Abstract
AIM: To evaluate the role of endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) in the preoperative diagnosis of gastrointestinal stromal tumor (GIST).
METHODS: From September 2002 to June 2006, Fifty-three consecutive EUS-FNAs of GI tract subepithelial hypoechoic tumors with continuity to proper muscle layer suspected as GIST by standard EUS were evaluated prospectively. The reference standards for the final diagnosis were surgery (n = 31), or clinical follow-up (n = 22). Additionally, immunophenotyping of specimens obtained by EUS-FNA and surgical resection specimens were compared.
RESULTS: In 2 cases puncture was not performed because of anatomical problems. The collection rate of adequate specimens from the GI tract subepithelial hypoechoic tumor with continuity to proper muscle layer was 82% (42/51). The diagnostic rate for the tumor less than 2 cm, 2 to 4 cm, and 4 cm or more were 71% (15/21), 86% (18/21), and 100% (9/9), respectively. In 29 surgically resected cases, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of EUS-FNA using immunohistochemical analysis of GIST were 100% (24/24), 80% (4/5), 96% (24/25), 100% (4/4), and 97% (28/29), respectively. No major complications were encountered.
CONCLUSION: EUS-FNA with immunohistochemical analysis is a safe and accurate method in the prethera-peutic diagnosis of GIST. It should be taken into consideration in decision making, especially in early diagnosis following minimal invasive surgery for GIST.
Keywords: Gastrointestinal stromal tumor, Ultrasound-guided fine needle aspiration, Immunohistochemical analysis
INTRODUCTION
Gastrointestinal stromal tumors (GIST) were described in 1983 as tumors in the gastrointestinal tract and mesentery, characterized by a specific histological and immunohistochemical pattern[1]. GIST has a risk of metastatic relapse, specifically in the liver and peritoneum, after initial surgery for localized disease[1-6]. Since every GIST is now considered as potentially malignant, all GISTs may need to be resected, even small intramural lesions of the gastrointestinal tract[1,5,6]. However, since not all intramural lesions of the gastrointestinal tube are GISTs, a preoperative pathological diagnosis should be obtained. However, even when a biopsy is performed during a conventional endoscopy, usually the GIST is covered by normal mucosa, leading to insufficient endoscopic biopsy specimens from deeper layers. Endoscopic ultrasonography (EUS), enabling intramural scanning of the GI tract, has been reported to be useful in the diagnosis of submucosal tumor (SMT) and in differentiating SMT from extraluminal lesions[7-9]. Nevertheless, the diagnosis on the basis of EUS is presumptive and cannot replace a histological diagnosis of GIST. Therefore, definitive tissue diagnosis of SMT and extraintestinal lesions is elusive without surgery, and a less invasive method of obtaining tissue diagnosis is desirable. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has emerged as a minimally invasive technique that allows identification and sampling of various SMT and extraintestinal mass lesions[10-15]. In accordance with current knowledge, the diagnosis of GIST should be based on immunohistochemical analysis. The purpose of the present study was to determine the diagnostic usefulness of EUS-FNA using immunohistochemical analysis for preoperative diagnosis of GIST.
MATERIALS AND METHODS
From September 2002 to June 2006, 53 consecutive patients with subepithelial hypoechoic tumors originating in the fourth sonographic layer of the GI tract wall suspected as GIST by standard EUS underwent EUS-FNA for histologic diagnosis at Aso Iizuka Hospital. There were 14 males and 39 females, and the mean age was 66 years (range 34-91 years). Informed written consent for the study was obtained from all patients. One attending endosonographer (KA) performed all EUS and EUS-FNA procedures.
Standard EUS was performed on an outpatient basis, with the patient under conscious sedation, using a conventional radial scanner echoendoscope GF-UM20 (Olympus, Tokyo, Japan) or 12 MHz ultrasound catheter probe SP-702 (Fujinon, Saitama, Japan). EUS-FNA was performed on a one-day inpatient basis, with conscious sedation, using PEF-708FA (Toshiba-Fujinon, Tokyo, Japan) convex array echoendoscope[16] (Figure 1). The echoendoscope was connected to a Toshiba ultrasound scanner SSA-550A (Toshiba, Tokyo, Japan). Color flow and Doppler sonography were performed to exclude intervening vascular structures and to select a vessel-free needle track. All FNA procedures were performed using the Olympus needle (NA-11J-KB) (Figure 2) consisting of a 180 cm long steel needle 0.8 mm in diameter (22G) with a stylet passing through a metal catheter with an outer diameter of 1.6 mm. It has a power-shot style that advances to a maximum of 4 cm instantly with the push of a button. The needle is inserted into the working channel of the echoendoscope. Once the tip of the catheter was visualized, the needle was advanced from the catheter sheath through the wall of the GI tract and into the target lesion under ultrasonographic guidance (Figure 3). The stylet was removed and continuous suction applied with a 20-mL syringe. The needle was moved back and forth within the lesion under ultrasonographic guidance. The suction was then released and the needle removed from the biopsy channel.
Figure 1.
Toshiba-Fujinon curved linear array echoendoscope PEF-708FA.
Figure 2.
Olympus power shot type metallic needle NA-11J-KB.
Figure 3.
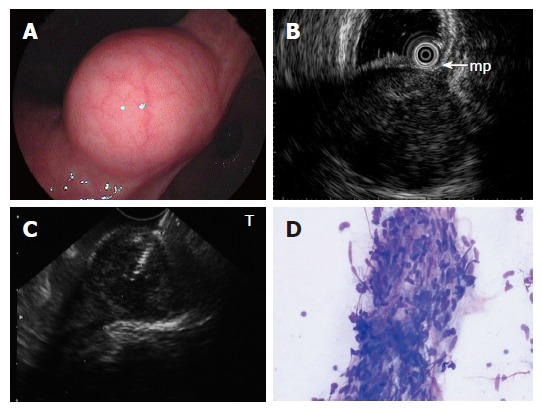
A: Submucosal lesion in the angulus of the stomach shown on endoscopy; B: EUS using ultrasound catheter probe reveals 3 cm subepithelial hypoechoic tumor with continuity to proper muscle layer (arrow-mp); C: Puncture of the small GIST under direct endosonographic visualization. The needle can be visualized; D: EUS-FNA smear of GIST showing a small tissue fragment composed of ovoid to spindle-shaped nuclei with minimal to no atypia arranged in fascicles (modified Giemsa stain).
The aspirates were placed on glass slides, and both air-dried and alcohol-fixed smears were prepared. Air dried smears were stained with a modified Giemsa stain (Figure 3) and reviewed immediately by a cytopathologist on site to ensure specimen adequacy. The remaining prepared histologic specimens were processed at a later time in the pathology laboratory for hematoxylin and eosin staining and additional ancillary studies such as immunochemistry (Figure 4). Immunohistochemical analysis was performed as follows: Both the EUS-FNA and surgical resection specimens were fixed in 10% formaldehyde and tissue blocks were embedded in paraffin. Sections were stained with hematoxylin and eosin. The polymer method was used for immunohistochemical staining with the following antibodies: c-kit (polyclonal, 1:200; Dako North America Inc, Carpenteria CA, USA), CD34 (QBend 10, monoclonal, 1:100; Novocastra, Benton Lane, UK), muscle actin (1A4, monoclonal, 1:100; Dako A/S, Glostrup, Denmark), S-100 (polyclonal, 1:12; Dako A/S, Glostrup, Denmark). A tumor with positive reaction to c-kit and/or CD34 was diagnosed as GIST. A tumor with a negative reaction to c-kit, CD34, and S-100 and a positive reaction for muscle actin was diagnosed as a myogenic tumor (leiomyoma). A tumor with a negative reaction to c-kit, CD34, and muscle actin and positive reaction for S-100 was diagnosed as a neurogenic tumor (neurinoma).
Figure 4.
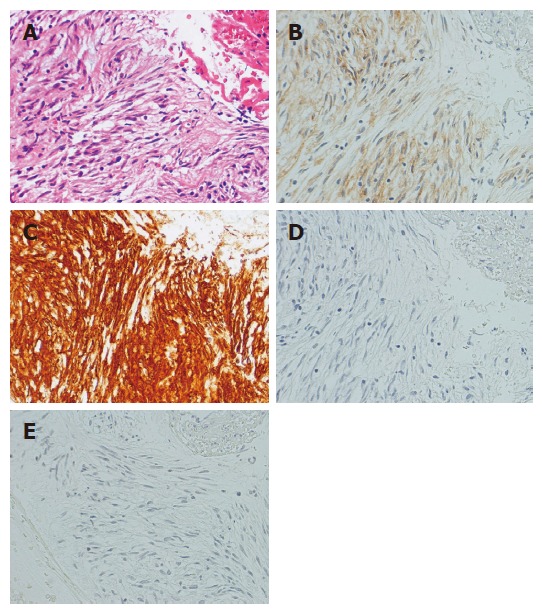
Photomicrographs of EUS-FNA specimen of GIST. A: Hematoxylin-eosin stain; B: Immunohistochemical stain for c-kit; C: Immunohistochemical stain for CD34; D: Immunohistochemical stain muscle actin; E: Immunohistochemical stain for S-100. The tumor is diffusely positive for c-kit and CD34 and negative for muscle actin and S-100. The immunohistochemical pattern diagnosis is GIST.
EUS-FNA diagnoses were analyzed for correlations with final diagnoses, which were based on histological examination of surgically resected pathology materials and/or clinical follow-up (mean; 8 mo, range; 3 to 36 mo) findings. To determine the operating characteristics of EUS-FNA in the diagnosis of subepithelial hypoechoic tumors originating in the fourth sonographic layer of the GI tract suspected as GIST by standard EUS, histologic diagnostic rate according to tumor size and sensitivity, specificity, positive predictive value, negative predictive value and accuracy for the differential diagnosis of GIST or non-GIST were calculated. This study was reviewed and approved by our institutional review board.
RESULTS
Clinicopathological characteristics of the lesions are summarized in Table 1. All patients had previously had lesions detected with gastrointestinal endoscopy and EUS that had prompted their referral for EUS-FNA for tissue diagnosis. There were 4 FNA specimens (8%) from the esophagus, 43 (81%) from the stomach, 1 (2%) from the duodenum, 4 (8%) from the rectum, and 1 (2%) from the pancreas. Final diagnosis after EUS-FNA were GIST (n = 33), leiomyoma (n = 2), neurinoma (n = 3), ectopic pancreas (n = 2), accessory pancreas (n = 1), esophageal cancer (n = 1), pancreatic cancer (n = 1), and not diagnostic (n = 8). Surgical resection was performed in 32 patients and followed up in 21 patients (2 cases with recurrence of rectal cancer were treated by chemotherapy. 6 cases with GIST refused surgery.)
Table 1.
Clinicopathological characteristics (n = 53)
| Anatomic sites of the lesions | ||||
| Esophagus | n = 4 | Stomach | n = 43 | |
| Duodenum | n = 1 | Rectum: | n = 4 | |
| Pancreas | n = 1 | |||
| Final diagnosis after EUS-FNA | ||||
| Surgically resected cases (n = 32) | ||||
| GIST: | n = 27 | Leiomyoma: | n = 1 | |
| Neurinoma: | n = 2 | Accessory spleen:1 | n = 1 | |
| Esophageal cancer: | n = 1 | |||
| Not surgically rsected cases (n = 21) | ||||
| GIST: | n = 6 | Ectopic pancreas: | n = 2 | |
| Recurrence of rectal cancer: | n = 2 | Neurinoma: | n = 1 | |
| Leiomyoma: | n = 1 | Pancreatic cancer: | n = 1 | |
| Not diagnostic: | n = 8 | |||
Laparotomic biopsy only.
Table 2 summarizes the technical results of EUS-FNA. Puncture was not performed because of anatomical problems (intervening vessel and tumor location) in two cases. The collection rate of adequate specimens was 82% (42 of 51 patients), and histological diagnosis was successfully performed in all 42 adequate specimens. We encountered no severe complications associated with this procedure.
Table 2.
Technical results of EUS-FNA (n = 53)
| Puncture not performed | 12/53 (4%) |
| Puncture success rate | 51/51 (100%) |
| Number of EUS-FNA passes | 1 to 6 passes (Average, 2.4) |
| Collection of adequate specimen | 42/51 (82%) |
| Complications | 0/53 (0%) |
Reason: 1 intervening vessel, 1 tumor location.
Representative EUS and EUS-FNA findings in a patient with GIST are shown in Figure 3. The result of immunohistochemical analysis of the tumor (Figure 4) was positive reaction for c-kit and CD34, and negative reaction for muscle actin and S-100. The tumor was diagnosed as GIST, and then the patient underwent local resection. The immunohistochemical staining pattern in the surgically resected lesion was similar (diagnosed as GIST). Figure 5 shows EUS and EUS-FNA findings in a patient with non-GIST. The tumor was diagnosed as ectopic pancreas by the histologic result of EUS-FNA specimen, and then follow-up was performed in the patient. Diagnostic rate of GI tract subepithelial hypoechoic tumor with continuity to proper muscle layer according to tumor size appears in Table 3. When the size of the tumors was classified into three grades, using 2-cm intervals, the larger the size of the lesion, the higher the diagnostic rate, the overall diagnostic rate was 82% (42 of 51 patients). GIST or non-GIST was correctly diagnosed by EUS-FNA using immunohistochemical analysis in 28 of the 29 surgically resected cases (Table 4). The sensitivity, specificity, positive and negative predictive values and accuracy for diagnosis of GIST were 100%, 80%, 96%, 100% and 97%, respectively.
Figure 5.
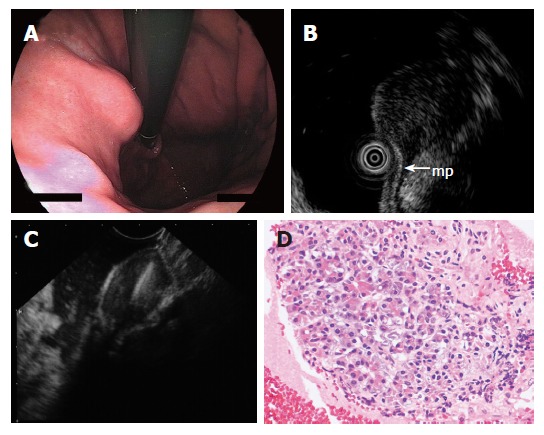
A: Endoscopy showing submucosal lesion in the upper body of the stomach; B: EUS using ultrasound catheter probe demonstrating 1.5 cm subepithelial hypoechoic tumor with continuity to proper muscle layer (arrow-mp); C: The hypoechoic mass shown on EUS was punctured under real-time EUS guidance; D: The FNAB specimen consisted of acinar cells. The histologic diagnosis was ectopic pancreas.
Table 3.
Diagnostic rate according to tumor size
| Tumor size (mean) | Diagnostic rate (%) |
| 0-2 cm (1.5) | 15/21 (71) |
| 2-4 cm (3.3) | 18/21 (86) |
| 4- cm (6.6) | 9/9 (100) |
| Total (3.0) | 42/51 (82) |
Table 4.
Diagnosis of GIST or non-GIST using immunohisto-chemical analysis in the 29 surgically resected cases
| Surgery |
EUS-FNA |
|
| GIST | Non-GIST | |
| GIST | 24 | 0 |
| Non-GIST | 1 | 4 |
Overall accuracy 97% (28 of 29); sensitivity 100% (24 of 24); specificity 80% (4 of 5); positive predictive value 96% (24 of 25); negative predictive value 100% (4 of 4).
Figure 6 shows EUS-FNA diagnosis and following management. Local resection was performed in 25 out of 31 patients diagnosed as GIST by EUS-FNA. The remaining 6 patients rejected surgery. Appropriate management, including follow-up, surgery, and chemotherapy, was perfomed in the all 11 patients diagnosed as non-GIST. It was impossible to diagnose the remaining 11 patients because of insufficient material, but eight patients were carefully followed up and three patients (GI bleeding 1, patient’s wish 2) received local resection.
Figure 6.
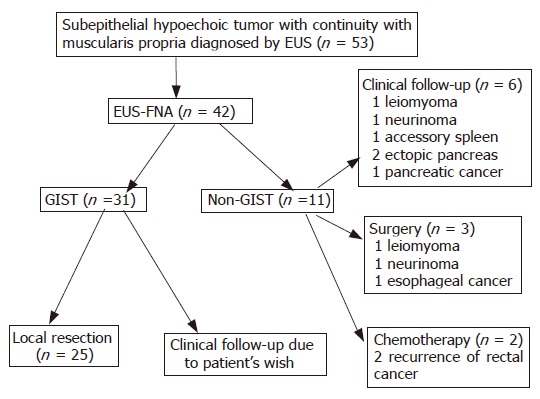
EUS-FNA diagnosis and following management.
DISCUSSION
GISTs are uncommon abdominal malignancies. Despite being the most common GI sarcomas, GISTs represent only 1% of alimentary tract tumors[17]. Identification of GISTs has been facilitated by the recent application of c-kit immunohistchemistry that identifies the c-kit proto-oncogene product that is overexpressed in nearly all GIST and distinguishes these neoplasms from leiomyomas, leiomyosarcomas, or schwannomas[5]. C-kit has become the predominant immunomarker for the identification of GISTs. Although a number of clinicopathologic features have been implicated in the determination of malignancy potential, even those that demonstrate the best correlation only allow the prediction of relative risk. The 2 strongest predictors of GIST’s behavior remain tumor size and mitotic activity[1,6]. However, predicting their behavior remains difficult. Since every GIST is now considered as potentially malignant, all GIST may need to be resected, even small intramural lesions of the gastrointestinal tract. Surgery has been and continues to be the primary treatment modality for GIST. The overall 5-year survival rate for patients with primary gastric GISTs who underwent complete resection ranges from 20% to 63%, with a recurrence rate of 17 to 76%[18,19]. Miettinen reported that small GIST (< 2 cm) occurred no metastasis in 1765 cases broken down into prognostic categories, with follow-up information[6]. In other words, it means that surgical complete resection of GIST smaller than 2 cm produces 100% cure. In our study, postoperative metastasis of GIST occurred in one case with a 3 cm tumor, and in all GISTs less than 2 cm there was no postoperative relapse. So, early diagnosis and early surgical resection is vital to improve the prognosis of this disease.
However, preoperative pathological diagnosis must be obtained because all subepithelial lesions of the gastrointestinal tract are not GIST. Endoscopy alone has suboptimal accuracy of as low as 40% for identifying the cause of submucosal bulges[20]. Usually the mucosal surface is normal, and conventional forceps biopsy results are frequently negative. Other noninvasive imaging methods such as transabdominal ultrasound and computed tomography are also suboptimal for evaluating submucosal indentations[21]. EUS combines the endoscopic view with ultrasonographic images generated by a high-frequency intraluminal probe. This allows clear imaging of the gastrointestinal wall layers and precise evaluation of the submucosal tumor whether from extrinsic compression or the layer in which the intramural lesion originates. Although EUS provides important morphologic information from submucosal lesions, including some features suggestive of malignancy (size > 3-4 cm, irregular margins, internal echogenic foci or cystic spaces, and rapid growth rate at follow-up EUS)[22,23], it cannot establish a final pathologic diagnosis. In the diagnostic process of GIST, immunohistochemical analysis of tissue sample such as c-kit is vital for confirmation of this disease. Preoperative percutaneous biopsy carries the theoretical risk of peritoneal seeding or tumor rupture, and is indicated only for clearly irresectable disease or when treatment needs to be altered, as would be the case if the mass proved to be lymphoma or germ cell tumor[24]. One alternative technique is EUS-FNA, which has been used increasingly for the evaluation of different types of intraabdominal and intrathoracic lesions[7-15]. Observations to date indicate that EUS-FNA is a safe and accurate procedure.
The majority of reports on EUS-FNA, however, have focused on pancreatic lesions and lymphadenopathy. Few reports have specifically investigated the use of EUS-FNA in evaluating intramural and extramural structures of the GI tract[15]. Furthermore, the clinical usefulness of EUS-FNA for GIST is unclear in previous literature[10-15]. In this study, the collection rate of adequate specimens from a GI tract subepithelial hypoechoic tumor with continuity to proper muscle layer was 82%. No major complications were encountered. The diagnostic rate for the tumor less than 2 cm, 2 to 4 cm, and 4 cm or more were 71%, 86%, and 100%, respectively. In our 29 surgically resected cases, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnosctic accuracy of EUS-FNA using immunohistochemical analysis of GIST were 100%, 80%, 96%, 100%, and 97%, respectively. Reported accuracy of preoperative diagnosis of EUS-FNA using immunohistochemical analysis for surgically resected GIST cases ranged from 91% to 100%[11,25]. The diagnostic accuracy of EUS-FNA using immunohistochemical analysis is excellent. This accurate preoperative histological proof of GIST facilitates the surgeon’s decision making for early local resection.
At present, a precise algorithm for diagnostic or therapeutic workup of GIST is still lacking. A large group of submucosal lesions such as lipomas, cysts, and submucosal varices has typical features that allow accurate diagnosis based solely on the data gathered from endoscopy and EUS imaging[7,8,12]. However, an important subset of submucosal lesions such as GISTs, granular cell tumors, carcinoid tumors, ectopic pancreas, neurofibromas, adenocarcinomas, glomus tumors, lymphoid mass tissues, squamous cell cancer, recurrent neoplasms, and metastases may have overlapping echo and endoscopic features and cannot be accurately determined without a biopsy sample. A precise preoperative histological diagnosis has the potential to directly affect clinical treatment[12]. This study supports the role of EUS in the evaluation of lesions within, or adjacent to, the gastrointestinal tract. EUS-FNA can be carried out safely, and the results of FNA can significantly alter management of the patients. In our study, management decisions were simplified and delineated when GIST (local resection) or non-GIST such as ectopic pancreas, recurrent cancer, and SMT-like cancer (follow up, chemotherapy, appropriate surgery, etc) was confirmed by EUS-FNA. In the author’s experience and discussions with surgeons at our institution, crucial preoperative planning and management are facilitated by the histological diagnoses provided with EUS-FNA. Operative planning and the type of surgery conducted vary dramatically in relation to the diagnosis[1,17-19,23-29]. For example, a patient with a GIST can be cured with a wedge resection, or if inoperable, SMT-like anastomotic recurrence can receive chemotherapy; however, a patient with SMT-like advanced esophageal carcinoma would undergo esophagectomy with lymph-node dissection and might need postoperative chemotherapy or radiotherapy. A patient with benign SMT could avoid surgery completely because of histological confirmation of benignancy such as ectopic pancreas. In addition, EUS-FNA can provide a definitive histological diagnosis, which is routinely requested by oncologists before initiating any chemotherapy, radiotherapy, or palliative treatment[26]. Figure 7 outlines a proposed diagnosis and treatment algorithm for patients who are diagnosed with SMT or SMT-like lesion by conventional endoscopy. EUS-FNA thus evidently has a significant positive impact on clinical management of patients by providing a definitive histological diagnosis.
Figure 7.
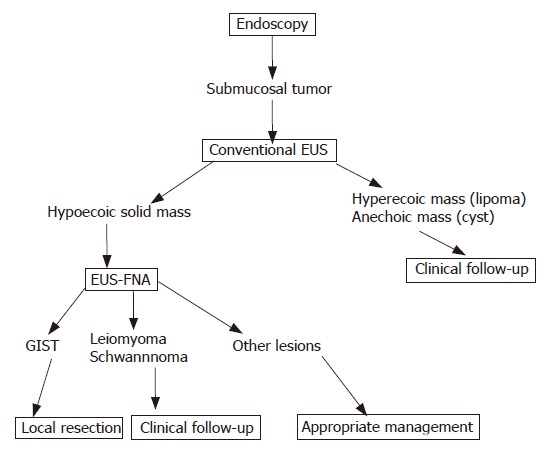
Algorithm of diagnosis and treatment of gastrointestinal submucosal tumor.
Footnotes
S- Editor Wang J L- Editor Li M E- Editor Chen GJ
References
- 1.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213–1220. doi: 10.1016/s0046-8177(99)90040-0. [DOI] [PubMed] [Google Scholar]
- 3.Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293–1301. doi: 10.14670/HH-15.1293. [DOI] [PubMed] [Google Scholar]
- 4.Rubin BP, Fletcher JA, Fletcher CD. Molecular Insights into the Histogenesis and Pathogenesis of Gastrointestinal Stromal Tumors. Int J Surg Pathol. 2000;8:5–10. doi: 10.1177/106689690000800105. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 7.Akahoshi K, Harada N, Nawata H. The current state of endoscopic ultrasonography. In: Pandalai SG, editor. Recent research developments in radiology. Kerala: Transworld Research Network; 2003. pp. 1–22. [Google Scholar]
- 8.Byrne MF, Jowell PS. Gastrointestinal imaging: endoscopic ultrasound. Gastroenterology. 2002;122:1631–1648. doi: 10.1053/gast.2002.33576. [DOI] [PubMed] [Google Scholar]
- 9.Role of endoscopic ultrasonography. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 2000;52:852–859. [PubMed] [Google Scholar]
- 10.Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration of intramural and extraintestinal mass lesions: diagnostic accuracy, complication assessment, and impact on management. Endoscopy. 2005;37:984–989. doi: 10.1055/s-2005-870272. [DOI] [PubMed] [Google Scholar]
- 11.Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 12.Arantes V, Logroño R, Faruqi S, Ahmed I, Waxman I, Bhutani MS. Endoscopic sonographically guided fine-needle aspiration yield in submucosal tumors of the gastrointestinal tract. J Ultrasound Med. 2004;23:1141–1150. doi: 10.7863/jum.2004.23.9.1141. [DOI] [PubMed] [Google Scholar]
- 13.Logrono R, Bhanot P, Chaya C, Cao L, Waxman I, Bhutani MS. Imaging, morphologic, and immunohistochemical correlation in gastrointestinal stromal tumors. Cancer. 2006;108:257–266. doi: 10.1002/cncr.21918. [DOI] [PubMed] [Google Scholar]
- 14.Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720–726. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vander Noot MR, Eloubeidi MA, Chen VK, Eltoum I, Jhala D, Jhala N, Syed S, Chhieng DC. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2004;102:157–163. doi: 10.1002/cncr.20360. [DOI] [PubMed] [Google Scholar]
- 16.Aibe T, Yamanaka T. EUS-guided biopsy of submucosal tumors using a newly developed needle. Endoscopia Digestiva. 2000;12:289–294. [Google Scholar]
- 17.Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705–712. doi: 10.1007/s10434-000-0705-6. [DOI] [PubMed] [Google Scholar]
- 18.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehnert T. Gastrointestinal sarcoma (GIST)--a review of surgical management. Ann Chir Gynaecol. 1998;87:297–305. [PubMed] [Google Scholar]
- 20.Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856–862. [PubMed] [Google Scholar]
- 21.Nesje LB, Laerum OD, Svanes K, Ødegaard S. Subepithelial masses of the gastrointestinal tract evaluated by endoscopic ultrasonography. Eur J Ultrasound. 2002;15:45–54. doi: 10.1016/s0929-8266(01)00166-5. [DOI] [PubMed] [Google Scholar]
- 22.Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92. doi: 10.1136/gut.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen EF, Arnott ID, Plevris J, Penman ID. Endoscopic ultrasonography in the diagnosis and management of suspected upper gastrointestinal submucosal tumours. Br J Surg. 2002;89:231–235. doi: 10.1046/j.0007-1323.2001.02002.x. [DOI] [PubMed] [Google Scholar]
- 24.Connolly EM, Gaffney E, Reynolds JV. Gastrointestinal stromal tumours. Br J Surg. 2003;90:1178–1186. doi: 10.1002/bjs.4352. [DOI] [PubMed] [Google Scholar]
- 25.Okubo K, Yamao K, Nakamura T, Tajika M, Sawaki A, Hara K, Kawai H, Yamamura Y, Mochizuki Y, Koshikawa T, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of gastrointestinal stromal tumors in the stomach. J Gastroenterol. 2004;39:747–753. doi: 10.1007/s00535-004-1383-0. [DOI] [PubMed] [Google Scholar]
- 26.Wu PC, Langerman A, Ryan CW, Hart J, Swiger S, Posner MC. Surgical treatment of gastrointestinal stromal tumors in the imatinib (STI-571) era. Surgery. 2003;134:656–665; discussion 665-666. doi: 10.1016/s0039-6060(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 27.Iwahashi M, Takifuji K, Ojima T, Nakamura M, Nakamori M, Nakatani Y, Ueda K, Ishida K, Naka T, Ono K, et al. Surgical management of small gastrointestinal stromal tumors of the stomach. World J Surg. 2006;30:28–35. doi: 10.1007/s00268-005-7944-4. [DOI] [PubMed] [Google Scholar]
- 28.Otani Y, Ohgami M, Igarashi N, Kimata M, Kubota T, Kumai K, Kitajima M, Mukai M. Laparoscopic wedge resection of gastric submucosal tumors. Surg Laparosc Endosc Percutan Tech. 2000;10:19–23. [PubMed] [Google Scholar]
- 29.Chan KH, Chan CW, Chow WH, Kwan WK, Kong CK, Mak KF, Leung MY, Lau LK. Gastrointestinal stromal tumors in a cohort of Chinese patients in Hong Kong. World J Gastroenterol. 2006;12:2223–2228. doi: 10.3748/wjg.v12.i14.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]


