Abstract
AIM: To examine the effects of vascular endothelial growth factor (VEGF)-targeted small interfering RNA (siRNA) on proliferation of gastric cancer cells in vitro.
METHODS: Several siRNAs were transfected into human gastric cancer cell line SGC-7901 with Lipofectamine 2000. Cells not transfected with LipofectamineTM 2000 or scrambled (SCR) siRNA served as controls. The inhibitory effect of siRNA on the expression of VEGF mRNA and protein was detected by RT-PCR and ELISA. MTT assay was used to examine the inhibition rate of cell growth. The change in cell cycling of siRNA-treated cells was detected by flow cytometry.
RESULTS: siRNA targeting human VEGF effectively inhibited the proliferation of gastric cancer cell line SGC-7901 and the distribution of cell cycle. The percentage of G0/G1 phase was significantly higher in siRNA1- and siRNA2-transfected cells than in control cells. The expression of VEGF mRNA was significantly inhibited in siRNA1- and siRNA2-transfected cells compared with that in control cells. VEGF protein notably decreased in siRNA-transfected cells, but had no effect on SCR siRNA.
CONCLUSION: VEGF siRNA inhibits proliferation of gastric cancer cells in vitro.
Keywords: siRNA, SGC-7901, Vascular endothelial growth factors, Gene therapy, RNA interference
INTRODUCTION
A number of tumor-released angiogenic cytokines affecting vessel formation, tumor growth, invasion, and metastasis have been identified. Vascular endothelial growth factor (VEGF) is one of the most important angiogenic factors[1-3].
Tumor neoangiogenesis plays an important role in identifying subsets of cancer patients with poor outcome. As neoangiogenesis is crucial for sustained tumor growth, angiogenesis inhibitors may be used as therapeutic agents in treatment of cancer. Long double-stranded RNAs (dsRNAs; typically > 200 nt) can be used to silence the expression of target genes in a variety of organisms and cell types (e.g., worms, fruit flies, and plants). Upon introduction, long dsRNAs enter a cellular pathway which is commonly referred to as the RNA interference (RNAi) pathway[4,5]. dsRNAs can be transformed into 21-23 nucleotide (nt) small interfering RNAs (siRNAs) by a RNase III-like enzyme called Dicer and silence the expression of target genes distinctively[6]. Gastric cancer is the most common cause of cancer-related death and one of the most malignant carcinomas in China[7-11]. In this study, we investigated the effects of VEGF siRNA on proliferation of gastric cancer cells in vitro.
MATERIALS AND METHODS
Materials
Human gastric cancer cell line (SGC-7901 was purchased from the Cell Center of Peking Union Medical College. Dulbecco's modified Eagle's medium (DMEM), LipofectamineTM 2000, TRIzol reagent, and RPMI medium1640 were purchased from Invitrogen Company. T7 RiboMAXTM express RNAi system kit and Access RT-PCR introductory system were purchased from Promega Company. MTT (thiazolyl blue tetrazolium bromide) was purchased from Sigma Compan. Human VEGF ELISA kit was purchased from Boster Biological Technology Company.
Preparation of siRNA
The sequence data of human VEGF mRNA (ACCESSION No: AB021221) used in this study were collected from GenBank. Two siRNAs targeting human VEGF and one scrambled siRNA were designed on line and obtained by in vitro transcription using a kit from Promega Company (Table 1). (The basic GC composition of scrambled siRNA was the same as siRNAs targeting human VEGF, but had no distinguished homology with human VEGF RNA, used as a negative control)[12].
Table 1.
Sequence of designed siRNA
| siRNA | Sense strand | Antisense strand |
| SiRNA1 | 5’-GAUCAAACCUCACC AAGGCUU-3’ | 5’-GCCUUGGUGAGG UUUGAUCUU-3’ |
| siRNA2 | 5’-GGAGUACCCUGAUG AGAUCUU-3’ | 5’-GAUCUCAUCAGG GUACUCCUU-3’ |
| siRNASCR | 5’-GCGUAACGCGGGAA UUUACUU-3’ | 5’-GUAAAUUCCCGC GUUACGCUU-3’ |
Cell culture
SGC-7901 cell line was maintained in RPMI medium 1640 supplemented with 100 mL/L newborn calf and incubated at 37°C with a humidified incubator containing 50 mL/L CO2.
MTT assay
Cells were divided into 7 groups: (1) 100 nmol/L siRNA1 or siRNA 2 group, (2) 200 nmol/L group, (3) 400 nmol/L group, (4) 1000 nmol/L group, (5) 200 nmol/L scrambled siRNA group, (6) lipofectin group with no siRNAs, (7) untreated group with no siRNAs and lipofection. LipofectamineTM2000 in transfection medium was used according to the manufacturer’s instructions (0.5 mL/well).
Cells (6 × 107/L cells) were suspended in RPMI medium 1640, plated onto gelatinized 96-well culture plates (0.1 mL/well), and incubated in a humidified incubator containing 50 mL/L CO2 at 37°C for 24 h. The media were replaced with transfection medium or Opti-MEM (0.1 mL/well). After incubated for 6 h, newborn calf serum were added (10 μL/well) and incubated for 24 h, Cell proliferation was determined by MTT assay.
Cell cycle assay
Cells (1.5 × 105/L cells) were suspended in RPMI medium 1640 and then plated onto 25 cm2 culture flasks. After gene transferring for 2 d, cells were collected, suspended in 0.01 mol/L phosphate-buffer saline (PBS) and fixed in 70 mL/L ethanol for 30 min. The cells were washed once with PBS, digested by 200 μL RNase (1 g/L) at 37°C for 30 min, and stained with propidium iodide (PI) at room temperature for 30 min. DNA histograms were assayed by flow cytometry (EPICS-ELITE-ESP). In each sample, a minimum of 11 000 cells was counted and stored in list mode. Data analysis was performed using standard Cell Quest software (Becton-Dickinson).
Semi-quantitative reverse transcription-PCR analysis
The sequences of PCR primers used for VEGF amplification are 5′-TCCGGGCCTCCG AAACC-3' and 5′-CCTGGTGAGATCTGG-3′. The predicted size of PCR products was 421 bp. Glyceraldehyde-3-phosphate -dehydrogenase (GAPDH) served as a positive control (Terada et al, 1992). The sequences of PCR primers used for GAPDH amplification are 5′-ACTGC CACCCAGAAGACT-3′ and 5'-GCTCAGTGTAGCCCAGGAT-3′. The predominant cDNA amplification product was 292 bp in length.
Cells (1.0 × 105/L cells) were suspended in RPMI medium 1640, plated onto 6-well culture plates, and incubated with a humidified incubator containing 50 mL/L CO2 for 24 h at 37°C. After transfected with siRNAs for 24 h, cells were collected and total RNA was extracted.
Thirty cycles of PCR were performed at 94°C for 30 s, at 53°C for 60 s, at 72°C for 60 s, and a final extension at 72°C for 5 min. After electrophoresis and ethidium bromide staining, DNA bands were visualized with an ultraviolet transilluminator. The results were scanned into computer to measure DNA band intensities by Tianneng Technology Company.
Concentration of VEGF in cell culture
After gene transferring for 24 h, supernatant was collected. The concentration of VEGF in cell culture supernatants was determined using sandwich enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions.
Statistical analysis
Results were presented as mean ± SD. Data were analyzed using analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
RESULTS
Inhibition of proliferation of SGC-7901 cells
The OD value of MTT reduction assay showed that siRNA1 and siRNA 2 markedly inhibited the growth of SGC-7901 cells while the scrambled siRNA showed no inhibitory effects on the growth of SGC-7901 cells. Compared with the untransfected group, there was a significant difference between siRNA1 and siRNA2 groups (Figure 1). The scrambled siRNA or lipofectin group (no siRNAs) had no statistical significance compared with untreated group (no siRNAs and no lipofectin)(P > 0.05, Table 2).
Figure 1.
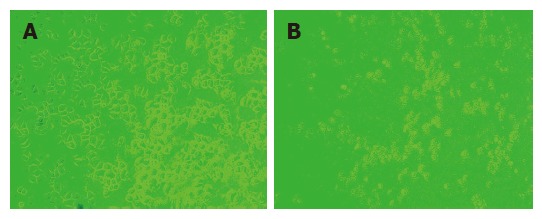
Growth of SGC-7901 cells after transfected by siRNA for 24 h in lipofectin group (A) and siRNA2 group (B).
Table 2.
Data of MTT assay after transfected by siRNA for 24 h (mean ± SD, n = 5)
| Grouping | Absorption | Inhibitory rate (%) |
| Untreaded group | 0.444 ± 0.027 | - |
| Lipofectin group | 0.440 ± 0.029 | 0.9 |
| siRNASCR group | 0.438 ± 0.023 | 1.3 |
| siRNA1-100 nm group | 0.377 ± 0.024b | 15.1 |
| siRNA1-200 nm group | 0.279 ± 0.023d | 37.2 |
| siRNA1-400 nm group | 0.383 ± 0.022b | 13.7 |
| siRNA1-1000 nm group | 0.395 ± 0.040 | 11.0 |
| siRNA2-100 nm group | 0.379 ± 0.031b | 14.6 |
| siRNA2-100 nm group | 0.278 ± 0.025d | 37.3 |
| siRNA2-100 nm group | 0.383 ± 0.026b | 13.7 |
| siRNA2-100 nm group | 0.394 ± 0.033 | 11.3 |
P < 0.01,
P < 0.001 vs the lipofectin group.
Influence of cell cycle
The change of cell cycle is listed in Table 3. Compared with the untreated group, the percentage of cells increased in the G0/G1 phase and decreased in the S and G2/M phase in siRN-treated group. The scrambled siRNA group and lipofection group (no siRNAs) had no significant change compared with the untreated group, suggesting that siRNA inhibited the growth of SGC-7901 cells.
Table 3.
Change of cell cycle measured by flow cytometry
| Group | G0/G1 phase (%) | S phase (%) | G2/M phase (%) |
| Untreaded group | 58.37 | 39.52 | 2.11 |
| Lipofectin group | 58.76 | 40.18 | 1.06 |
| siRNASCR group | 58.23 | 41.34 | 0.43 |
| siRNA1 group | 75.04b | 17.82b | 7.14 |
| siRNA2 group | 76.52b | 16.73b | 6.75 |
P < 0.01 vs untreated group.
Expresson of VEGF mRNA after tranfected with siRNA
RT-PCR data showed that VEGF mRNA expression was markedly down-regulated in SGC-7901 cells transfected with siRNA1 and siRNA2 for 24 h, while the scrambled siRNA and lipofectin showed no effects on the VEGF mRNA expression in SGC-7901 cells (Table 4) .
Table 4.
Data of absorbance (AVEGF/AGAPDH) (mean ± SD, n = 3)
| Group | AVEGF/AGAPDH |
| Normal control group | 0.856 ± 0.009 |
| Lipofectin group | 0.834 ± 0.020 |
| siRNASCR group | 0.815 ± 0.017 |
| siRNA1 group | 0.456 ± 0.030b |
| siRNA2 group | 0.468 ± 0.019b |
P < 0.01 vs lipofectin group.
Change of VEGF protein in supernatant
The ELISA data showed that secretion of VEGF protein in the supernatant of SGC-7901 cells transfected with siRNA1 and siRNA 2 for 24 h was significantly lower than that of untransfected cells, while the scrambled siRNA showed no such effects (Table 5) .
Table 5.
Change of VEGF protein in supernatant (mean ± SD, n = 3)
| Group | VEGF (p/ng per L) | Inhibition efficiency (%) |
| Normal control group | 414.0 ± 61.5 | - |
| Lipofectin group | 366.3 ± 25.5 | - |
| siRNASCR group | 350.0 ± 25.7 | 4.46 |
| siRNA1 group | 164.6 ± 22.7b | 55.1 |
| siRNA2 group | 166.3 ± 26.6b | 54.6 |
P < 0.01 vs lipofectin group; Inhibition efficiency = (lipofectin group- siRNA group)/lipofectin group.
DISCUSSION
Gastric adenocarcinoma remains the second common cause of cancer-related death in the world[13]. Although surgery can cure it in some patients, the majority of the patients lose their surgical chance when they visit hospitals. With the rapid development of molecular biology, potential treatment methods for gastric cancer are available[14]. Solid tumors need adequate blood supply to grow and metastasize. VEGF has therefore been extensively studied in many types of tumor (Please provide the references for the studies ).
VEGF has been shown to be a critical angiogenic cytokine involved in blood supply for different tumors[15]. VEGF is a highly potent angiogenic agent that increases vessel permeability and endothelial cell growth, proliferation, migration and differentiation. In addition, VEGF induces vasodilation and increases permeability of vascular beds[12]. VEGF, also known as VEGF-A, belongs to a gene family consisting of placental growth factor (PlGF), VEGF-B, VEGF-C, VEGF-D and VEGF-E. VEGF gene splicing generates four main VEGF isoforms that differ in their molecular mass and biological activities. In humans, it corresponds to isoforms VEGF121, VEGF165, VEGF189 and VEGF206[12,16]. Secreted VEGF121can freely diffuse in extracellular matrix without binding to heparin or extracellular matrix. Secreted VEGF165 can also bind to cell surface heparin and extracellular matrix. VEGF189 and VEGF206 have a higher affinity to heparin and heparin sulphate than VEGF165. The secreted forms of VEGF induce proliferation of endothelial cells, in vivo angiogenesis and expression in carcinomas including gastric adenocarcinoma. VEGF165 is one of the most common VEGF isoforms and plays an important role in angiogenesis[17,18]. RNAi technology is a powerful reverse genetic tool that has been widely employed to silence gene expression in mammalian and human cells. RNAi-based gene therapy has become a promising therapy for cancer[19,20]. This therapy targeting the molecular mechanism of angiogenesis, especially those interacting with VEGF and its receptors or antibody against VEGF and its receptors may prove useful in the management of cancer[21,5].
By using molecular biology technology, we obtained two kinds of siRNA by in vitro transcription, and then transfected them into SGC-7901 cells with lipofectin to inhibit the expression of VEGF. The results showed that siRNAs could significantly inhibit the expression of VEGF mRNA and the seceretion of VEGF protein. At the same time, the growth of SGC-7901 cells was markedly inhibited, indicating that VEGF is an effective target site in the gene therapy for gastric adenocarcinoma. The two kinds of siRNA obtained by in vitro transcription had a high purity and could be used to transtfect cultured cells. Cationic liposome is a potential candidate for gene delivery. Formation of RNA-cationic liposome complex and its adherence to the surface of cells may be the mechanism of gene transfection[22].
In conclusion, RNAi gene therapy is effective against gastric adenocarcinoma. The accessory genes in cancers take part in cell growth, proliferation and migration. Silencing these genes is a good strategy for the treatment of cancers. RNAi inhibits the expression of VEGF in human gastric cancer cells[23]. Further study is required before VEGF technology can be used in treatment of gastric adenocarcinoma.
COMMENTS
Background
Gastric cancer is the most common cause of cancer-related death in China. Since there is no satisfactory therapy for it, we studied the effects of VEGF siRNA on proliferation of gastric cancer cells in vitro.
Research frontiers
Tumor needs adequate blood supply to grow and metastasize. VEGF has been shown to be a critical angiogenic cytokine in different tumors including gastric cancer. Secreted VEGF165 induces proliferation of endothelial cells and in vivo angiogenesis in gastric adenocarcinoma and plays an important role in angiogenesis. RNAi technology is a powerful reverse genetic tool that has been widely used to silence gene expression in mammalian and human cells. RNAi-based gene therapy has become one of the promising therapies.
Innovations and breakthroughs
Studies showed that therapies targeting VEGF may be useful in the management of cancer. We obtained two kinds of siRNAs by in vitro transcription, and then transfected them into human gastric cancer cells (SGC-7901) with lipofectin to inhibit the expression of VEGF. The results indicate that VEGF is an effective target site in gene therapy for astric adenocarcinoma. RNAi can inhibit the expression of VEGF in human gastric cancer cells, thus providing a chance to develop more effective therapies for gastric adenocarcinoma.
Applications
siRNA targeting VEGF can inhibit proliferation of gastric cancer cells, which may used as a new strategy against gastric cancer.
Peer review
This paper describes that siRNA targeting human VEGF can effectively inhibit proliferation of gastric cancer cells. In this study, the authors investigated the effects of VEGF siRNA on cell proliferation of gastric cancer cells in vitro. VEGF expression was suppressed by RNAi, suggesting that our method might be effective in the treatment of cancer. Further study is required before VEGF technology can be used to in the treatment of gastric adenocarcinoma.
Footnotes
Supported by Science Project of Qingdao Science and Technology Administration, No. 03-1-YN-14
S- Editor Liu Y L- Editor Wang XL E- Editor Chen GJ
References
- 1.Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–220. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- 2.O'Byrne KJ, Koukourakis MI, Giatromanolaki A, Cox G, Turley H, Steward WP, Gatter K, Harris AL. Vascular endothelial growth factor, platelet-derived endothelial cell growth factor and angiogenesis in non-small-cell lung cancer. Br J Cancer. 2000;82:1427–1432. doi: 10.1054/bjoc.1999.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giatromanolaki A, Koukourakis MI, Sivridis E, O'Byrne K, Gatter KC, Harris AL. 'Invading edge vs. inner' (edvin) patterns of vascularization: an interplay between angiogenic and vascular survival factors defines the clinical behaviour of non-small cell lung cancer. J Pathol. 2000;192:140–149. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH693>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Fire A. RNA-triggered gene silencing. Trends Genet. 1999;15:358–363. doi: 10.1016/s0168-9525(99)01818-1. [DOI] [PubMed] [Google Scholar]
- 5.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 6.Yang G, Cao GJ, Li J, etc Inhibition of SARS-CoV Infection in Vero-E6 Cells by siRNAs. Journal of Medical Molecular Biology. 2004;1:270–273. [Google Scholar]
- 7.Li SB, Wu QM, Wang Q. Effects of adenovirus-mediated human cox-2 antisense RNA on synthesis of DNA and proteins in esophgeal carcinoma cell line. World J Gastroenterol. 2003;11:517–521. [Google Scholar]
- 8.Li GF, Xie SB, Sun H, Yang XH, Liu WJ, Zhai Q, Zhou YX, Li ZH, Zhang GM. An investigation of intra-arterial chemotherapy infusion and embolization combined with abdominal chemotherapy for advanced gastric cancer. World J Gastroenterol. 1998;4(Suppl 2):71–73. [Google Scholar]
- 9.Popiela T, Kulig J, Kolodziejczyk P, Sierzega M. Long-term results of surgery for early gastric cancer. Br J Surg. 2002;89:1035–1042. doi: 10.1046/j.1365-2168.2002.02156.x. [DOI] [PubMed] [Google Scholar]
- 10.Forman D, Morris E, Eastwood A, Kleijnen J. Guidelines for treatment of upper gastrointestinal cancer. Lancet. 2003;361:80. doi: 10.1016/S0140-6736(03)12141-1. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama M. Treatment results of gastric cancer staged by the TNM classification. In: Maruyama M, Kimura K, editors. Review of clinical research in gastroenterology. Tokyo: Igaku-Shoin; 1998. p. 112. [Google Scholar]
- 12.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 13.El-Rifai W, Powell SM. Molecular biology of gastric cancer. Semin Radiat Oncol. 2002;12:128–140. doi: 10.1053/srao.2002.30815. [DOI] [PubMed] [Google Scholar]
- 14.Waters JS, Ross PJ, Popescu RA, Cunningham D. New approaches to the treatment of gastro-intestinal cancer. Digestion. 1997;58:508–519. doi: 10.1159/000201494. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 16.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 17.Köllermann J, Helpap B. Expression of vascular endothelial growth factor (VEGF) and VEGF receptor Flk-1 in benign, premalignant, and malignant prostate tissue. Am J Clin Pathol. 2001;116:115–121. doi: 10.1309/1LBM-6X32-JH6W-ENUD. [DOI] [PubMed] [Google Scholar]
- 18.Ma ZL, Deng H. Research development of VEGF and its effect on angiogenesis in tumour. Jiangsu Yixue Zazhi. 2004;30:50–51. [Google Scholar]
- 19.Wu YM, Chen SM. Recent Advances in RNA Interference. Zhongguo Shengwuhuaxue yr Fenzishengwu Xuebao. 2003;19:411–417. [Google Scholar]
- 20.Yang J. Advance of anti-sense RNA technology and tumor therapy. Foreign Med Sci Cancer Sec. 2002;29:83–86. [Google Scholar]
- 21.Yang JC. Bevacizumab for patients with metastatic renal cancer: an update. Clin Cancer Res. 2004;10:6367S–6370S. doi: 10.1158/1078-0432.CCR-050006. [DOI] [PubMed] [Google Scholar]
- 22.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 23.Dave RS, Pomerantz RJ. RNA interference: on the road to an alternate therapeutic strategy! Rev Med Virol. 2003;13:373–385. doi: 10.1002/rmv.407. [DOI] [PubMed] [Google Scholar]


