Abstract
Background
Long-term hemodialysis patients are prone to an exceptionally high burden of cardiovascular disease and mortality. The novel temperature based technology of Digital Thermal Monitoring (DTM) of vascular reactivity appears associated with the severity of coronary artery disease in asymptomatic population. We hypothesized that in hemodialysis patients the DTM and coronary artery calcium (CAC) score have a gradient association that follows that of subjects without kidney disease.
Methods
We examined the cross-sectional DTM-CAC associations in a group of long-term hemodialysis patients and their 1:1 matched normal counterparts, Area under the curve for temperature (TMP-AUC), the surrogate of the DTM index of vascular function, was assessed after a 5-minute arm-cuff reactive hyperemia test. Coronary calcium score was measured via EBCT or MDCT scan.
Results
We studied 105 randomly recruited hemodialysis patients (age:58±13 years, 47 % men) and 105 age- and gender-matched controls. In hemodialysis patients vs. controls TMP-AUC was significantly worse (114±72 vs. 143±80. p=0.001) and CAC score was higher (525±425 vs. 240±332, p<0.001). Hemodialysis patients were 14 times more likely to have CAC score >1000 as compared with controls. After adjustment for known confounders, the relative risk for case vs. control for each standard deviation decrease in TMP-AUC was 1.46 (95%CI: 1.12-1.93, p=0.007).
Conclusions
Vascular reactivity measured via the novel DTM technology is incrementally worse across CAC scores in hemodialysis patients, in whom both measures are even worse than their age- and gender matched controls. The DTM technology may offer a convenient and radiation-free approach to risk-stratify hemodialysis patients.
Keywords: Chronic Kidney Disease, Coronary Calcification, Digital Thermal Monitoring, Hemodialysis, Vascular Disease
Introduction
Long-term hemodialysis patients are prone to an exceptionally high burden of cardiovascular disease and mortality (1,2). Traditional cardiovascular risk factors which are helpful in risk stratification of asymptomatic population without chronic kidney disease, may not fully account for this increased risk of adverse cardiovascular disease burden in end stage renal disease (ESRD) patients undergoing maintenance hemodialysis (MHD) (3). This fact highlights the need to find novel nontraditional risk factors useful for identification of at risk patients. A number of marker like hyperhomocysteinemia, elevated C-reactive protein, low albumin and coronary artery calcium have been proposed to account for this increased cardiovascular risk in ESRD patients (4-6).
Coronary artery calcium (CAC), an anatomic disease marker of calcified coronary atherosclerosis, is one of the strongest predictors of future adverse cardiovascular events and has been suggested to be incorporated into national guidelines for risk stratification of asymptomatic population (7). It is also an independent predictor of mortality in long term MHD patients beyond traditional cardiovascular risk factors.(8) Fingertip digital thermal monitoring (DTM) of vascular reactivity is a non-invasive, operator independent test based on changes in fingertip temperature during and after arm cuff occlusion. The fingertip DTM of vascular function during arm cuff reactive hyperemia test correlates with the severity and extent of coronary artery disease in both asymptomatic and symptomatic population (11, 12). Vascular dysfunction is an early step in the development of atherosclerotic plaque formation that can be used for cardiovascular risk assessment (13,14). The functional changes in vascular function precede the development of structural changes and reverse more quickly in response to therapies (15, 16).
The current study evaluates the hypothesis that CAC is associated with fingertip temperature based DTM of vascular function in long term MHD patients compared to an age- and gender- matched cohort without CKD (non-CKD). We also hypothesized that in MHD patients the DTM and CAC score have a gradient association that follows that of subjects without kidney disease. However, we expected that this gradient be steeper in long term MHD patients compared with subjects without chronic kidney disease. Since vascular dysfunction represents earlier stage in the atherosclerosis process and since MHD patients have higher burden of cardiovascular disease compared with the general population, we expected to find lower (worse) DTM in MHD patients, even in MHD patients with CAC score of zero, compared to non-CKD controls.
Subject and Methods
Study Population
The study population consisted of 105 long term MHD patients who underwent a number of elaborate tests including both CAC and DTM. MHD patients were selected from 10 Da Vita long-term dialysis facilities in South Bay Los Angeles area. Inclusion criteria were patients who had been on maintenance hemodialysis for at least 6 months, had an average monthly Kt/Vsp >1.4 with a dialysis time between 3.5 and 5 hours, functioning AV graft or fistula and signed the institutional review board approved consent form. Exclusion criteria for MHD patients included (1) Peritoneal dialysis; (2) Terminal illnesses with life expectancy <6 months, e.g. stage IV cancer or full-blown AIDS; (3) MHD <6 months after switching from Peritoneal dialysis or renal transplant failure; (4) Likelihood of pregnancy or intention to become pregnant; (5) Acute wasting condition or active systemic disease (e.g. acute flares of SLE, rheumatoid arthritis or vasculitis); (6) Pulse chemo-therapy; (7) Non-compliance with dialysis treatment; and (8) dialysis catheter.
The matched cohort for comparison was selected from a previous study population (11) that included 129 consecutive patients with suspected coronary artery disease who were referred to the cardiac imaging laboratory. All subjects underwent CAC scanning and DTM as a part of the study. Patients with established cardiovascular disease, stroke, diabetic retinopathy, end-stage renal disease, Reynaud’s syndrome, infection, cancer, immunosuppression, systemic inflammation, and end-stage liver disease were excluded. All non-CKD patients had normal GFR at study entry and no history of renal disease. All patients had signed the informed consent approved by the Institutional Review Board of Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. These patients were matched on one-on-one basis with MHD patients based on age and gender. A total of 105 patients from MHD group and 105 patients from matched non-CKD group were selected. All subjects underwent CAC scan and DTM test on the same day at Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. These measurements were performed on non-dialysis days.
Digital Thermal Monitoring
All DTM measurements were performed in a quiet, dimmed room at a controlled ambient temperature between 23.0 and 25.0°C (11). Studies were conducted after an overnight fast of at least 10 h (water was permitted) and abstinence from tobacco, alcohol, caffeine, vasoactive medications, exercise, high-fat foods and vitamin C. The measurements were obtained with the subjects in sitting position and after 30 min of rest. Blood pressure measurement was recorded in the non-arteriovensous-fistula arm in a sitting position 5 min before the DTM test (IntelliSense_Professional Digital Blood Pressure Monitor, HEM-907XL, Omron Inc, Illinois) in MHD patients and in control arm in non-CKD patients. DTM of both hands was obtained during 5 min stabilization, 5 min cuff inflation to 30-50 mmHg greater than systolic blood pressure, and 5 min deflation using an automated, operator-independent protocol (VENDYS, Endothelix Inc., Houston, TX). Thermal changes during a 5 min arm-cuff induced reactive hyperemia test were monitored continuously in the fingertip of both the occluded and non-occluded arms using VENDYS software. The device consists of a computer based thermometry system with two fingertip RTD (Resistance Temperature Detector) fast response probes designed to minimize the skin-probe contact area and fingertip pressure, attached to the pulp of the index finger on both hands. The system includes a common automated sphygmomanometer cuff, cuff-inflation pump, and release valve to permit noninvasive measurement of arterial pressure and the control of occlusive hyperemia.
Coronary Artery Calcium Score Measurement
Coronary artery calcium scans were performed on E-Speed electron beam scanner (EBCT) (GE-Imatron, South San Francisco, CA, USA) or 64-multidetector computed tomography (MDCT) (General Electric, Milwaukie, Wisconsin) scanners. The coronary arteries were imaged with 30-48 contiguous 2.5-3 mm slices during mid-diastole using ECG-triggering during a 35 sec breath hold. CAC measurements were performed on non-contrast studies by an experienced reader blinded to the patient information. Coronary artery calcium was defined as a plaque of at least 3 contiguous pixels (area 1.02 mm2) with a density of >130 Hounsfield units. The lesion score was calculated by multiplying the lesion area by a density factor derived from the maximal Hounsfield unit within this area, as described by Agatston et al (17). Total calcium score was determined by summing individual lesion scores from each of the four main coronary arteries (left main coronary, left anterior descending coronary, left circumflex coronary and right coronary arteries). CAC (Agatston Score, AS) were categorized as CAC 0, 1-100, 101-400, 401-1000 and >1000 AS.
Statistical Analysis
The clinical information of the patient population will be compared using t-test and chi-square test. The continuous variables will be mentioned as mean ± standard deviation whereas the categorical variables will be presented as percentages or absolute numbers. CAC scores will be categorized as increasing quartiles as follows: 0, 1-100,101-400,401-1000 & >1000. The association between CAC score quartiles and DTM of MHD and non-CKD cohort will be presented using a bar graph. The relative risk of CAC will be assessed for MHD patients according to the CAC score categories of 1-100,101-400, 401-1000 & >1000 using relative risk regression analysis. The non-CKD patients will be used as reference group for this analysis. The results will be adjusted for traditional cardiovascular risk factors including age, gender, diabetes mellitus, hypertension, hypercholesterolemia, smoking, ethnicity and race. Similarly for DTM, we will determine the relative risk per standard deviation decrease in TMP-AUC in MHD patients compared with the non-CKD patients.
Results
General characteristics of the patients are given in Table 1. Mean age of the MHD population was 58±13 (range: 27-84 years; men 47%), and the non-CKD matched cohort was 59±8 (men 47%). There were no statistically significant differences based on age, gender, body mass index or diabetes among subjects with and without CKD (Table 1). Mean heart rate for MHD and non-CKD patients during DTM test was 71±10/min. and 62±10/min., respectively (p<0.001). Mean systolic and diastolic blood pressure for MHD and non-CKD patients during DTM test was 156±26 mmHg, 145±21 mmHg (p=0.002) and 81±13 mmHg, 79±11 mmHg (p=0.21), respectively. The prevalence of patients with CAC score of zero was 15 (14%) vs. 39 (37%) in MHD compared with non-CKD patients. There were 72 (69%) patients with CAC >100AS in MHD group compared with non-CKD patients (26 patients, 25%). Mean hemoglobin level for MHD patients was 12.01 ±1.16.
Table 1.
represents demographic information of ACKD and matched non-CKD cohort
| Matched non-CKD Cohort (n=105) |
ACKD Cohort (n=105) |
P | |
|---|---|---|---|
| Age (years) | 59±8 | 58±13 | 0.50 |
| Male, n(%) | 48(46.6) | 48(46.6) | 1.0 |
| Height (cm) | 169.6±7.5 | 161.7±21.0 | 0.0004 |
| Weight (kg) | 90.5±10.8 | 78.4±22.8 | <.0001 |
| BMI (kg/m2) | 31.6±4.4 | 30.7±15.7 | 0.59 |
| Hypertension, n(%) | 101(98.1) | 81(85.3) | 0.01 |
| Diabetes, n(%) | 58(56.3) | 65(64.4) | 0.24 |
| Family history of CAD, n(%) | 40(38.8) | 23(23.2) | 0.02 |
| Dyslipidemia, n(%) | 65 (63.1) | 34(35.8) | <0.001 |
| CAC Score | 240±332 | 525±425 | 0.0001 |
| TMP-AUC | 143±80 | 114±72 | 0.001 |
ACKD-Advanced Chronic Kidney Disease, BMI-Body Mass Index, CAD-Coronary Artery Disease, CKD-Chronic Kidney Disease, CAC-Coronary Artery Calcium, TMP-AUC-Area the Temperature Curve
In long-term MHD patients vs. controls, TMP-AUC was significantly lower or worse (114±72 vs. 143±80. p=0.001), and CAC score was higher (525±425 vs. 240±332, p<0.001). After adjustment for age, gender, diabetes mellitus, hypertension, hypercholesterolemia, smoking, ethnicity and race, the risk for each standard deviation decrease in TMP-AUC was 1.46 (95%CI 1.12-1.93, p=0.007) in MHD compared to non-CKD. TMP-AUC was lower in each CAC score category (CAC 0, 1-100, 101-400, 401-1000 & >1000 AS) in MHD population compared to the non-CKD group (p < 0.05) (Figure 1). The lowest mean value of TMP-AUC was among the MHD group with CAC >1000 AS (figure 1). When we compared the TMP-AUC of MHD patients with CAC score of zero, the TMP-AUC was found to be significantly lower than the non-CKD matched cohort.
Figure 1. Association between CAC and DTM of hemodialysis patients and matched non-CKD subjects.
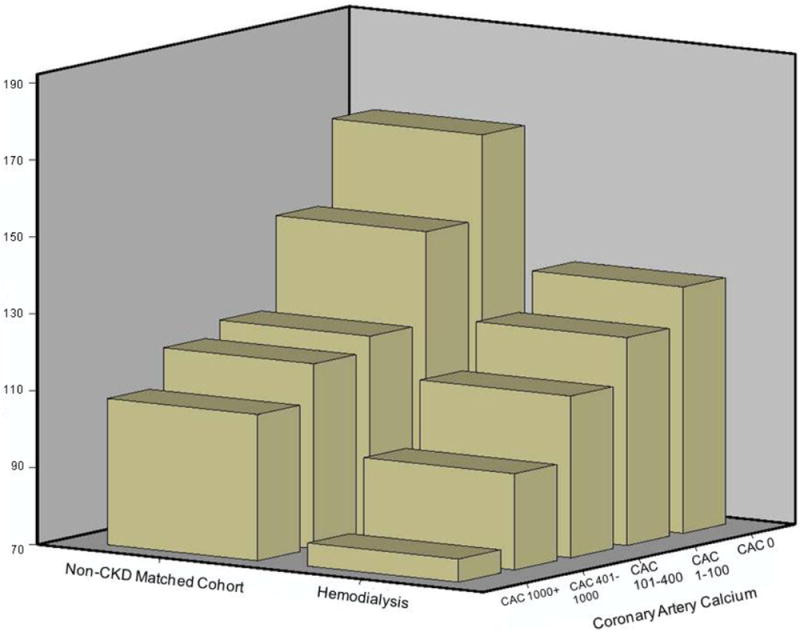
Figure shows association between CAC and DTM of endothelial function measured in hemodialysis patients and matched non-CKD cohort patients. DTM is lower (worse) within each CAC score category in hemodialysis patients compared to matched non-CKD controls, with lowest being in patients with CAC score >1000 AS.
(AS-Agatston Score, CAC-Coronary Artery Calcium, CKD-Chronic Kidney Disease, DTM-Digital Thermal Monitoring)
The relative risk of CAC increase from 0 to 1-100, 101-400, 401-1000 and >1000 AS was 5.29 (95%CI 1.82- 15.38, p=0.002), 7.49 (95%CI 2.45- 22.85, p=0.0001), 10.88 (95%CI 3.82- 31.03, p=0.0001), and 13.98 (95%CI 4.86- 40.14, p=0.0001) respectively, in MHD compared to non-CKD (table 2). These results were adjusted for age, gender, diabetes, hypertension, hyperlipidemia, smoking, ethnicity and race.
Table 2.
showing Relative Risk€ of CAC scores for ACKD patients compared with matched non-CKD patients
| Non-CKD Matched Cohort | ACKD Cohort | |
|---|---|---|
| CAC 1-100 | 1.0 (Ref) | 5.29 (95%CI 1.82- 15.38), p=0.002 |
| CAC 101-400 | 1.0 (Ref) | 7.49 (95%CI 2.45- 22.85), p=0.0001 |
| CAC 401-1000 | 1.0 (Ref) | 10.88 (95%CI 3.82- 31.03), p=0.0001 |
| CAC >1000 | 1.0 (Ref) | 13.98 (95%CI 4.86- 40.14), p=0.0001 |
These results were adjusted for age, gender, diabetes, hypertension, hyperlipidemia, smoking, ethnicity and race.
ACKD-Advanced Chronic Kidney Disease, CKD-Chronic Kidney Disease, CAC-Coronary Artery Calcium, CI-Confidence Interval
Discussion
In this study of 105 MHD patients we found that vascular reactivity measured via the novel DTM technology is incrementally worse across CAC scores in these patients, in whom both measures are even worse than their age- and gender matched controls. Given these unprecedented findings, the novel DTM technology may offer a convenient and radiation-free approach to risk-stratify hemodialysis patients.
Traditional cardiovascular risk factors are important risk stratification screening parameters used in the general population such as obesity, hypercholesterolemia and hypertension, but they appear to play a protective role in dialysis patients; a concept called ‘reverse epidemiology’ where under-nutrition is associated with adverse cardiovascular outcomes in long term dialysis patients compared to the well known association between over-nutrition and poor outcome in the general population (3). A possible explanation for this is that small numbers of patients reach MHD stage and may not share same constellation of risk factors as those of their predecessor CKD patients. This fact highlights the need to identify novel markers of coronary atherosclerosis in long term dialysis patients. The current shows the presumptive role of novel non-invasive, operator independent, radiation free DTM of vascular reactivity in risk stratification of at risk long term MHD patients.
CAC is an anatomic marker of coronary atherosclerosis that correlates with the presence and extent of coronary atherosclerosis (18). CAC has been shown to be independent predictor of mortality in a multivariable model after adjusting for age, gender, ethnicity and cardiac risk factors in a registry of 25,253 asymptomatic populations (19). Addition of CAC to traditional risk factors resulted in a higher concordance index compared with traditional risk factors alone (0.81 vs. 0.61, p<0.0001 respectively). Detrano et al., in a population based study of 6,722 participants followed for a median period of 3.8 years for coronary events, demonstrated a CAC score >300 AS was associated with adjusted risk of 9.67 (p<0.001). CAC has also been shown to predict all cause mortality in MHD patients. MHD patients having CAC scores of 101-400 and >400 were associated with hazard ratio for death of 8.5 and 13.3 compared with CAC score of zero, independent of demographics, co-morbidities, lipids and other cardiovascular risks, surrogates of bone disease, nutritional and inflammatory markers and dialysis dose (8). There were only two deaths reported in patients with CAC score zero compared with 30 deaths in patients with CAC score >400. In the present study, we found that the relative risk of CAC increase in MHD patients with CAC score 101-400 was 7.5% and 11% for >400 AS compared with the non-CKD population. The relative risk for >1000 AS CAC score was 14% in MHD population compared with matched non-CKD population.
Vascular dysfunction is an early step in atherosclerosis process and precedes the actual development of atherosclerotic plaques (13,14). Pathophysiology of endothelial dysfunction includes reduced bioavailability of nitric oxide, elevated levels of asymmetric Dimethylarginine (ADMA), oxidative excess, angiotensinogen II, hyperhomocysteinemia and various mechanisms in diabetics (20). The novel technology of DTM measures skin vascular response based on fingertip temperature reactivity. Skin vascular response is believed to be primarily due to micro-vascular reactivity. Studies have been done regarding the pathophysiology of skin vascular reactivity and its significance in patients with vascular disease (21-24). Our previous study showed that vascular dysfunction measured by DTM was strongly correlated with Framingham risk score and CAC independent of age, sex, and traditional cardiac risk factors and was superior to Framingham risk score for the prediction of significant CAC in a group of 230 asymptomatic subjects (12). This study also showed the superior and incremental predictive power of DTM in addition to FRS in prediction of significant CAC ≥100 (0.79 for DTM vs. 0.66 for FRS vs. 0.89 for DTM+FRS respectively). Similarly, in another study, DTM was shown to be associated with the presence and severity of coronary artery disease measured by cardiac computed tomography angiography (11). Endothelial dysfunction in coronary arteries has been shown to associate with cardiovascular events in patients with and without coronary artery disease (14,25,26). Caglar et al (10) showed presence of endothelial dysfunction in all stages (1-5) of CKD and association with reduction in estimated glomerular filtration rate using Flow mediated dilatation determined by high-resolution brachial ultrasonography. Perticone et al. (27) showed impaired vasodilator response to acetylcholine associated with renal function loss in patients with essential hypertension. These studies show the presence of vascular dysfunction in patients with different stages of CKD. Chung et al. (28) showed a differential response of the vasculature obtained from non-dialyzed and dialyzed CKD patients, to various stimuli like depolarization, agonist and acetylcholine. The current study shows that vascular reactivity measured via the novel DTM technology is incrementally worse across CAC score categories, even worse than their age and gender matched controls. The worse vascular function was noted in MHD patients with CAC score 1000+. DTM vascular reactivity was also worse than their age- and gender matched control group in MHD patients with absence of CAC (CAC=0). The risk for each standard deviation decrease in TMP-AUC was 1.46 in MHD compared to non-CKD, after adjustment for cardiovascular risk factors. The cuff induced reactive hyperemia DTM test of vascular reactivity, by virtue of its standardized, operator independent and automated technique is easy to use for risk assessment of long term MHD patients. The ease of use and radiation-free approach, this novel fingertip temperature DTM of vascular function is well suited for repeated measurements and following the effect of various therapeutic treatments in at risk long term MHD patients.
The following limitations of our study should be considered: (1) Despite sample size, we were able to show significant differences in CAC and DTM of MHD patients compared with non-CKD patients. Further outcome studies with larger sample size are needed to definitively demonstrate the prognostic importance of these measures in the MHD population. (2) The study did not include various stages of renal dysfunction; it only included end stage renal disease patients undergoing MHD. The reason we selected only MHD patients is because of the amount of cardiovascular burden and mortality in this group and we were clearly able to show the high prevalence of CAC score and lower DTM in each CAC score category compared with the control non-CKD population. Also, MHD patients were found to be at much higher risk of significant CAC scores (100-400 & >1000) with relative risk of 7.5 and 14 respectively. (3) Systemic markers of inflammation like CRP and homocystine measurements were not included in the study. (4) The design of the study was cross-sectional; no cause–effect relationship can be derived from this. But this study clearly gives an idea of the higher burden of coronary atherosclerosis and more vascular dysfunction in MHD patients compared with the control non-CKD patients.
Conclusions
Long-term MHD patients have significantly worse vascular dysfunction as reflected by novel DTM metrics when compared with their age- and gender matched non-CKD subjects. The DTM has a gradient and incremental association with CAC score. The per standard deviation decrease of DTM measured vascular dysfunction was significantly higher in long-term MHD patients compared with age- and gender matched non-CKD control subjects, independent of traditional cardiovascular risk factors. The novel operator independent, radiation-free, automated technique of DTM vascular function may provide a very useful tool for risk assessment and follow up of therapeutic treatments in at risk long term MHD patients.
Acknowledgments
We thanks DaVita Clinical Research and DaVita dietitians in Wild West, Gold Coast, and Surf and Sun divisions for supporting the study and the staff at Harbor-UCLA General Clinic Research Center Core Laboratories for the management of blood samples and measuring inflammatory markers.
Funding Source:
The manuscript was supported by the research grants R21-DK078012 and K24-DK091419 from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health for the authors, a philanthropic grant from Mr. Harold C. Simmons and by the General Clinical Research Center grant M01- RR00425 from the NIH National Centers for Research Resources.
Support:
This study was supported by a National Institutes of Health grant (R21 DK078012) from NIH-NIDDK to Dr Kalantar-Zadeh and by the General Clinical Research Center grant M01- RR00425 from the NIH National Centers for Research Resources.
Footnotes
Disclosure:
There is no industry financial disclosure for any authors involved in this study.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–23. [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 4.Mallamaci F, Zoccali C, Tripepi G, et al. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney Int. 2002;61:609–14. doi: 10.1046/j.1523-1755.2002.00144.x. [DOI] [PubMed] [Google Scholar]
- 5.Soriano S, Gonzalez L, Martin-Malo A, Rodriguez M, Aljama P. C-reactive protein and low albumin are predictors of morbidity and cardiovascular events in chronic kidney disease (CKD) 3-5 patients. Clin Nephrol. 2007;67:352–7. doi: 10.5414/cnp67352. [DOI] [PubMed] [Google Scholar]
- 6.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Shantouf RS, Budoff MJ, Ahmadi N, et al. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. 2010;31:419–25. doi: 10.1159/000294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoppet M, Shroff RC, Hofbauer LC, Shanahan CM. Exploring the biology of vascular calcification in chronic kidney disease: what’s circulating? Kidney Int. 2008;73:384–90. doi: 10.1038/sj.ki.5002696. [DOI] [PubMed] [Google Scholar]
- 10.Caglar K, Yilmaz MI, Saglam M, et al. Serum fetuin-a concentration and endothelial dysfunction in chronic kidney disease. Nephron Clin Pract. 2008;108:c233–40. doi: 10.1159/000120209. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadi N, Nabavi V, Nuguri V, et al. Low fingertip temperature rebound measured by digital thermal monitoring strongly correlates with the presence and extent of coronary artery disease diagnosed by 64-slice multi-detector computed tomography. Int J Cardiovasc Imaging. 2009;25:725–38. doi: 10.1007/s10554-009-9476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadi N, Hajsadeghi F, Gul K, et al. Relations between digital thermal monitoring of vascular function, the Framingham risk score, and coronary artery calcium score. J Cardiovasc Comput Tomogr. 2008;2:382–8. doi: 10.1016/j.jcct.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 14.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 15.Schmieder RE, Schobel HP. Is endothelial dysfunction reversible? Am J Cardiol. 1995;76:117A–121A. doi: 10.1016/s0002-9149(05)80032-3. [DOI] [PubMed] [Google Scholar]
- 16.Westphal S, Abletshauser C, Luley C. Fluvastatin treatment and withdrawal: effects on endothelial function. Angiology. 2008;59:613–8. doi: 10.1177/0003319708316005. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 19.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–70. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 20.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–92. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 21.Lenasi H, Strucl M. The effect of nitric oxide synthase and cyclooxygenase inhibition on cutaneous microvascular reactivity. Eur J Appl Physiol. 2008;103:719–26. doi: 10.1007/s00421-008-0769-8. [DOI] [PubMed] [Google Scholar]
- 22.Rossi M, Cupisti A, Di Maria C, Galetta F, Barsotti G, Santoro G. Blunted post-ischemic increase of the endothelial skin blood flowmotion component as early sign of endothelial dysfunction in chronic kidney disease patients. Microvasc Res. 2008;75:315–22. doi: 10.1016/j.mvr.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Berghoff M, Kathpal M, Kilo S, Hilz MJ, Freeman R. Vascular and neural mechanisms of ACh-mediated vasodilation in the forearm cutaneous microcirculation. J Appl Physiol. 2002;92:780–8. doi: 10.1152/japplphysiol.01167.2000. [DOI] [PubMed] [Google Scholar]
- 24.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 25.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 26.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 27.Perticone F, Maio R, Tripepi G, Zoccali C. Endothelial dysfunction and mild renal insufficiency in essential hypertension. Circulation. 2004;110:821–5. doi: 10.1161/01.CIR.0000138745.21879.27. [DOI] [PubMed] [Google Scholar]
- 28.Chung AW, Yang HH, Sigrist MK, et al. Differential microvasculature dysfunction in living kidney donor transplant recipients: nondialyzed versus dialyzed chronic kidney disease patients. J Vasc Res. 2010;47:128–38. doi: 10.1159/000235967. [DOI] [PubMed] [Google Scholar]


