Abstract
AIM: To investigate the role of major non-protein and protein sulfhydryls and disulfides in chemically induced gastric hemorrhagic mucosal lesions (HML) and the mechanism of gastroprotective effect of sucralfate.
METHODS: Rats were given 1 mL of 75% ethanol, 25% NaCl, 0.6 mol/L HCl, 0.2 mol/L NaOH or 1% ammonia solutions intragastrically (i.g.) and sacrificed 1, 3, 6 or 12 min later. Total (reduced and oxidized) glutathione (GSH + GSSG), glutathione disulfide (GSSG), protein free sulfhydryls (PSH), protein-glutathione mixed disulfides (PSSG) and protein cystine disulfides (PSSP) were measured in gastric mucosa and liver.
RESULTS: Reduced glutathione (GSH) was depleted in the gastric mucosa after ethanol, HCl or NaCl exposure, while oxidized glutathione (GSSG) concentrations increased, except by HCl and NaOH exposure. Decreased levels of PSH after exposure to ethanol were observed, NaCl or NaOH while the total protein disulfides were increased. Ratios of reduced to oxidized glutathione or sulfhydrils to disulfides were decreased by all chemicals. No changes in thiol homeostasis were detected in the liver after i.g. abbreviation should be spelled out the first time here administration of ethanol. Sucralfate increased the concentrations of GSH and PSH and prevented the ethanol-induced changes in gastric mucosal thiol concentrations.
CONCLUSION: Our modified methods are now suitable for direct measurements of major protein and non-protein thiols/disulfides in the gastric mucosa or liver. A common element in the pathogenesis of chemically induced HML and in the mechanism of gastroprotective drugs seems to be the decreased ratios of reduced and oxidized glutathione as well as protein sulfhydryls and disulfides.
Keywords: Non-protein and protein thiol, Gastric mucosal injury, Gastroprotection, Sucralfate
INTRODUCTION
The biochemical basis of chemically induced acute gastroduodenal hemorrhagic mucosal lesions (HML) and protection remains unclarified. Injury to gastric mucosa and isolated gastric mucosal cells by damaging chemicals or H pylori involves a complex sequence of biochemical and morphological events, e.g., activation of oxidative stress pathways, diminished prostaglandin (PG) and GSH metabolisms and early microvascular injury[1-10].
In addition to the PG-dependent mucosal protec-tion[11,12] increasing evidence indicates that endogenous sulfhydryls (SH) play an important role in the maintenance of gastroduodenal integrity and in the protection against chemically-induced lesions in cells, tissues and organs[13-15]. The relatively high concentration of non-protein sulfhy-dryls (NPSH) which is mostly reduced glutathione (GSH, γ-glutamyl-cysteinyl glycine) besides cysteine (CSH), coenzyme A and other thiols in the gastric mucosa also indicates their possible implications for gastroprotection[16].
The direct organo- or cytoprotective effects of reduced GSH, N-acetylcysteine (NAC) or other SH compounds, (e.g., taurin) were demonstrated biochemically and pharmacologically in vivo against concentrated ethanol[2,13,16-20], non-steroidal anti-inflammatory drugs (NSAID)[1,21,22] and acrylonitrile[23] as well as in vitro with cultured or isolated gastric mucosal cells against ethanol[24-27] or HCl[28].
Rapid activation and release of SH protease cathepsin B was detected after ethanol exposure in the rat stomach. Both the increase of protease activity and development of acute gastric mucosal lesions were prevented by sulfhydryl alkylators iodoacetate, iodoacetamide and N-ethylmaleimide (NEM)[29,30]. In addition, inactivation of endogenous protease inhibitors and activation of catepsins B, L and H were demonstrated after ammonia-solution and ethanol exposures in the stomach. It has been concluded that various cysteine proteases and their endogenous inhibitors may have a role in the pathogenesis of chemically induced gastric mucosal lesion and protection[30-32].
Other studies also indicate that changes in PSH, PSSG and PSSP are also involved in both mechanisms of tissue damage and defense[33,34].
Various chemicals such as ethanol (50% or 75%), NaCl (25%), HCl (0.6 N), NaOH (0.2 N) or ammonia-solution (1%) provoke acute HML in minutes[3,5,11,31]. Since previous biochemical and pathophysiologic studies with endogenous SH compounds involved only ethanol and NSAID, we tested the hypothesis that changes in PSH, NPSH and disulfides (especially depletion of GSH in the rat gastric mucosa) might represent common pathogenic factors in the mechanisms of mucosal injury induced by endogenous chemicals such as HCl or ammonia (a product of H pylori), as well as exogenous NaOH, ethanol and hypertonic NaCl. In addition, we also investigated the possibility, whether non-protein and protein sulfhydryls might be protective mechanisms by which gastroprotective drugs such as sucralfate overcome these damaging substances.
MATERIALS AND METHODS
Animals
Female Sprague-Dawley (S-D) rats (Taconic Farms, Germantown, NY), weighing 160-200 g were fasted for 24 h before experiment, but water was available ad libidum. Groups of rats (n = 4-6) were given 1 mL of deionized water (in controls) or 1 mL of 75% (v/v) ethanol, 0.6 mol/L HCl, 0.2 mol/L NaOH, 25% (w/v) NaCl and 1% (v/v) ammonia water solution intragastrically (i.g.) by using gavage with a rubber stomach tube (Rusch No. 8). Rats were killed by cervical dislocation 1, 3, 6 or 12 min later. The abdomen was opened and the stomach was rapidly removed, opened along the greater curvature, rinsed in cold saline and blotted. A small piece of liver was also removed. The mucosa of the glandular stomach was scraped off with a blunt knife, weighed, then put in 5% (w/v) of ice-cold trichloroacetic acid (TCA) solution prepared with 0.01 mol/L HCl containing 5 mmol/L EDTA making a 1:20 w/v dilution. The liver samples were also rapidly weighed and put in TCA solution which was kept on ice.
Chemicals
N-Ethylmaleimide (NEM), 5,5’-dithio-bis(nitrobenzoic acid) (DTNB), glutathione, reduced form, crystalline (GSH), glutathione, oxidized form (GSSG), L-cysteine HCl (CSH), glutathione reductase (GSSGR) (EC 1.6.4.2) type III from baker’s yeast (155 units/mg protein), nicotinamide adenine dinucleotide phosphate, reduced form (NADPH), guanidine HCl, bovine serum albumin (BSA), n-octyl alcohol, sodium borohydride (NaBH4), Trizma base, sodium phosphate dibasic and sodium hydroxide, were purchased from Sigma Chemical, (St. Louis, MO). Ammonium hydroxide (ammonia water), hydrochloric acid, ethylenediamine-tetraacetate (EDTA) disodium, ethyl ether, sodium chloride, sodium hydroxide, sodium phosphate monobasic, TCA were obtained from Fisher Scientific (Pittsburgh, PA). Ethanol was purchased from Florida Distillers (Lake Alfred, FL). Sucralfate was obtained from Marion Laboratories (Kansas City, MO).
Preparation of samples
Figure 1 shows the flow chart of all major steps of biochemical procedures. Tissue samples were homogenized in TCA (1:20, w/v) solution for 60 s in plastic conic tubes using an ultra turrax homogenizer (Tissumizer, Tekmar Co., Cincinnati, OH) driven at 50 000 r/min for 10 s three times. The homogenates were kept on ice and centrifuged at 4°C, 6000 × g for 15 min. Supernatants and pellets were separated for further preparation and biochemical assays of NPSH and protein SH fractions.
Figure 1.
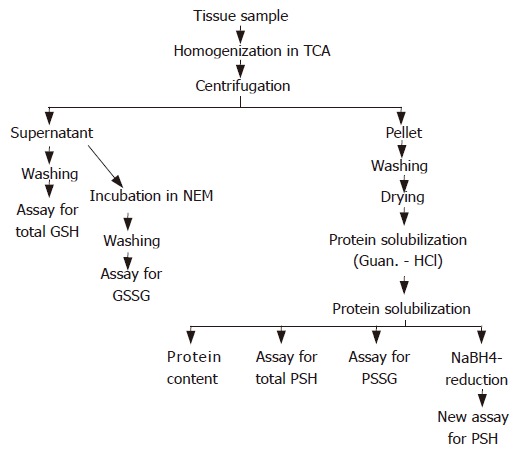
Flow diagram of sample preparation and measurement of thiols in the rat gastric mucosa and liver.
The supernatants were washed 5 times with ethyl ether (1:2, v/v) at 0°C. With this procedure triglycerides and TCA could be removed and the remaining 0.01 mol/L HCl could maintain slight acidity of solution to minimize the spontaneous oxidation of GSH. One portion of diluted (1:10-100, v/v) supernatants with 0.1 mol/L phosphate buffer saline (PBS, pH 7.4) at 0°C without further treatment was used for enzymatic and spectrophotometric measurement of the total glutathione (GSH + GSSG) levels.
Another 0.5-1.0 mL portion of the original supernatant was incubated (1:1, v/v) with 0.05 mol/L NEM in 0.1 mol/L PBS containing 5 mmol/L EDTA (pH 7.4) for 1 h at room temperature. Using this procedure the tissue concentration of GSSG could be determined following preliminary reaction of reduced GSH with excess of NEM. The rapid and complete reaction between GSH and the SH alkylator NEM prevents the participation of reduced GSH in the enzymatic assay as well as its further spontaneous oxidation to GSSG[35,36]. After removal of non-reactive NEM by 10 extractions with cold ether (1:3, v/v) the GSSG could be measured enzymatically with GSSGR with or without further dilution.
The protein pellets were vigorously washed with 3.0 mL of ice-cold 5% (w/v) TCA containing 5 mmol/L EDTA in 0.01 mol/L HCl, centrifuged at 4000 × g for 15 min then washed 3 times again in 3.0 mL of ethyl ether. The vacuum-dried pellets were suspended for protein denaturation in 2.0 mL of 6.0 mol/L guanidine HCl plus 1.0 mL of 0.1 mol/L PBS, pH 7.4, containing 5 mmol/L EDTA with vigorous agitation for 10 min producing an almost complete dissolution of pellet. The clear supernatant (i.e., “protein solution”) was separated by centrifugation at 4000 × g for 20 min for further preparation of samples or for biochemical assays.
Detection of protein disulfide groups (PSSP) was based on the reduction of disulfide bridges with 0.1 mol/L sodium borohydride (NaBH4) as reducing agent, and DTNB (Ellman’s reagent) as thiol disulfide exchanger on previously denaturated protein samples[37,38]: 200 μL of 0.1 mol/L NaBH4 in 0.1 mol/L NaOH containing 0.02% Na2EDTA and a drop of octyl alcohol as an antifoaming agent was added to an 50 μL aliquot of diluted (50%) “protein solution”. The reduction was performed for 1.5 h at 37°C in a shaking water bath. The excess of sodium borohydride was removed by adding 30 μL of 6 mol/L HCl. Since DTNB color should be developed in a mild alkaline environment, the pH of the reaction mixture was brought to about pH 8 with 400 μL of 2.0 mol/L Tris-HCl buffer, pH 8.3, containing 0.02% EDTA.
Enzymatic assays of both the total glutathione and oxidi-zed glutathione
This sensitive spectrophotometric measurement of SH groups was based on formation of color product resulting from reaction of DTNB and GSH[39,40]. The components, dissolved in 0.1 mol/L PBS (pH 7.4) containing 5 mmol/L EDTA were added in the following amounts: 100 μL of original or NEM-treated supernatant (containing 0.05-0.5 mg tissue) mixed with 600 μL of PBS (0.1 mol/L, pH 7.4), 100 μL of 6 mmol/L Ellman’s reagent (0.6 μmol) and 100 μL of GSSGR solution (10 μg). The reaction was initiated with 100 μL of 2 mmol/L NADPH (0.2 μmol). The mixture (1.0 mL) was incubated while shaking at 37°C for exactly 6 min, and the absorption at 412 nm was measured 10 min later against water[41] using a cuvette with 1 cm light path (standard assay for all GSH-containing disulfides).
The difference between the original and NEM-treated supernatants indicates the level of GSSG.
Measurement of free protein SH
One hundred μL of diluted (50%) “protein solution” was mixed with 800 μL of PBS (0.1 mol/L, pH 7.4) containing 5 mmol/L EDTA and 100 μL of 6 mmol/L DTNB solution. Absorbance at 412 nm was measured spectrophotometrically 10 min later.
Determination of protein-GSH mixed disulfides
The diluted (50%) “protein solution”, 100 μL, 10 μg GSSGR in 100 μL PBS, 600 μL of PBS (0.1 mol/L, pH 7.4, containing 5 mmol/L EDTA), 100 μL of 6 mmol/L DTNB and 0.2 μmol NADPH in 100 μL PBS were mixed and kept at 37°C in shaking water bath for exactly 6 min. Absorption at 412 nm was read immediately afterwards.
Measurement of protein cystine disulfides
The half cystine residues of proteins were quantified using Ellman’s reagent after guanidine-induced protein denaturation plus sodium borohydride reduction of cystine disulfide bonds, using untreated “protein solution” samples as control as described earlier in this paper.
Assay of protein concentration
Protein concentration was determined using the Coomassie blue (BIO-RAD) method[42] after 50% dilution of the “protein solution” sample. Absorption at 595 nm was measured with samples, blanks and known concentrations of bovine serum albumin.
Investigations with sucralfate
The experiments with gastroprotective doses of sucralfate were carried out in additional groups of fasted rats which were given 1 mL of 75% ethanol by gavage and were killed sacrificed 12 min later. Two of the groups received sucralfate at 10 or 50 mg/100g i.g. alone or (at 50 mg/100 g) 30 min before the administration of ethanol. The concentrations of all five types of non-protein and protein thiols in homogenates of mucosal scrapings of glandular stomach were measured immediately after autopsy as described above.
Data analysis and statistical evaluation
The nmol GSH/mg wet tissue and nmol GSSG/mg wet tissue were calculated from freshly prepared GSH dose response curves (5-100 ng). The dose curves of GSH and GSSG following reduction were identical. Protein SH data were expressed as nmol cysteine/mg protein corrected for the background absorbance measured in samples without protein solution. Protein disulfides were expressed as nmol cysteine (half cystine)/mg protein after reducing disulfides with sodium borohydride. Protein-GSH mixed disulfides were expressed as nmol GSSG equivalent/mg protein calculated from a GSSG (or GSH) dose curves prepared in the standard assay.
All experiments were carried out 3 times, the bio-chemical measurements were performed in duplicates and the results were pooled. Arithmetic means and standard errors of mean (SEM) were calculated. Statistical evaluation was performed using Mann-Whitney U-test, ANOVA and two-tailed Student’s t-test for unpaired comparisons. The level of significance was P < 0.05. All experiments were carried out according to the protocol approved by the Ethics Committee.
RESULTS
The total glutathione (GSH + GSSG) concentration in the glandular stomach mucosa of control rats was 2.3-3.0 nmol GSH/mg tissue measured by the enzymatic method. In these experiments the GSH level in normal rat liver was slightly higher (3.8 + 0.5 nmol GSH/mg tissue) than in the gastric mucosa (Table 1).
Table 1.
Total glutathione (GSH + GSSG) in the mucosa of rat glandular stomach and liver following i.g. administration of gastrotoxic chemicals
| 0 min | 1 min | 3 min | 6 min | 12 min | |
| Stomach | |||||
| Ethanol | 2.6 ± 0.3 | 1.7 ± 0.5 | 1.6 ± 0.3 | 1.4 ± 0.3a | 1.2 ± 0.2b |
| HCl | 2.3 ± 0.7 | 1.5 ± 0.6 | 0.9 ± 0.4 | 0.9 ± 0.3 | 0.5 ± 0.2a |
| NaOH | 2.6 ± 0.5 | 2.4 ± 0.4 | 1.9 ± 0.6 | 1.9 ± 0.6 | 1.9 ± 0.5 |
| NaCl | 2.9 ± 0.6 | 2.5 ± 0.5 | 2.0 ± 0.2 | 1.8 ± 0.1 | 1.5 ± 0.3a |
| Ammonia | 3.0 ± 0.7 | 2.6 ± 0.5 | 2.3 ± 0.4 | 2.8 ± 0.4 | 2.9 ± 0.7 |
| Liver | |||||
| Ethanol | 3.8 ± 0.5 | 3.9 ± 1.0 | 4.1 ± 0.6 | 4.3 ± 0.8 | 4.2 ± 0.5 |
The damaging agent, 1 mL of 75% ethanol, 0.6 mol/L HCl, 0.2 mol/L NaOH, 25% NaCl or 1% ammonia-water was given by gavage to fasted S-D rats (n = 4-6), and the animals were killed subsequently at time-points indicated. Data are expressed as mean ± SEM of nmol GSH mg wet tissue.
P < 0.05,
P < 0.01, vs corresponding control values (0 min).
Very rapid and time-dependent depletion-which reached statistical significance within 6-12 min - were found in total GSH concentrations in the gastric mucosa after i.g. administration of ethanol, HCl or hypertonic NaCl solution. In contrast, the diminished GSH levels in the gastric mucosa did not reach the level of statistical significance following 0.2 mol/L NaOH or 1% ammonia solution. Similarly, no significant alterations were found in GSH concentrations in the liver at any time after i.g. injection of ethanol (Table 1).
GSSG represents 0.7%-4.0% of total GSH pools. The concentrations of GSSG were significantly and time-dependently increased in the glandular stomach from 0.7%-1.4% to 3.2%-4.0% after ethanol, ammonia water or hypertonic NaCl treatments. However, these parameters did not increase markedly after exposure to HCl or NaOH solutions. Ethanol did not substantially modify glutathione disulfide levels in the rat liver at any time during the 12 min period studied, and the proportion of GSSG remained below 1% (Table 2).
Table 2.
Oxidized glutathione (GSSG) in the mucosa of rat glandular stomach and liver following i.g. administration of gastrotoxic chemicals
| 0 min | 1 min | 3 min | 6 min | 12 min | |
| Stomach | |||||
| Ethanol | 20.0 ± 3.0 | 17.0 ± 3.5 | 26.0 ± 4.0 | 32.0 ± 5.1 | 39.0 ± 2.1b |
| HCl | 19.0 ± 6.0 | 18.0 ± 4.0 | 18.0 ± 2.8 | 22.0 ± 4.8 | 20.0 ± 4.5 |
| NaOH | 20.0 ± 2.0 | 25.0 ± 2.1 | 26.0 ± 3.7 | 25.0 ± 4.6 | 24.0 ± 3.1 |
| NaCl | 27.0 ± 1.9 | 25.0 ± 2.1 | 35.0 ± 5.7 | 35.7 ± 6.9 | 58.0 ± 8.9b |
| Ammonia | 28.1 ± 8.9 | 34.7 ± 7.1 | 57.0 ± 9.9 | 63.0 ± 10.3a | 60.0 ± 9.7a |
| Liver | |||||
| Ethanol | 74.0 ± 13.1 | 81.4 ± 15.2 | 64.0 ± 11.3 | 58.9 ± 10.8 | 66.6 ± 13.3 |
The damaging agent, 1 mL of 75% ethanol, 0.6 mol/L HCl, 0.2 mol/L NaOH, 25% NaCl or 1% ammonia-water was given by gavage to fasted S-D rats (n = 4-6), and the animals were killed subsequently at time-points indicated. Data are expressed as mean ± SEM of pmol GSSG in 1 mg wet tissue.
P < 0.05,
P <0.01, vs corresponding control values (0 min).
The moderate or marked changes in GSH and GSSG levels resulted in significant decreases of the GSH/GSSG ratios after the administration of all gastrotoxic chemicals used. However, lower ratio values were found in the gastric mucosal thiol concentrations following ethanol, HCl or NaCl than after ammonia or NaOH administration. The relatively small changes in GSH or GSSG levels in the mucosa after ammonia or NaOH exposures resulted in statistically significant decreases in the GSH/GSSG ratios (Figure 2).
Figure 2.
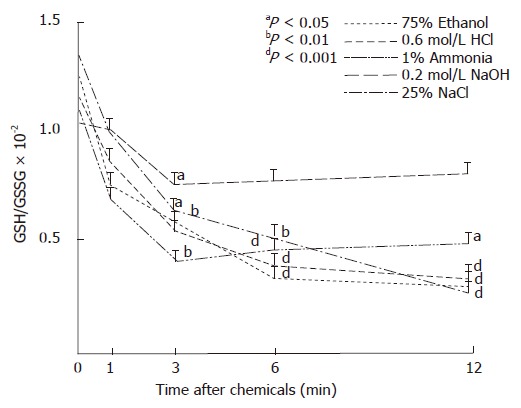
Ratios of reduced glutathione (GSH) and glutathione disulfide (GSSG) in mucosa of glandular stomach after i.g. administration of gastrotoxic chemicals in rats.
The concentrations of PSH in the gastric mucosa decreased rapidly and significantly following exposure to ethanol, nevertheless, were also diminished after NaOH or hypertonic NaCl. These values remained unchanged in the liver samples following ethanol and in the gastric mucosa after exposure to ammonia or HCl solutions (Table 3).
Table 3.
Protein SH (PSH) in the mucosa of rat glandular stomach and liver following i.g. administration of gastrotoxic chemicals
| 0 min | 1 min | 3 min | 6 min | 12 min | |
| Stomach | |||||
| Ethanol | 32.9 ± 1.2 | 29.6 ± 1.9 | 22.4 ± 1.1b | 15.9 ± 1.3d | 13.7 ± 1.0b |
| HCl | 34.2 ± 3.0 | 39.8 ± 2.1 | 38.7 ± 1.3 | 35.7 ± 3.3 | 31.7 ± 4.2 |
| NaOH | 26.9 ± 1.0 | 22.3 ± 2.1 | 13.0 ± 4.1b | 11.8 ± 3.5b | 14.0 ± 4.9a |
| NaCl | 26.7 ± 3.6 | 26.5 ± 2.1 | 23.1 ± 6.9 | 13.0 ± 6.7 | 15.1 ± 2.1a |
| Ammonia | 29.8 ± 2.9 | 22.6 ± 2.0 | 26.6 ± 3.9 | 30.9 ± 2.1 | 26.3 ± 4.9 |
| Liver | |||||
| Ethanol | 55.4 ± 4.8 | 51.2 ± 2.4 | 53.0 ± 1.8 | 54.3 ± 4.1 | 50.6 ± 4.5 |
The damaging agent, 1 mL of 75% ethanol, 0.6 mol/L HCl, 0.2 mol/L NaOH, 25% NaCl or 1% ammonia-water was given by gavage to fasted S-D rats (n = 4-6), and the animals were killed subsequently at time-points indicated. Data are expressed as mean ± SEM of nmol cysteine in 1 mg protein.
P < 0.05,
P < 0.01,
P < 0.001, vs corresponding control values (0 min).
Table 4 shows the concentrations of PSSG in protein samples of the gastric mucosa and liver of control and treated rats. The concentration of PSSG was higher in the liver at all the times examined than in the gastric mucosa of control rats or during the development of gastric mucosal damage. Furthermore, time-dependent and significant increments of these mixed disulfides were found in the mucosa after administration of ethanol or HCl. Since these proteins contained moderately (e.g. HCl) or markedly (e.g. ethanol, NaOH, NaCl exposures) less free SH groups than those obtained from control groups, the ratios of PSH to PSSG groups were rapidly and significantly decreased by the gastrotoxic chemicals used, except by ammonia solution (Figure 3).
Table 4.
Protein-GSH mixed disulfides (PSSG) in rat glandular stomach mucosa and liver following various chemicals given i.g.
| 0 min | 1 min | 3 min | 6 min | 12 min | |
| Stomach | |||||
| Ethanol | 0.17 ± 0.04 | 0.28 ± 0.08 | 0.37 ± 0.03b | 0.35 ± 0.02b | 0.49 ± 0.04d |
| HCl | 0.20 ± 0.02 | 0.62 ± 0.11a | 0.83 ± 0.15a | 0.83 ± 0.16a | 0.84 ± 0.09b |
| NaOH | 0.22 ± 0.03 | 0.31 ± 0.10 | 0.36 ± 0.09 | 0.45 ± 0.12 | 0.43 ± 0.15 |
| NaCl | 0.20 ± 0.02 | 0.19 ± 0.04 | 0.17 ± 0.04 | 0.20 ± 0.01 | 0.22 ± 0.05 |
| Ammonia | 0.26 ± 0.03 | 0.25 ± 0.04 | 0.27 ± 0.04 | 0.36 ± 0.11 | 0.32 ± 0.04 |
| Liver | |||||
| Ethanol | 0.68 ± 0.03 | 0.86 ± 0.03 | 0.74 ± 0.03 | 0.64 ± 0.11 | 0.81 ± 0.10 |
The damaging agent, 1 mL of 75% ethanol, 0.6 mol/L HCl, 0.2 mol/L NaOH, 25% NaCl or 1% ammonia-water was given by gavage to fasted S-D rats (n = 4-6), and the animals were killed subsequently at time-points indicated. Data are expressed as mean ± SEM of nmol GSSG in 1 mg protein.
P < 0.05,
P <0.01,
P < 0.001, vs corresponding control values (0 min).
Figure 3.
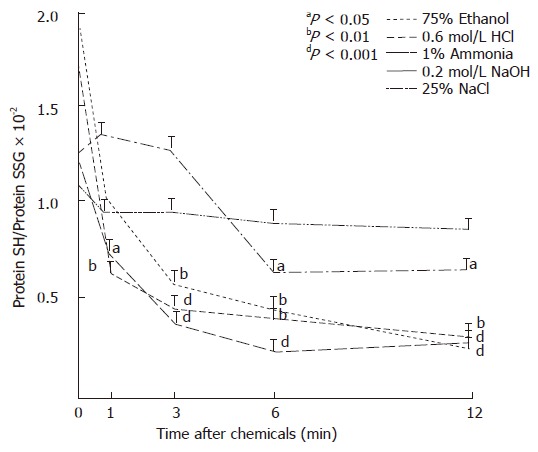
Ratios of protein SH (PSH) to protein-GSH mixed disulfides (PSSG) in the gastric mucosa after i.g. administration of gastrotoxic chemicals in rats.
The concentrations of cystine disulfides in the proteins were also significantly increased in the gastric mucosa by ethanol, HCl or NaCl measured after denaturation and borohydride reduction. No significant changes were found in the liver after ethanol or in the gastric mucosa following ammonia water or NaOH administration (Table 5).
Table 5.
Protein disulfides (PSSP) in rat glandular stomach mucosa and liver following various chemicals given i.g.
| 0 min | 1 min | 3 min | 6 min | 12 min | |
| Stomach | |||||
| Ethanol | 0.55 ± 0.08 | 0.94 ± 0.06b | 1.36 ± 0.20b | 1.72 ± 0.05d | 1.71 ± 0.13d |
| HCl | 0.54 ± 0.20 | 0.57 ± 0.04a | 0.90 ± 0.30 | 1.33 ± 0.45 | 1.69 ± 0.09b |
| NaOH | 0.44 ± 0.07 | 0.52 ± 0.17 | 0.55 ± 0.04 | 0.66 ± 0.14 | 0.59 ± 0.20 |
| NaCl | 0.57 ± 0.02 | 0.53 ± 0.02 | 1.00 ± 0.12b | 1.01 ± 0.18b | 1.35 ± 0.05d |
| Ammonia | 0.39 ± 0.07 | 0.52 ± 0.17 | 0.56 ± 0.15 | 0.78 ± 0.20 | 0.64 ± 0.15 |
| Liver | |||||
| Ethanol | 0.89 ± 0.25 | 0.75 ± 0.20 | 0.95 ± 0.30 | 0.86 ± 0.17 | 0.69 ± 0.05 |
The damaging agent, 1 mL of 75% ethanol, 0.6 mol/L HCl, 0.2 mol/L NaOH, 25% NaCl or 1% ammonia-water was given by gavage to fasted S-D rats (n = 4-6), and the animals were killed subsequently at time-points indicated. Data are expressed as mean ± SEM of nmol half cystine residue in 1 mg protein.
P < 0.01,
P < 0.001, vs corresponding control values (0 min).
Figure 4 shows the changes in the ratios of PSH to PSSP prepared from rat gastric mucosa. In contrast to moderate alterations by ammonia solution, the ratio of PSH to PSSP in the proteins rapidly decreased by all other chemicals calculated from the protein thiol data in the gastric mucosa.
Figure 4.
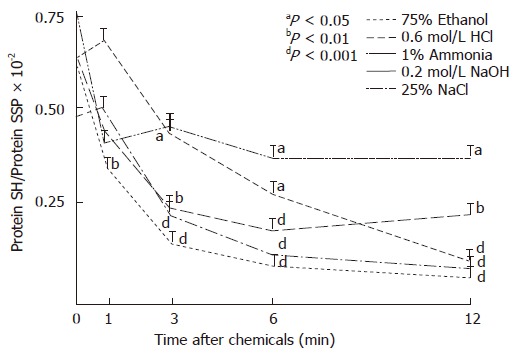
Ratios of protein SH (PSH) to protein disulfides (PSSP) in the gastric mucosa following i.g. administration of gastrotoxic chemicals in rats.
All measured alterations in PSH, NPSH and disulfides of the gastric mucosa as well as the calculated ratios are summarized in Table 6. The common elements seem to be the trend in decreased GSH and increased GSSG levels both of which reached level of statistical significance with three out of five gastrotoxic chemicals tested, very often in an overlapping manner. Similar changes were apparent with decrease in PSH and increase in PSSP, i.e., in 3/5 and 4/5 cases, respectively. Consequently, the most consistent patterns of alterations were, however, detected in the slight or marked decreases in GSH/GSSG and the PSH/PSSP ratios involving all the five tested and commonly used gastrotoxic chemicals. One should also stress that these biochemical changes preceded the full development of hemorrhagic erosions which were virtually absent at 1 min, barely detectable at 3 and 6 min, and visible at 12 min.
Table 6.
Changes in the concentrations of nonprotein and protein sulfhydryls and disulfides in the rat glandular stomach in the early phase of chemically-induced mucosal injury
| Agent | Total GSH | GSSG | GSH/GSSG | PSH | PSSG | PSSP | PSH/PSSG | SH/PSSP |
| Ethanol | DD | II | DD | DD | III | III | DD | DD |
| HCl | D | I | DD | D | No | II | No | DD |
| NaOH | DD | No | D | No | I | I | DD | DD |
| NaCl | No | No | D | D | No | I | DD | D |
| Ammonia | No | I | D | No | No | No | No | D |
Total GSH: GSH + GSSG; GSSG: glutathione disulfide; PSH: protein SH; PSSG: protein-GSH mixed disulfide; PSSP: protein disulfides; I: increase; D: decrease, Ior D: P < 0.05; II or DD: P < 0.01; III or DDD: P < 0.001; No: no significant change in comparison with corresponding controls in the 12 min experiments.
Our direct pharmacologic results demonstrate that the gastric mucosal levels of reduced GSH and the ratio of GSH/GSSG increased dose-dependently increased following sucralfate administration. In addition, the ethanol-induced decrease in GSH concentration was almost com-pletely prevented by pretreatment with gastroprotective dose (50 mg/100 g body weight) of sucralfate (Figure 5). The decrease of mucosal PSH/PSSP caused by ethanol was also abolished after pretreatment with sucralfate.
Figure 5.
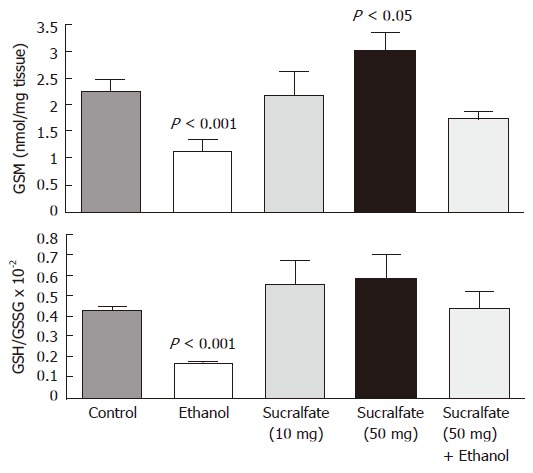
Concentration of GSH (nmol/mg tissue) and ratio of GSH/GSSG in the glandular mucosa of rat stomach after i.g. administrations of sucralfate (10 and 50 mg/100 g, b.w.), 1 mL of 75% ethanol, and sucralfate (50 mg/100 g, b.w.) plus ethanol (n = 5). Data are expressed as means SEM.
DISCUSSION
Because of previous observations with gastric mucosal thi-ols[12,13] and protein sulfhydryls (including SH-proteases)[30,31] we wanted to study the early changes in tissue levels of major sulfhydryls and disulfides in the mucosa of the glandular stomach in a time-dependent manner after the administration of various gastrotoxic chemicals, i.e., before the development of HML.
Our results of the present study revealed rapid changes in SH and disulfide concentrations in the rat gastric mucosa after exposure to damaging chemicals such as ethanol, hypertonic NaCl, HCl, NaOH or ammonia solutions. The depletion of reduced GSH and the elevation of GSSG concentration were more marked after ethanol, HCl or NaCl, than following base solutions such as NaOH and ammonia water. The ratios of reduced to oxidized glu-tathione were significantly decreased by all gastrotoxic chemicals. No changes in the thiol homeostasis were found in the liver.
Previous investigations demonstrated that concentra-ted ethanol, acid and base solutions and other chemicals may induce time-dependent severe acute mucosal le-sions in the rat stomach within minutes[2,3,5,6,31]. Differ-ent biochemical events, i.e., generation of oxygen free radicals, lipid peroxidation, impaired prostaglandin meta-bolism, endothelial injuries have been implicated in the pathogenesis of chemically-induced acute mucosal lesions or epithelial cell damages[2,3,5,7,9,11]. Lipid peroxides and peroxiradicals in the gastric mucosa can be detoxified by GSH peroxidase via formation of GSSG from reduced GSH. Cytotoxins can also be eliminated by GSH con-jugation catalyzed by glutathione S-transferase. Other findings revealed decreased GSH and increased GSSG in the gastric mucosa, thus increased oxidative stress might be one of the first mechanisms of the mucosal damage. The role of lipid peroxidation in the development of HML seems to be more limited than previously thought[2,3,43].
Several researchers found a significant decrease of reduced GSH in gastric mucosa following chemicals such as NSAID and acrylonitrile[1,13,16,21-23]. Acute depletion of GSH during gastric mucosal injury may be due to: (1) direct oxidation of GSH to GSSG; (2) formation of mixed disulfides with protein SH groups or cysteine; (3) non-enzymatic conjugation with GSH[44].
Exogenously administered SH agents such as L-cysteine, N-acetylcysteine, cystamine, cysteamine, penicillamine, GSH exert dose-dependent protection against the ethanol-induced HML in the rat stomach [1,2,13,17]. Direct cytoprotective effect of GSH was demonstrated in vitro against ethanol[25] or acid-induced[28] injury in cultured rat gastric mucosal cells. On the other hand, s.c. injection of SH alkylators such as iodoacetate or NEM 10 min before or after gastroprotective thiol or PG compounds eliminated the protective effect of SH drugs in ethanol model[45,46]. These results also show the importance of reduced SH groups in gastroprotection.
We detected significant increase of GSSG concentration in the glandular stomach after ethanol, HCl or hypertonic NaCl exposures, but this increment was minor following ammonia or NaOH solutions. The increased level of oxidized GSH is one of the elements in pathogenesis of mucosal injury. It is known that GSSG is rapidly reduced to GSH by NADPH-dependent GSSGR[47]. When the rate of GSH oxidation to GSSG exceeds the capacity of GSSGR, GSSG is actively transported out of the cell. GSSG selectively destroys cysteine protease inhibitors[48], consequently it may activate lysosomal thiol protease cathepsins B, H, L in gastric mucosal which participate in the early development of HML[29-31,45].
Thus, our present studies revealed a rapid and significant decrease in the concentration of gastric mucosal protein SH following i.g. administration of ethanol, hypertonic NaCl or NaOH. Tissue levels of PSH remained unchanged during the development of ammonia or HCl-induced gastric lesions. Furthermore, slight or profound increases of PSSG or PSSP were detected in gastric mucosa using GSSGR enzymic method or borohydride reduction of disulfide bridges in the protein samples, respectively. But ratios of protein SH and disulfides (PSSG + PSSP) were significantly decreased by all chemicals used. No changes were found in liver protein SH concentrations after i.g. administration of ethanol. Thus, protein thiol changes are also involved in the pathogenesis of chemically-induced gastric mucosal lesions in rats. What are the possible mechanisms?
Protein SH groups play a critical role in the catalytic mechanisms of numerous enzymes including metabolic enzymes such as creatine kinase and glyceraldehyde-3-phosphate dehydrogenase. A large group of these enzymes play an important role in cell metabolism or membrane transport. PSH has been implicated in the maintenance of plasma membrane integrity and ion fluxes, of mitochondrial permeability[49], of membrane-bound ATP-ase[50], and of various receptors and G protein which might be one of the universal mediators of gastric mucosal protection. PSH are important for the maintenance of protein structure and functions in the gastric mucosa, e.g., their modifications via oxidation, alkylation and conjugation may change the functions of mucosal parenchymal or endothelial cells[13,51]. On the other hand, numerous thiol proteases such as calpain in the plasma membranes and cytosol, and the lysosomal cysteine protease cathepsins B, H and L may be activated during the early phase of acute gastric HML[30,32].
The results presented here have revealed that exposure of gastric mucosa to toxic concentrations of chemicals also caused a rapid loss of total protein SH groups. During this period cysteine proteases can be activated and released[30-32]. Our group demonstrated previously that formation of reversible protein disulfides might be advantageous in the maintenance of integrity of gastric mucosa due to: (1) their possible antioxidant or reactive metabolite-eliminating effects; (2) a more stable tertiary structure integrity and functions of several structure (membrane) protein or metabolic enzymes might be more resistant against noxious influences; (3) inhibition of PSH may be associated with decreased activity of cysteine proteases.
Our results with rats given sucralfate alone revealed a significant and dose-dependent increase in both the GSH concentration and the GSH/GSSG ratio in the gastric mucosa. In addition, a gastroprotective dose of sucralfate almost completely prevented the ethanol-induced decrease of GSH concentration and ratio of GSH/PSSP. These results are also in agreement with our previous pharmacologic experiments demonstrating an SH-sensitive step in the mechanism of gastroprotection by sucralfate[52].
We conclude that rapid and time-dependent depletion of total GSH in the gastric mucosa precedes the develop-ment of erosions induced by ethanol, HCl or NaCl. This biochemical change was not evident after exposure to ammonia or NaOH solutions. However, GSSG concen-trations in glandular mucosa increased, except after HCl or NaOH exposure. Thus, the ratios of reduced to oxidized GSH concentrations markedly decreased by all gastrotoxic chemicals studied. Considerable decreases of PSH concentration were also detected after concentrated ethanol, NaCl and NaOH solutions, while the protein disulfides were increased, resulting in diminished ratios of PSH and protein disulfides in the gastric mucosa. We also conclude that sucralfate increases the concentration of GSH in the gastric mucosa and this biochemical effect may be one of the mechanisms of gastroprotection by sucralfate and possible other similar drugs.
ACKNOWLEDGMENTS
The authors thank Gabor Nagy, Joanne M Jenkins, Romeo Morales and Ken Green for their technical assistance during these studies. The secretarial help of Mónika Rebman and Tibor Temesvári is also greatly appreciated.
Footnotes
S- Editor Liu Y L- Editor Alpini D E- Editor Liu Y
References
- 1.Strubelt O, Hoppenkamps R. Relations between gastric glutathione and the ulcerogenic action of non-steroidal anti-inflammatory drugs. Arch Int Pharmacodyn Ther. 1983;262:268–278. [PubMed] [Google Scholar]
- 2.Szabó S. Role of sulfhydryls and early vascular lesions in gastric mucosal injury. Acta Physiol Hung. 1984;64:203–214. [PubMed] [Google Scholar]
- 3.Szabo S. Mechanisms of gastric mucosal injury and protection. J Clin Gastroenterol. 1991;13 Suppl 2:S21–S34. [PubMed] [Google Scholar]
- 4.Shirin H, Pinto JT, Liu LU, Merzianu M, Sordillo EM, Moss SF. Helicobacter pylori decreases gastric mucosal glutathione. Cancer Lett. 2001;164:127–133. doi: 10.1016/s0304-3835(01)00383-4. [DOI] [PubMed] [Google Scholar]
- 5.Mózsik G, Morón F, Fiegler M, Jávor T, Nagy L, Patty I, Tárnok F. Interrelationships between membrane-bound ATP-dependent energy systems, gastric mucosal damage produced by NaOH, hypertonic NaCl, HCl, and alcohol, and prostacyclin-induced gastric cytoprotection in rats. Prostaglandins Leukot Med. 1983;12:423–436. doi: 10.1016/0262-1746(83)90032-x. [DOI] [PubMed] [Google Scholar]
- 6.Kusterer K, Pihan G, Szabo S. Role of lipid peroxidation in gastric mucosal lesions induced by HCl, NaOH, or ischemia. Am J Physiol. 1987;252:G811–G816. doi: 10.1152/ajpgi.1987.252.6.G811. [DOI] [PubMed] [Google Scholar]
- 7.Vincze A, Garamszegi M, Jávor T, Sütö G, Tigyi A, Tóth G, Zsoldos T, Mózsik G. The free radical mechanisms in beta-carotene induced gastric cytoprotection in HCl model. Acta Physiol Hung. 1989;73:351–355. [PubMed] [Google Scholar]
- 8.Peskar BM, Ehrlich K, Peskar BA. Role of ATP-sensitive potassium channels in prostaglandin-mediated gastroprotection in the rat. J Pharmacol Exp Ther. 2002;301:969–974. doi: 10.1124/jpet.301.3.969. [DOI] [PubMed] [Google Scholar]
- 9.Hiraishi H, Shimada T, Ivey KJ, Terano A. Role of antioxidant defenses against ethanol-induced damage in cultured rat gastric epithelial cells. J Pharmacol Exp Ther. 1999;289:103–109. [PubMed] [Google Scholar]
- 10.Szabo S, Tarnawski A, Vincze Á, Nagy L, Glavin G, Kusstatscher R. Isolated gastric mucosal cells and glands as models for mechanistic and pharmacologic studies of acute injury. In: Gaginella T.S., ed. Experimental Models of Mucosal Inflammation. Boca Raton-New York-London-Tokyo: CRC Press 1996, 43-65 [Google Scholar]
- 11.Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761–767. [PubMed] [Google Scholar]
- 12.Robert A, Eberle D, Kaplowitz N. Role of glutathione in gastric mucosal cytoprotection. Am J Physiol. 1984;247:G296–G304. doi: 10.1152/ajpgi.1984.247.3.G296. [DOI] [PubMed] [Google Scholar]
- 13.Szabo S, Trier JS, Frankel PW. Sulfhydryl compounds may mediate gastric cytoprotection. Science. 1981;214:200–202. doi: 10.1126/science.7280691. [DOI] [PubMed] [Google Scholar]
- 14.Boyd SC, Sasame HA, Boyd MR. Gastric glutathione depletion and acute ulcerogenesis by diethylmaleate given subcutaneously to rats. Life Sci. 1981;28:2987–2992. doi: 10.1016/0024-3205(81)90276-9. [DOI] [PubMed] [Google Scholar]
- 15.Hoppenkamps R, Thies E, Younes M, Siegers CP. Glutathione and GSH-dependent enzymes in the human gastric mucosa. Klin Wochenschr. 1984;62:183–186. doi: 10.1007/BF01731642. [DOI] [PubMed] [Google Scholar]
- 16.Miller TA, Li D, Kuo YJ, Schmidt KL, Shanbour LL. Nonprotein sulfhydryl compounds in canine gastric mucosa: effects of PGE2 and ethanol. Am J Physiol. 1985;249:G137–G144. doi: 10.1152/ajpgi.1985.249.1.G137. [DOI] [PubMed] [Google Scholar]
- 17.Bálint GA, Varró V. On the cytoprotective action of sulfhydryl-containing substances. Acta Physiol Acad Sci Hung. 1982;60:139–142. [PubMed] [Google Scholar]
- 18.Dupuy D, Szabo S. Protection by metals against ethanol-induced gastric mucosal injury in the rat. Comparative biochemical and pharmacologic studies implicate protein sulfhydryls. Gastroenterology. 1986;91:966–974. doi: 10.1016/0016-5085(86)90701-8. [DOI] [PubMed] [Google Scholar]
- 19.Ezer E. Orally administered aspirin prevents the severe gastric damage induced by acidified ethanol and sulfhydryl blocker pretreatment counteracts this protective effect in the rat. Gastroenterology. 1985;88:1377. [Google Scholar]
- 20.Konturek SJ, Brzozowski T, Piastucki I, Radecki T, Dupuy D, Szabo S. Gastric mucosal protection by agents altering gastric mucosal sulfhydryls. Role of endogenous prostaglandins. Digestion. 1987;37:67–71. [PubMed] [Google Scholar]
- 21.Stubelt O. The role of sulfhydryls in the ulcerogenic action of nonsteroidal antirheumatics. In: Szabo S, Mózsik Gy, editors. New Pharmacology of Ulcer Disease. Experimental and New Therapeutic Approaches. New York: Elsevier; 1987. pp. 437–446. [Google Scholar]
- 22.Szabo S, Spill WF, Rainsford KD. Non-steroidal anti-inflammatory drug-induced gastropathy. Mechanisms and management. Med Toxicol Adverse Drug Exp. 1989;4:77–94. doi: 10.1007/BF03259905. [DOI] [PubMed] [Google Scholar]
- 23.Ghanayem BI, Boor PJ, Ahmed AE. Acrylonitrile-induced gastric mucosal necrosis: role of gastric glutathione. J Pharmacol Exp Ther. 1985;232:570–577. [PubMed] [Google Scholar]
- 24.Romano M, Razandi M, Ivey KJ. Effect of cimetidine and ranitidine on drug induced damage to gastric epithelial cell monolayers in vitro. Gut. 1989;30:1313–1322. doi: 10.1136/gut.30.10.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutoh H, Hiraishi H, Ota S, Yoshida H, Ivey KJ, Terano A, Sugimoto T. Protective role of intracellular glutathione against ethanol-induced damage in cultured rat gastric mucosal cells. Gastroenterology. 1990;98:1452–1459. doi: 10.1016/0016-5085(90)91075-h. [DOI] [PubMed] [Google Scholar]
- 26.Nagy L, Szabo S, Morales RE, Plebani M, Jenkins JM. Identification of subcellular targets and sensitive tests of ethanol-induced damage in isolated rat gastric mucosal cells. Gastroenterology. 1994;107:907–914. doi: 10.1016/0016-5085(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 27.Nagy L, Morales RE, Beinborn M, Vattay P, Szabo S. Investigation of gastroprotective compounds at subcellular level in isolated gastric mucosal cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1201–G1208. doi: 10.1152/ajpgi.2000.279.6.G1201. [DOI] [PubMed] [Google Scholar]
- 28.Mutoh H, Ota S, Hiraishi H, Ivey KJ, Terano A, Sugimoto T. Reduced glutathione protects cultured gastric mucosal cells from suckling rats against acid. Am J Physiol. 1991;261:G65–G70. doi: 10.1152/ajpgi.1991.261.1.G65. [DOI] [PubMed] [Google Scholar]
- 29.Szabo S, Nagy L, Plebani M. Glutathione, protein sulfhydryls and cysteine proteases in gastric mucosal injury and protection. Clin Chim Acta. 1992;206:95–105. doi: 10.1016/0009-8981(92)90010-n. [DOI] [PubMed] [Google Scholar]
- 30.Nagy L, Kusstatscher S, Hauschka PV, Szabo S. Role of cysteine proteases and protease inhibitors in gastric mucosal damage induced by ethanol or ammonia in the rat. J Clin Invest. 1996;98:1047–1054. doi: 10.1172/JCI118865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagy L, Johnson BR, Hauschka P, Szabo S. Characterization of proteases and protease inhibitors in the rat stomach. Am J Physiol. 1997;272:G1151–G1158. doi: 10.1152/ajpgi.1997.272.5.G1151. [DOI] [PubMed] [Google Scholar]
- 32.Bühling F, Peitz U, Krüger S, Küster D, Vieth M, Gebert I, Roessner A, Weber E, Malfertheiner P, Wex T. Cathepsins K, L, B, X and W are differentially expressed in normal and chronically inflamed gastric mucosa. Biol Chem. 2004;385:439–445. doi: 10.1515/BC.2004.051. [DOI] [PubMed] [Google Scholar]
- 33.Raza A, Szabo S. Gastroprotection by glutathione depletors is not due to low gastric glutathione: role for protein sulfhydryl modification. Fed Proc. 1987;46:1453. [Google Scholar]
- 34.Matsumura M, Signor G, Matthews BW. Substantial increase of protein stability by multiple disulphide bonds. Nature. 1989;342:291–293. doi: 10.1038/342291a0. [DOI] [PubMed] [Google Scholar]
- 35.Gregory JD. The stability of N-ethylmaleimide and its reaction with sulfhydryl groups. J Am Chem Soc. 1955;77:3922–3923. [Google Scholar]
- 36.Güntherberg H, Rost J. The true oxidized glutathione content of red blood cells obtained by new enzymic and paper chromatographic methods. Anal Biochem. 1966;15:205–210. doi: 10.1016/0003-2697(66)90025-x. [DOI] [PubMed] [Google Scholar]
- 37.Cavallini D, Graziani MT, Dupré S. Determination of disulphide groups in proteins. Nature. 1966;212:294–295. doi: 10.1038/212294a0. [DOI] [PubMed] [Google Scholar]
- 38.Kuzuhara T, Nakajima Y, Matsuyama K, Natori S. Determination of the disulfide array in sapecin, an antibacterial peptide of Sarcophaga peregrina (flesh fly) J Biochem. 1990;107:514–518. doi: 10.1093/oxfordjournals.jbchem.a123077. [DOI] [PubMed] [Google Scholar]
- 39.ELLMAN GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 40.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 41.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 42.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 43.Pihan G, Regillo C, Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987;32:1395–1401. doi: 10.1007/BF01296666. [DOI] [PubMed] [Google Scholar]
- 44.Murphy ME, Kehrer JP. Oxidation state of tissue thiol groups and content of protein carbonyl groups in chickens with inherited muscular dystrophy. Biochem J. 1989;260:359–364. doi: 10.1042/bj2600359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabo S, Pihan G, Dupuy D. The biochemical pharmacology of sulfhydryl compounds in stric mucosal injury and protection. In: Szabo S, Mózsik Gy, editors. New Pharmacology of Ulcer Disease. Experimental and New Therapeutic Approaches. New York: Elsevier; 1987. pp. 424–436. [Google Scholar]
- 46.Szabo S, Pihan G, Raza A, Muller AE, Hauschka PV. Multiple mechanisms of cell injury in the gastric mucosa. Fed Proc. 1987;46:1152. [Google Scholar]
- 47.Jaeschke H. Glutathione disulfide as index of oxidant stress in rat liver during hypoxia. Am J Physiol. 1990;258:G499–G505. doi: 10.1152/ajpgi.1990.258.4.G499. [DOI] [PubMed] [Google Scholar]
- 48.Lenney JF, Liao JR, Sugg SL, Gopalakrishnan V, Wong HC, Ouye KH, Chan PW. Low molecular weight inhibitors of cathepsins B, H and T in human serum, synovial fluid and CSF. Biochem Biophys Res Commun. 1982;108:1581–1587. doi: 10.1016/s0006-291x(82)80088-0. [DOI] [PubMed] [Google Scholar]
- 49.Lê-Quôc K, Lê-Quôc D. Control of the mitochondrial inner membrane permeability by sulfhydryl groups. Arch Biochem Biophys. 1982;216:639–651. doi: 10.1016/0003-9861(82)90254-5. [DOI] [PubMed] [Google Scholar]
- 50.Pfleger H, Wolf HU. Activation of membrane-bound high-affinity calcium ion-sensitive adenosine triphosphatase of human erythrocytes by bivalent metal ions. Biochem J. 1975;147:359–361. doi: 10.1042/bj1470359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shertzer HG, Sainsbury M, Berger ML. Importance of protein thiols during N-methyl-N'-nitro-N-nitrosoguanidine toxicity in primary rat hepatocytes. Toxicol Appl Pharmacol. 1990;105:19–25. doi: 10.1016/0041-008x(90)90355-x. [DOI] [PubMed] [Google Scholar]
- 52.Szabo S, Brown A. Prevention of ethanol-induced vascular injury and gastric mucosal lesions by sucralfate and its components: possible role of endogenous sulfhydryls. Proc Soc Exp Biol Med. 1987;185:493–497. doi: 10.3181/00379727-185-4-rc1. [DOI] [PubMed] [Google Scholar]


