Abstract
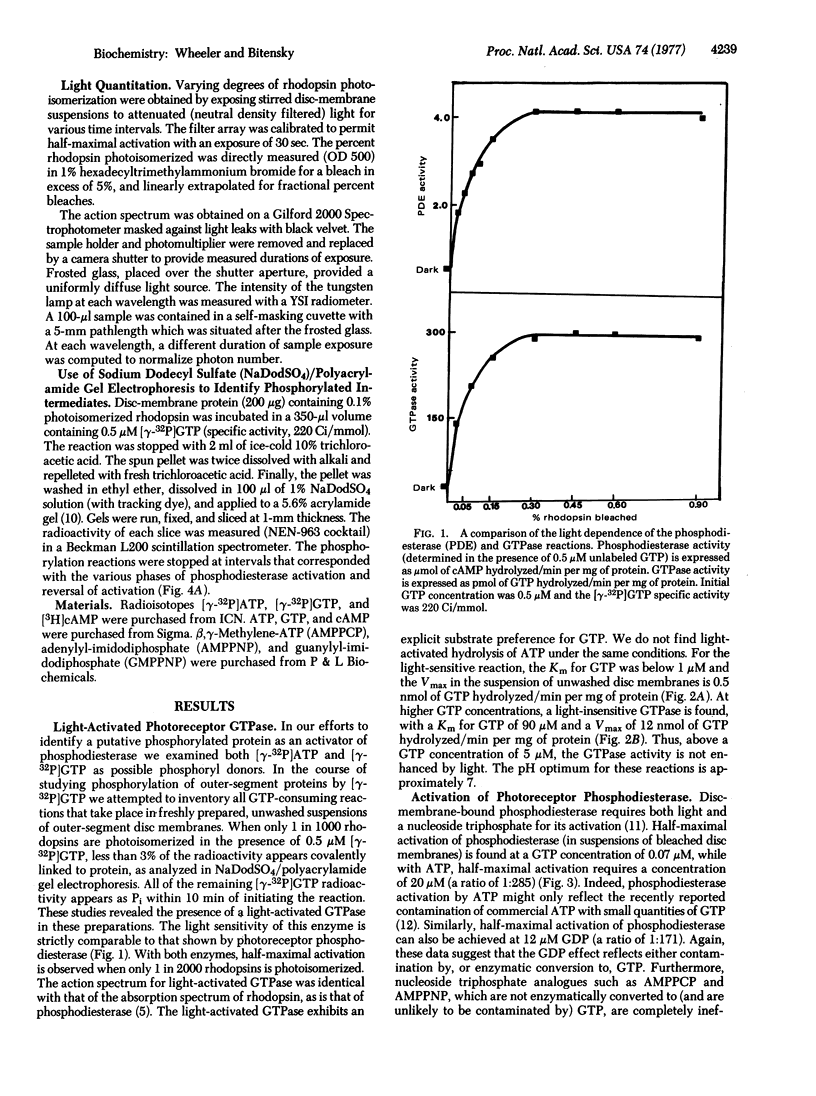
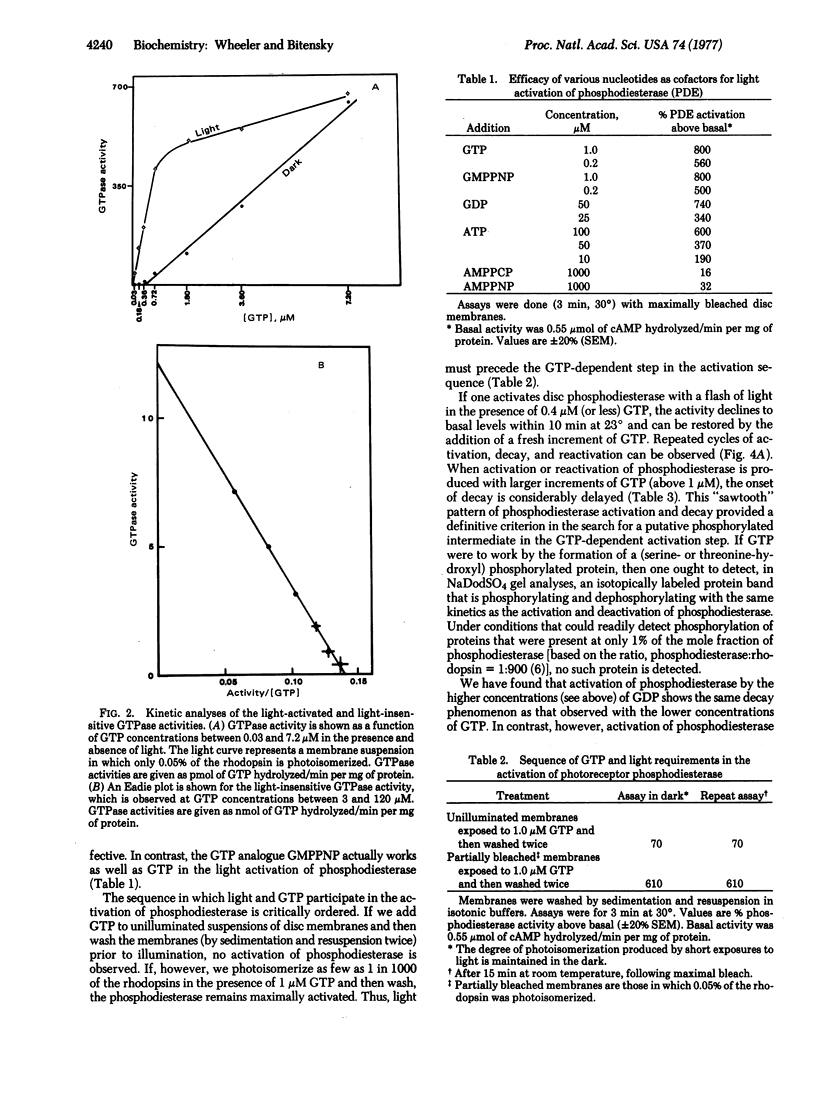
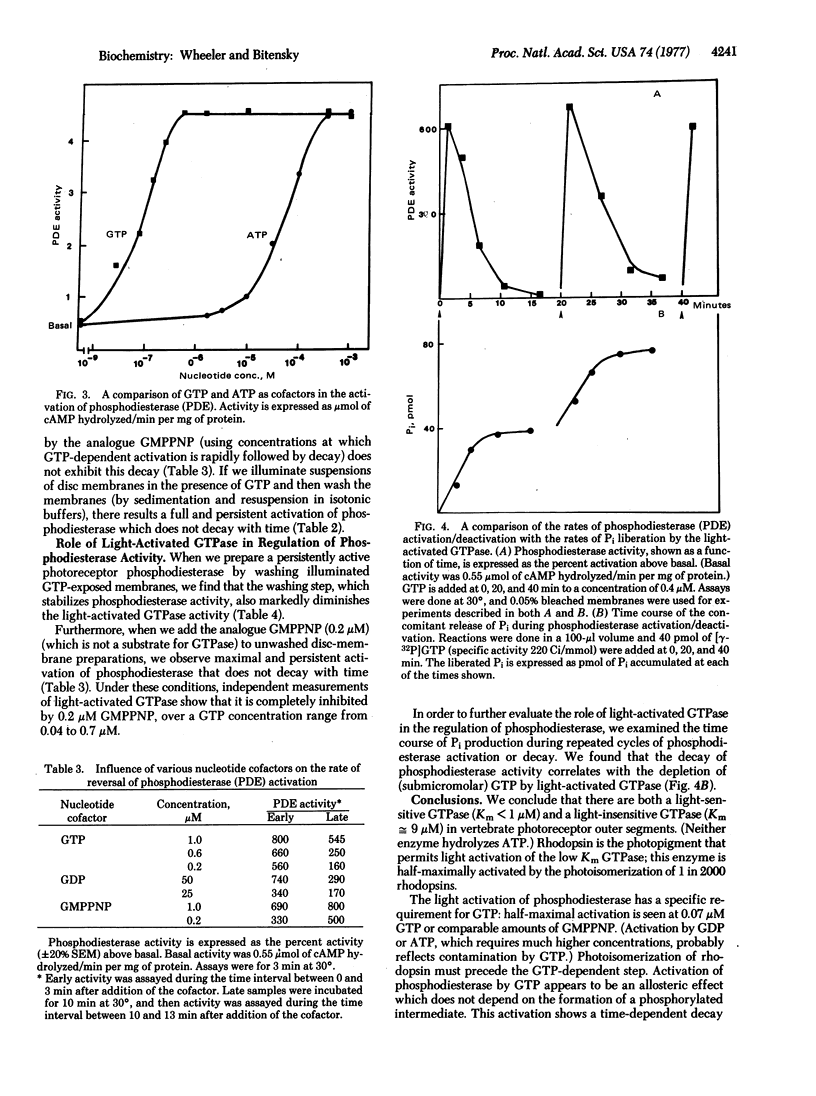
We have been studying the mechanism by which light and nucleoside triphosphates activate the discmembrane phosphodiesterase (oligonucleate 5′-nucleotidohydrolase; EC 3.1.4.1) in frog rod outer segments. GTP is orders of magnitude more effective than ATP as a cofactor in the light-dependent activation step. GTP and the analogue guanylyl-imidodiphosphate function equally as allosteric activators of photoreceptor phosphodiesterase rather than participating in the formation of a phosphorylated activator. Moreover, we have found a light-activated (5-fold) GTPase which participates in the modulation of photoreceptor phosphodiesterase. This GTPase activity appears necessary for the reversal of phosphodiesterase activation in vitro and may play a critical role in the in vivo regulation of light-sensitive phosphodiesterase. The Km for GTP in the light-activated GTPase reaction is <1 μM. The light sensitivity of this GTPase (number of photons required for half-maximal activation) is identical to that of light-activated phosphodiesterase. The GTPase action spectrum corresponds to the absorption spectrum of rhodopsin. There is, in addition, a light-insensitive GTPase activity with a Km for GTP of 90 μM. At GTP concentrations above 5 μM, there is no appreciable activation of GTPase activity by light. The substrate Km values for guanylate cyclase, light-activated GTPase, and light-activated phosphodiesterase order an enzyme array that might permit light to simultaneously cause the hydrolysis of both the substrate and product of guanylate cyclase. These findings reveal yet another facet of light regulation of photoreceptor/cyclic GMP levels and also provide a striking analogy to the GTP regulation of nonphotoreceptor, hormone-sensitive adenylate cyclase.
Keywords: retina, rhodopsin, cyclic nucleotides, adenylate cyclase, guanosine nucleotides
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurbach G. D., Spiegel A. M., Gardner J. D. Beta-adrenergic receptors, cyclic AMP, and ion transport in the avian erythrocyte. Adv Cyclic Nucleotide Res. 1975;5:117–132. [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E., Miller W. H. Adenyl cyclase as a link between photon capture and changes in membrane permeability of frog photoreceptors. Proc Natl Acad Sci U S A. 1971 Mar;68(3):561–562. doi: 10.1073/pnas.68.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitensky M. W., Miki N., Keirns J. J., Keirns M., Baraban J. M., Freeman J., Wheeler M. A., Lacy J., Marcus F. R. Activation of photoreceptor disk membrane phosphodiesterase by light and ATP. Adv Cyclic Nucleotide Res. 1975;5:213–240. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Goridis C., Virmaux N. Light-regulated guanosine 3',5'-monophosphate phosphodiesterase of bovine retina. Nature. 1974 Mar 1;248(5443):57–58. doi: 10.1038/248057a0. [DOI] [PubMed] [Google Scholar]
- Goridis C., Virmaux N., Urban P. F., Mandel P. Guanyl cyclase in a mammalian photoreceptor. FEBS Lett. 1973 Mar 1;30(2):163–166. doi: 10.1016/0014-5793(73)80642-8. [DOI] [PubMed] [Google Scholar]
- Keirns J. J., Miki N., Bitensky M. W., Keirns M. A link between rhodopsin and disc membrane cyclic nucleotide phosphodiesterase. Action spectrum and sensitivity to illumination. Biochemistry. 1975 Jun 17;14(12):2760–2766. doi: 10.1021/bi00683a032. [DOI] [PubMed] [Google Scholar]
- Kimura N., Nagata N. The requirement of guanine nucleotides for glucagon stimulation of adenylate cyclase in rat liver plasma membranes. J Biol Chem. 1977 Jun 10;252(11):3829–3835. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levinson S. L., Blume A. J. Altered guanine nucleotide hydrolysis as basis for increased adenylate cyclase activity after cholera toxin treatment. J Biol Chem. 1977 Jun 10;252(11):3766–3774. [PubMed] [Google Scholar]
- Lolley R. N., Farber D. B. Cyclic nucleotide phosphodiesterases in dystrophic rat retinas: guanosine 3',5' cyclic monophosphate anomalies during photoreceptor cell degeneration. Exp Eye Res. 1975 Jun;20(6):585–597. doi: 10.1016/0014-4835(75)90225-0. [DOI] [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Miki N., Keirns J. J., Marcus F. R., Bitensky M. W. Proceedings: Light regulation of adenosine 3',5' cyclic monophosphate levels in vertebrate photoreceptors. Exp Eye Res. 1974 Mar;18(3):281–297. doi: 10.1016/0014-4835(74)90155-9. [DOI] [PubMed] [Google Scholar]
- Neufeld A. H., Levy H. M. A second ouabain-sensitive sodium-dependent adenosine triphosphate in brain microsomes. J Biol Chem. 1969 Dec 10;244(23):6493–6497. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y., Londos C., Harwood J. P., Martin B. R., Rendell M., Berman M. The role of adenine and guanine nucleotides in the activity and response of adenylate cyclase systems to hormones: evidence for multi-site transition states. Acta Endocrinol Suppl (Copenh) 1974;191:11–37. doi: 10.1530/acta.0.077s0011. [DOI] [PubMed] [Google Scholar]
- Schramm M., Rodbell M. A persistent active state of the adenylate cyclase system produced by the combined actions of isoproterenol and guanylyl imidodiphosphate in frog erythrocyte membranes. J Biol Chem. 1975 Mar 25;250(6):2232–2237. [PubMed] [Google Scholar]
- Schramm M. The catecholamine-responsive adenylate cyclase system and its modification by 5'guanylylimidodiphosphate. Adv Cyclic Nucleotide Res. 1975;5:105–115. [PubMed] [Google Scholar]


