Abstract
Presacral ganglioneuromas are so rare benign tumors that only 17 cases have been reported in the literature. They are abdominal masses growing slowly and differential diagnoses have to be considered. Surgical resection is important for definitive diagnosis because it represents the only therapeutic choice. Because of the benign nature of ganglioneuroma, adjuvant chemo- or radiotherapy is not indicated but regular follow-up is necessary for an early diagnosis of potential local recurrence. We report a case of a 64-year-old man with a presacral ganglioneuroma.
Keywords: Ganglioneuroma, Neuroblastoma, Presacral tumor
INTRODUCTION
Ganglioneuromas are rare tumors in the neuroblastoma group[1-6]. They are benign lesions arising from sympathetic ganglion cells and complete surgical resection is considered to be curative[7-17]. They are rarely located in the presacral space, and so far, only 17 cases have been reported in the literature.
CASE REPORT
A 64-year-old healthy man sought medical advice in September 2005 because a routine abdominal ultrasonography for benign prostatic hypertrophy incidentally revealed a pelvic mass. He did not complain of any back pain or discomfort, and no neurologic symptoms were present. There was no evidence for endocrinological symptoms like diarrhea. He was admitted to our institute. Hereditary background for syndromes such as MEN2, cowden syndrome, tuberous sclerosis or familial polyposis coli were definitely excluded. Physical examination confirmed the presence of an abdominal mass, and neurologic examination was negative. There was no proctorrhagia, and both digital rectal examination and colonscopy excluded any origin of the pelvic mass from the rectum. Routine blood tests were made and tumor markers were detected (CEA, CA 19-9, CA 125, AFP, CA 72-4), but all values were normal. Laboratory studies showed no evidence of catecholamine excess. Computed tomography (CT) and magnetic resonance (MR) imaging were performed (Figure 1). Abdominal CT scan revealed a disomogeneous mass (12 cm × 9 cm × 8 cm) with irregular enhancement in the different contrastographic phases, arising from the sacral canal through the third right sacral foramen. Differential diagnosis had to be considered but a first diagnosis of Schwannoma or Chordoma was suggested by the radiologist. Pelvic MR confirmed the origin of the lesion from sacral canal in S2 through the third right sacral foramen, and excluded any sign of sacral or coccygeal metameric infiltration or osteolysis. Pelvic MR also confirmed the first hypothesis of Schwannoma. The patient was submitted to surgical laparotomy: midline incision and transperitoneal exposure were performed; small bowel, distal sigmoid and rectum and their mesenteries were retracted to expose the tumor lesion. Pelvis was completely occupied by a big mass, measuring 12 cm × 9 cm, tenaciously sticked to sacral plane. Intraoperative frozen section excluded the malignancy of the lesion. A partial resection of mesocolon and rectal preparation were necessary in order to gain access to the tumor lesion. Tumor preparation and its resection were very difficult because of large size, anatomical deep location and origin from the third sacral foramen. A complete and curative resection was performed. Histopathologic examination noticed ganglioneuroma with microcalcifications and focal lymphoplasmocellular infiltration. Large tumor ganglion cells are well differentiated and embedded in a neuromatous stroma with microcalcifications (Figures 2 and 3).
Figure 1.
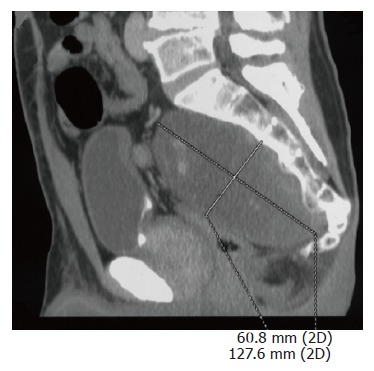
Abdominal CT scan shows a disomogeneous mass arising from the sacral canal through the third right sacral foramen.
Figure 2.
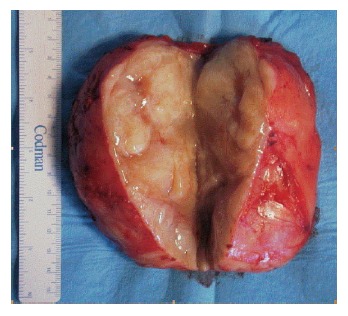
Tumor mass exposed after longitudinal section.
Figure 3.
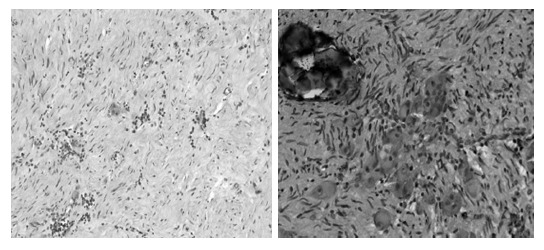
Hematoxylin and Eosin photomicrograph (× 10; × 20). Large ganglion cells are embedded in a neuromatous stroma with calcifications.
The patient had a regular postoperative hospital stay without complications and was discharged on the 7th postoperative day. After a 8-mo follow-up, he remains healthy with the exception of urinary retention, probably due to the benign prostatic hypertrophy.
DISCUSSION
Histologically ganglioneuromas are considered to be part of the neuroblastoma group together with neuroblastomas and ganglioneuroblastomas[1-6]. Ganglioneuromas are well-differentiated tumors, distinguished from the other groups because they are considered benign and constituted by mature sympathetic ganglion cells[1]. Some authors reported malignant transformation, spontaneously or after radiotherapy[15,18,19]. Arising along the sympathetic chain, ganglioneuromas are commonly localized in the posterior mediastinum followed by retroperitoneum, cervical region and adrenal gland[1,20]. Few cases have been reported in bone. The presacral location is very rare[7-14,17,21] (Table 1). An association with neurofibromatosis and multiple endocrine neoplasia syndrome Type II B has been reported by other authors[6]. They are common in young females[6,22,23] and such as abdominal benign masses, are usually asymptomatic until they reach a large size when they compress and displace adjacent structures. Moreover, several patients can present with constipation or pain due to local mass effect on the rectum, sacral root and lumbosacral plexus[1,23]. Ganglioneuromas have usually a mean diameter of 7 cm, so our patient is a rare case both for its presacral location and size[6]. On diagnostic evaluation, magnetic resonance represents the best non-invasive imaging method for preoperative study of this kind of lesions. Structural and morphological features of ganglioneuromas, such as the presence of mature sympathetic ganglion cells, can help distinguish them in differential diagnosis from other pelvic or abdominal lesions such as Schwannoma, neurofibroma, meningoma or cystic lesions. Lymphomas, chordomas, teratomas, soft-tissue sarcomas, Ewing sarcoma, osteosarcomas, chondrosarcomas or bone metastases are distinguished from ganglioneuromas because they are able to invade and erode bones. Preoperative study should be completed by FNAB samples of the lesion, but because of its anatomical location, technical approach is often difficult. Furthermore, an inaccurate diagnosis may occur with a single FNAB sample and multiple sites should be reached within the tumor[19]. So, we preferred intraoperative frozen section in order to define a correct diagnosis or discriminate between benign and malignant lesions. We preferred anterior laparotomic approach transperitoneally with rectum mobilization so as to achieve a complete tumor resection. A posterior trans-sacral approach was not preferred because of the potential iatrogenic morbidity to the dural sac and cauda equine. It was not necessary to perform laminectomy and foraminotomy because our patient was neurologically asymptomatic and intraoperative frozen section reported a benign lesion. To achieve this kind of surgical approach, we preserved nerve tissues avoiding morbidity and neurological dysfunction for the patient. If necessary, we could perform laminectomy and foraminotomy in case of recurrence with neurological symptoms. Because of the benign nature of ganglioneuromas, adjuvant systemic chemotherapy or local radiotherapy are not indicated. Moreover, surgical resection represents the only choice for both diagnosis and treatment, and a regular follow-up is necessary to assess local recurrence. Ganglioneuromas have a tendency to remain silent for a long time, and are often associated with a long-term disease-free survival[23]. Therefore, annual follow-up with neurologic examination and pelvic magnetic resonance is necessary, particularly when residual disease is present after surgical debulking.
Table 1.
Clinical and surgical data in our patient and 17 previously reported cases
| Authors | Age (yr)/Sex | Symptoms | Surgical approach | Resection |
| MacCarty (1965) | 37/M | None | Transperitoneal approach + sacral amputation | C |
| Andersen (1986) | 14/M | None | Transperitoneal approach | C |
| Richardson (1986) | 71/M | Neurogenic bladder, constipation | Sacral laminectomy | P |
| Leeson and Hite (1989) | 21/F | Pain, weight gain, urinary frequency | Transperitoneal approach | P |
| Stener (1989) | 20/F | Pain | Sacral amputation | C |
| Spirnak and Wood (1993) | 8/M | Constipation | Not specified | C |
| Okai (2001) | 70/M | Constipation, pain | Transperitoneal approach | P |
| Lam and Nagib (2002) | 11/M | Constipation | Transperitoneal approach + laminectomy | C |
| Marmor (2002) | 70/M | None | Transperitoneal approach | C |
| Modha (2005) | 65/F | Pain | Retroperitoneal exposure | P |
| 21/F | Pain | Retroperitoneal exposure | P | |
| 21/M | None | Transperitoneal approach | P | |
| 19/F | Constipation, low back pain | Transperitoneal approach | C | |
| 28/F | Low back pain | Transperitoneal approach | P | |
| Przkora (2005) | 17/F | Amenorrhoea, weight loss | Sacral resection+laminectomy | C |
| Cerullo (2005) | 64/M | None | Transperitoneal approach | C |
| Mounasamy (2006) | 64/M | Low back and leg pain | Transperitoneal approach + laminectomy | C |
| 21/F | None | Transperitoneal approach + laminectomy | P |
C: complete rection; P: partial resection; M: male; F: female.
Footnotes
S- Editor Liu Y L- Editor Ma JY E- Editor Zhou T
References
- 1.Ghali VS, Gold JE, Vincent RA, Cosgrove JM. Malignant peripheral nerve sheath tumor arising spontaneously from retroperitoneal ganglioneuroma: a case report, review of the literature, and immunohistochemical study. Hum Pathol. 1992;23:72–75. doi: 10.1016/0046-8177(92)90015-u. [DOI] [PubMed] [Google Scholar]
- 2.Hayes FA, Green AA, Rao BN. Clinical manifestations of ganglioneuroma. Cancer. 1989;63:1211–1214. doi: 10.1002/1097-0142(19890315)63:6<1211::aid-cncr2820630628>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Shimada H, Brodur G. Tumors of peripheral neuroblasts and ganglion cells. In: Bigner DD, McLendon RE, Bruner JM, editors. Russell and Rubinstein's Pathology of Tumors of the Nervous System. 6th ed. New York: Oxford University Press; 1998. pp. 493–533. [Google Scholar]
- 4.Spirnak JP, Wood BP. Radiological cases of the month. Presacral ganglioneuroma. Am J Dis Child. 1993;147:1119–1120. [PubMed] [Google Scholar]
- 5.Stener B. Complete removal of vertebrae for extirpation of tumors. A 20-year experience. Clin Orthop Relat Res. 1989:72–82. [PubMed] [Google Scholar]
- 6.Modha A, Paty P, Bilsky MH. Presacral ganglioneuromas. Report of five cases and review of the literature. J Neurosurg Spine. 2005;2:366–371. doi: 10.3171/spi.2005.2.3.0366. [DOI] [PubMed] [Google Scholar]
- 7.Goldman RL, Winterling AN, Winterling CC. Maturation of tumors of the sympathetic nervous system. Report of long-term survival in 2 patients, one with disseminated osseous metastases, and review of cases from the literature. Cancer. 1965;18:1510–1516. doi: 10.1002/1097-0142(196511)18:11<1510::aid-cncr2820181124>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Joshi VV. Peripheral neuroblastic tumors: pathologic classification based on recommendations of international neuroblastoma pathology committee (Modification of shimada classification) Pediatr Dev Pathol. 2000;3:184–199. doi: 10.1007/s100240050024. [DOI] [PubMed] [Google Scholar]
- 9.Keller SM, Papazoglou S, McKeever P, Baker A, Roth JA. Late occurrence of malignancy in a ganglioneuroma 19 years following radiation therapy to a neuroblastoma. J Surg Oncol. 1984;25:227–231. doi: 10.1002/jso.2930250402. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni AV, Bilbao JM, Cusimano MD, Muller PJ. Malignant transformation of ganglioneuroma into spinal neuroblastoma in an adult. Case report. J Neurosurg. 1998;88:324–327. doi: 10.3171/jns.1998.88.2.0324. [DOI] [PubMed] [Google Scholar]
- 11.Leeson MC, Hite M. Ganglioneuroma of the sacrum. Clin Orthop Relat Res. 1989:102–105. [PubMed] [Google Scholar]
- 12.Marmor E, Fourney DR, Rhines LD, Skibber JM, Fuller GN, Gokaslan ZL. Sacrococcygeal ganglioneuroma. J Spinal Disord Tech. 2002;15:265–268. doi: 10.1097/00024720-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Richardson RR, Reyes M, Sanchez RA, Torres H, Vela S. Ganglioneuroma of the sacrum. A case report. Spine (Phila Pa 1976) 1986;11:87–89. doi: 10.1097/00007632-198601000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Shekitka KM, Sobin LH. Ganglioneuromas of the gastrointestinal tract. Relation to Von Recklinghausen disease and other multiple tumor syndromes. Am J Surg Pathol. 1994;18:250–257. [PubMed] [Google Scholar]
- 15.Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86:349–363. [PubMed] [Google Scholar]
- 16.Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, et al. The International Neuroblastoma Pathology Classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 17.Stout AP. Ganglioneuroma of the sympathetic nervous system. Surg Gynecol Obstet. 1947;84:101–110. [PubMed] [Google Scholar]
- 18.Cushing H, Wolbach SB. The Transformation of a Malignant Paravertebral Sympathicoblastoma into a Benign Ganglioneuroma. Am J Pathol. 1927;3:203–216.7. [PMC free article] [PubMed] [Google Scholar]
- 19.Jain M, Shubha BS, Sethi S, Banga V, Bagga D. Retroperitoneal ganglioneuroma: report of a case diagnosed by fine-needle aspiration cytology, with review of the literature. Diagn Cytopathol. 1999;21:194–196. doi: 10.1002/(sici)1097-0339(199909)21:3<194::aid-dc9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Andersen HJ, Hansen LG, Lange P, Teglbjaerg PS. Presacral ganglioneuroma. Case report. Acta Chir Scand. 1986;152:777–778. [PubMed] [Google Scholar]
- 21.Przkora R, Perez-Canto A, Ertel W, Heyde CE. Ganglioneuroma : primary tumor or maturation of a suspected neuroblastoma? Eur Spine J. 2006;15:363–365. doi: 10.1007/s00586-005-0964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacCarty CS, Waugh JM, Coventry MB, Cope WF. Surgical treatment of sacral and presacral tumors other than sacrococcygeal chordoma. J Neurosurg. 1965;22:458–464. doi: 10.3171/jns.1965.22.5.0458. [DOI] [PubMed] [Google Scholar]
- 23.Mounasamy V, Thacker MM, Humble S, Azouz ME, Pitcher JD, Scully SP, Temple HT, Eismont F. Ganglioneuromas of the sacrum-a report of two cases with radiologic-pathologic correlation. Skeletal Radiol. 2006;35:117–121. doi: 10.1007/s00256-005-0028-6. [DOI] [PubMed] [Google Scholar]


