cub.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Roesch MR, Olson CR. Neuronal Activity in Macaque SEF and ACC During Performance of Tasks Involving Conflict. Journal of Neurophysiology. 2005;93:884–908. doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- 20.Critchley HD, et al. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27:885–95. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 21.Holroyd CB, et al. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nature neuroscience. 2004;7(5) doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- 22.Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nature neuroscience. 2011;14(10):1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307(5712):1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- 25.Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the neurosciences. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- 26.Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–8. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 27.Kennerley SW, et al. Optimal decision making and the anterior cingulate cortex. Nature neuroscience. 2006;9(7):940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 28.Swick D, Turken U. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proceedings of the National Academy of Sciences. 2002;99(25):16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. Vol. 4. McGraw-Hill; New York: 2000. [Google Scholar]
- 30.Ebitz RB, Pearson JM, Platt ML. Pupil size and social vigilance in rhesus macaques. Frontiers in neuroscience. 2014;8 doi: 10.3389/fnins.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anthony B, Graham F. Blink reflex modification by selective attention: Evidence for the modulation of ‘automatic’processing. Biological Psychology. 1985 doi: 10.1016/0301-0511(85)90052-3. [DOI] [PubMed] [Google Scholar]
- 32.Pandya DN, Van Hoesen GW, Mesulam MM. Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Research. 1981;42:319–30. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- 33.Rempel-Clower N, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1998;398(3):393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.Öngür D, An X, Price J. Prefrontal cortical projections to the hypothalamus in macaque monkeys. Journal of Comparative Neurology. 1998;401(4):480–505. [PubMed] [Google Scholar]
- 35.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual review of neuroscience. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 36.Anlezark G, Crow T, Greenway A. Impaired learning and decreased cortical norepinephrine after bilateral locus coeruleus lesions. Science. 1973;181(4100):682–684. doi: 10.1126/science.181.4100.682. [DOI] [PubMed] [Google Scholar]
- 37.Hu H, et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131(1):160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Carli M, et al. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behavioural Brain Research. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- 39.Witte EA, Marrocco RT. Alteration of brain noradrenergic activity in rhesus monkeys affects the alerting component of covert orienting. Psychopharmacology. 1997;132:315–23. doi: 10.1007/s002130050351. [DOI] [PubMed] [Google Scholar]
- 40.Nassar MR, et al. Rational regulation of learning dynamics by pupil-linked arousal systems. Nature neuroscience. 2012;15:1040–6. doi: 10.1038/nn.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nature neuroscience. 2013 doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilzenrat MS, et al. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cognitive, affective & behavioral neuroscience. 2010;10:252–69. doi: 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jepma M, Nieuwenhuis S. Pupil diameter predicts changes in the exploration-exploitation trade-off: evidence for the adaptive gain theory. Journal of cognitive neuroscience. 2011;23(7):1587–1596. doi: 10.1162/jocn.2010.21548. [DOI] [PubMed] [Google Scholar]
- 44.Burnham K,P, Anderson DR. Model Selection and Multi-model Inference: A Practical Information-Theoretic Approach. Springer; 2002. [Google Scholar]
- 45.Kahneman D, et al. Pupillary, heart rate, and skin resistance changes during a mental task. Journal of experimental psychology. 1969;79:164–7. doi: 10.1037/h0026952. [DOI] [PubMed] [Google Scholar]
- 46.Bradley M, et al. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008 doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokolov EN. Higher nervous functions: The orienting reflex. Annual review of physiology. 1963;25(1):545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- 48.Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318(5852):987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- 49.Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005;128(4):788–796. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- 50.Amemori K.-i., Graybiel AM. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nature neuroscience. 2012;15(5):776–785. doi: 10.1038/nn.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parvizi J, et al. The Will to Persevere Induced by Electrical Stimulation of the Human Cingulate Gyrus. Neuron. 2013;80(6):1359–1367. doi: 10.1016/j.neuron.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Critchley H. Volitional Control of Autonomic Arousal: A Functional Magnetic Resonance Study. NeuroImage. 2002;16:909–919. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- 53.Hodes R, Magoun H. Pupillary and other responses from stimulation of the frontal cortex and basal telencephalon of the cat. Journal of Comparative Neurology. 1942;76(3):461–473. [Google Scholar]
- 54.Paus T, et al. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport. 1998;9(9):R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 55.Grinband J, et al. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011;57(2):303–311. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldwater BC. Psychological significance of pupillary movements. Psychological bulletin. 1972;77(5):340. doi: 10.1037/h0032456. [DOI] [PubMed] [Google Scholar]
- 57.Procyk E, Tanaka YL, Joseph J-P. Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nature neuroscience. 2000;3(5):502–508. doi: 10.1038/74880. [DOI] [PubMed] [Google Scholar]
- 58.Quilodran R, Rothe M, Procyk E. Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron. 2008;57(2):314–325. doi: 10.1016/j.neuron.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 59.Karlsson MP, Tervo DG, Karpova AY. Network resets in medial prefrontal cortex mark the onset of behavioral uncertainty. Science. 2012;338(6103):135–139. doi: 10.1126/science.1226518. [DOI] [PubMed] [Google Scholar]
- 60.Johnston K, et al. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53(3):453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 61.Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282(5392):1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 62.Toda K, et al. Differential encoding of factors influencing predicted reward value in monkey rostral anterior cingulate cortex. PloS one. 2012;7(1):e30190. doi: 10.1371/journal.pone.0030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–11. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- 64.Richer F, Beatty J. Pupillary dilations in movement preparation and execution. Psychophysiology. 1985;22(2):204–207. doi: 10.1111/j.1469-8986.1985.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 65.Bijleveld E, Custers R, Aarts H. The unconscious eye opener pupil dilation reveals strategic recruitment of resources upon presentation of subliminal reward cues. Psychological Science. 2009;20(11):1313–1315. doi: 10.1111/j.1467-9280.2009.02443.x. [DOI] [PubMed] [Google Scholar]
- 66.Murphy PR, Vandekerckhove J, Nieuwenhuis S. Pupil-Linked Arousal Determines Variability in Perceptual Decision Making. PLoS computational biology. 2014;10(9):e1003854. doi: 10.1371/journal.pcbi.1003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nature neuroscience. 2013;16(8):1146–1153. doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Platt ML, Glimcher PW. Responses of intraparietal neurons to saccadic targets and visual distractors. Journal of neurophysiology. 1997;78:1574–89. doi: 10.1152/jn.1997.78.3.1574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.