Abstract
Recent studies have identified the presence of a novel Mep/Amt/Rh glycoprotein family of proteins that may play an important role in transmembrane ammonia transport. One of the mammalian members of this family, Rh C glycoprotein (RhCG), transports ammonia, is expressed in distal nephron sites that are critically important for ammonia secretion, exhibits increased expression in response to chronic metabolic acidosis, and originally was cloned as a tumor-related protein. The purpose of our studies was to determine the localization of RhCG in the normal and neoplastic human kidney. Immunoblot analysis of human renal cortical protein lysates demonstrated RhCG protein expression with a molecular weight of approximately 52 kD. Immunohistochemistry revealed both apical and basolateral Rhcg expression in the distal convoluted tubule, connecting segment, and initial collecting tubule and throughout the collecting duct. Co-localization with calbindin-D28k, H+-ATPase, aquaporin-2, and pendrin showed that distal convoluted tubule and connecting segment cells, A-type intercalated cells, and non-A, non-B cells express RhCG and that B-type intercalated cells, principal cells, and inner medullary collecting duct cells do not. In renal neoplasms, RhCG was expressed by chromophobe renal cell carcinoma and renal oncocytoma but not by clear cell renal cell carcinoma or by papillary renal cell carcinomas. These studies suggest that RhCG contributes to both apical and basolateral membrane ammonia transport in the human kidney. Furthermore, renal chromophobe renal cell carcinoma and renal oncocytoma seem to originate from the A-type intercalated cell.
Renal ammonia metabolism is the primary component of net acid excretion and thereby is critical for acidbase homeostasis (1-4). Ammonia is produced by the proximal tubule and is secreted preferentially into the luminal fluid. The thick ascending limb of the loop of Henle reabsorbs the majority of luminal ammonia into the renal interstitium, where the collecting duct secretes it into the luminal fluid. Under normal conditions, 70 to 80% of total urinary ammonia is secreted by the collecting duct, and almost the entire increase in net acid excretion in response to chronic metabolic acidosis is due to increased ammonia excretion. Importantly, this increased renal ammonia excretion is associated with substantial increases in collecting duct ammonia secretion. Accordingly, understanding the regulation of collecting duct ammonia transport is important.
Recent studies have identified a Mep/Amt/Rh glycoprotein family of proteins, which we have termed the ammonia transporter family, that may mediate an important role in ammonia transport (5-7). The mammalian members of this family include the erythroid-specific protein Rh A glycoprotein (RhAG/Rhag) and the nonerythroid proteins Rh B glycoprotein (RhBG/Rhbg) and Rh C glycoprotein (RhCG/Rhcg). Early studies identified that RhAG and RhCG were homologous to Mep/Amt proteins and that they restored ammonia-dependent growth in yeast that was deficient in endogenous ammonia transporters, suggesting that RhAG and RhCG could function as ammonia transporters (8). Further studies demonstrated that RhAG can transport ammonia and have characterized the transport as being functionally equivalent to facilitated NH3 transport and/or NH4+/H+ exchange (9-11).
The nonerythroid Rh glycoproteins, RhBG and RhCG, also transport ammonia (10-16) and are expressed in a wide variety of tissues in which ammonia metabolism is important, including kidneys, liver, central nervous system, gastrointestinal tract, and skeletal muscles (17-23). In the kidney, RhCG is expressed in the distal convoluted tubule (DCT), connecting segment (CNT), initial collecting tubule (ICT), and collecting duct in both rat and mouse kidneys (19,21,24), and Rhcg expression in the outer medullary collecting duct (OMCD) and inner medullary collecting duct (IMCD) increases in response to chronic metabolic acidosis (24,25). Therefore, RhCG may be important in renal ammonia transport.
To understand the specific role of RhCG in renal ammonia transport, it is important to know both its cellular and its membrane expression in the human kidney. Studies in the mouse and rat have suggested that there may be species-specific differences in the location of RhCG. In the mouse kidney, only apical immunoreactivity for RhCG has been reported (19). One study in the rat kidney also identified only apical RhCG immunoreactivity (21), whereas other studies, using both light microscopic immunohistochemistry and immunogold electron microscopy, have demonstrated RhCG expression in both the apical and basolateral plasma membranes (24,25). Therefore, it is possible that RhCG/Rhcg may play a central role in both apical and basolateral ammonia transport in the mammalian kidney. However, no previous studies have examined the expression of these proteins in the human kidney. Given the important differences in the expression of Rhcg, apical versus apical and basolateral, in the rat and mouse kidneys, respectively, understanding RhCG’s expression in the human kidney is important for understanding its specific role in human ammonia transport. Therefore, the purpose of our studies was to examine RhCG expression in the normal human kidney. We used nephrectomy specimens that were obtained as part of treatment for renal carcinomas and examined RhCG expression in uninvolved regions of the kidney and in neoplastic regions. To determine the specific cell types that express RhCG, we performed double-labeling immunohistochemistry using antibodies against calbindin D28K, H+-ATPase, pendrin, and aquaporin-2 (AQP-2).
Materials and Methods
Antibodies
Affinity-purified antibodies to RhCG that were generated in this laboratory have been characterized previously (17,19,20,24,25). Antibodies to H+-ATPase were obtained from Santa Cruz Biotechnology (SC-20943; Santa Cruz, CA), and antibodies to AQP-2 and calbindin- D28k were obtained from Chemicon (Temecula, CA). Antibodies to pendrin were provided by Ines E. Royaux, PhD (National Human Genome Research Institute, National Institutes of Health, Bethesda, MD).
Human Kidney Tissue
Normal human kidney tissues were obtained from the pathology laboratory of the Gainesville Veterans Affairs Medical Center (GVAMC; Gainesville, FL). Samples were obtained from unused portions of nephrectomy specimens that resulted from treatment of renal cell carcinoma. Tissue was fixed in 10% buffered formalin. Tissue from a total of 15 kidneys was used. These studies were approved by Subcommittee for Clinical Investigation, GVAMC, and the University of Florida.
Human Renal Cortical Protein Lysate
Normal human renal cortical protein lysates were obtained from Becton-Dickinson Biosciences (San Jose, CA).
Immunoblot Analysis
Immunoblot analysis was performed as described previously in detail (17,19,20,26). Briefly, renal tissue lysate protein (25 μg/lane) was electrophoresed on 10% PAGE ReadyGels (Bio-Rad, Hercules, CA). Proteins were transferred electrophoretically to nitrocellulose membranes; membranes then were blocked and incubated for 5 h with 7 μg/ml affinity-purified primary antibody. After washing, membranes were exposed to secondary antibody (goat anti-rabbit IgG conjugated to horseradish peroxidase; Promega, Madison, WI) diluted 1:5000. Sites of antibody-antigen reaction were visualized using enhanced chemiluminescence and a Kodak Image Station 440CF digital imaging system (Perkin-Elmer Life Sciences, Boston, MA).
Immunohistochemistry
Immunolocalization of RhCG was accomplished using immunoperoxidase procedures and a commercially available kit (Dako, DakoCytomation, Glostrup, Denmark) as we have reported in detail previously (17,19,20,24). RhCG antibody was used at either 1:1000 or 1:2000 dilution. Similar results were obtained with each dilution. In some experiments, sections were counterstained with hematoxylin. Sections were photographed using a Nikon E600 microscope equipped with DIC optics, a DXM1200F digital camera, and ACT-1 software (Nikon USA, Melville, NY).
Double-Labeling Procedure
Co-localization of RhCG with other proteins was performed using sequential immunoperoxidase procedures and a commercially available kit (Dako, DakoCytomation). The tissue sections were dewaxed; had endogenous peroxidase quenched using DAKO Peroxidase solution for 30 min; then were washed, incubated with DAKO serum-free blocking solution, washed, and incubated with RhCG antibody overnight at 4°C in a humidified chamber. The sections then were washed, incubated with biotinylated anti-mouse and anti-rabbit secondary antibody (DAKO LSAB2 kit) for 30 min, washed, incubated with streptavidin for 30 min, washed, exposed to diaminobenzidine for 5 min, and then washed. The above procedure then was repeated with the substitution of a second primary antibody (H+-ATPase, 1:200; AQP-2, 1:200; or, calbindin D28K, 1:5000) and the substitution of Vector SG for diaminobenzidine.
Nomenclature
The term ammonia is used to refer to the combination of the two molecular species, NH3 and NH4+. When referring specifically to the molecular species NH3, we specifically state “NH3,” and when referring to NH4+ we specifically state “NH4+”. According to standard nomenclature, RhAG refers to the human Rh A glycoprotein, and Rhag refers to nonhuman Rh A glycoproteins, and we use a similar pattern for Rh B and C glycoproteins. In the initial report, the term RhGK (Rh glycoprotein kidney) was used (8). RhCG and RhGK are alternative names for the same protein and mRNA. RhCG is the term that is used most frequently currently and is used in this report.
Results
Immunoblot Analysis
Immunoblot analysis of normal human renal cortical protein lysate identified expression of a 52-kD protein that was identical in apparent molecular weight to that observed in the normal mouse kidney (Figure 1A). A minor band with a molecular weight of approximately 48 kD also was present. This latter band is consistent with alternative glycosylation of RhCG, which was reported previously in the rat kidney (21). Specificity of the immunoblot immunoreactivity was confirmed both by using affinity-purified antibodies, which prevented the nonspecific immunoreactivity in mouse kidney protein that was reported previously when unpurified antisera were used (19), and by showing that preincubating the antibody with the immunizing peptide blocked protein recognition (Figure 1B).
Figure 1.
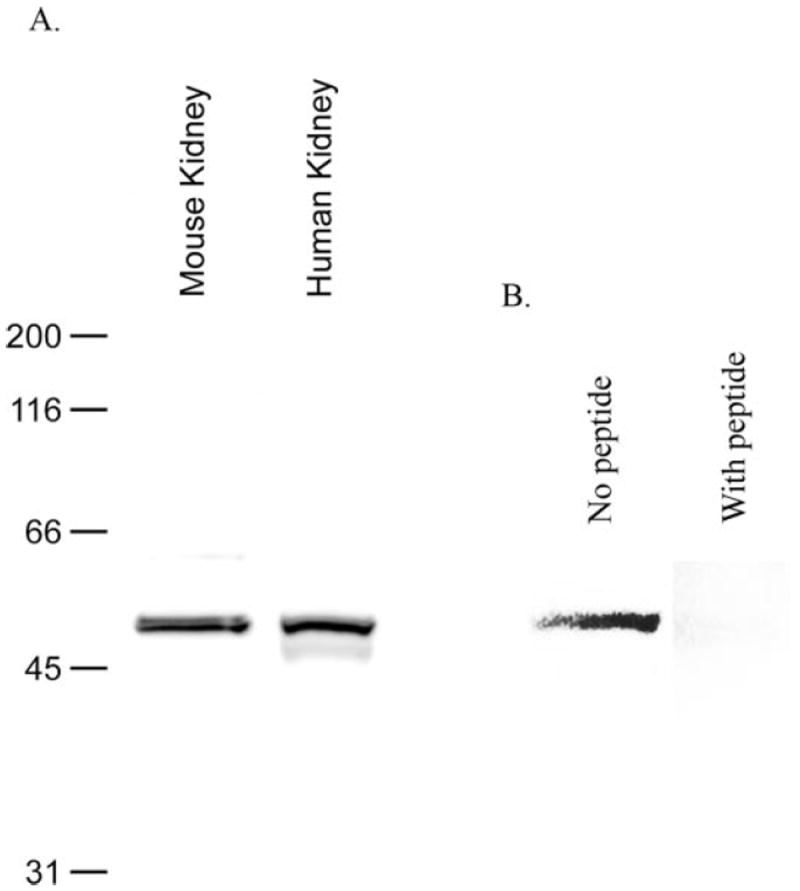
Immunoblot of normal human kidney protein. (A) Immunoblot of normal human and mouse renal cortical protein revealed expression of a 52-kD protein that was identical in size to mouse kidney Rh C glycoprotein (Rhcg). Lesser abundance of an approximately 48-kD protein also is observed, consistent with alternative glycosylation of RhCG. (B) Immunoblot specificity by showing the effect of preincubating the antibody with the immunizing peptide.
RhCG Immunolocalization
Immunohistochemical localization of RhCG in human kidney revealed expression in the DCT, CNT, and ICT and throughout the collecting duct (Figure 2). Preincubating the affinity-purified antibody with the immunizing peptide blocked detectable immunoreactivity (Figure 2B). Tissues that were treated with secondary antibody alone showed no detectable immunoreactivity (data not shown).
Figure 2.

Low-power micrograph of RhCG expression in the human kidney. (A) Low-power magnification of RhCG immunoreactivity in human kidney cortex. Immunoreactivity is present in a subset of convoluted tubule segment and in collecting duct epithelia. No expression is observed in proximal tubule epithelia (PT) or glomeruli (G). (B) Preincubation of the antibody with the immunizing peptide blocked immunoreactivity. (C) Rhcg expression in convoluted tubule segments in the human cortex. Both apical and basolateral Rhcg immunoreactivity is clearly present. Essentially all cells express Rhcg, and a subset of cells demonstrates increased immunoreactivity. (D) Expression in the cortical collecting duct (CCD). In contrast to convoluted tubule and initial collecting duct segments, only a subset of CCD cells expresses detectable RhCG immunoreactivity. These cells tend to be larger, protrude into the tubule lumen, and have morphologic characteristics suggestive of intercalated cells. (E) Expression in the outer medullary collecting duct in the inner stripe. Both apical and basolateral immunoreactivity is present in only a subset of cells (arrows). Basolateral immunoreactivity in these cells appears to be more intense than the apical immunoreactivity. (F) Expression in the inner medullary collecting duct. Only a minority of cells expresses Rhcg immunoreactivity (arrows); both apical and basolateral Rhcg immunoreactivity is present in these cells.
High-power observation of RhCG expression showed both apical and basolateral immunoreactivity. In the CNT and DCT, almost all cells expressed RhCG immunoreactivity (Figure 2C). In the cortical collecting duct (CCD), OMCD, and IMCD, only a minority of cells expressed detectable RhCG immunoreactivity (Figure 2, D through F). In general, basolateral RhCG immunoreactivity seemed more intense than apical immunoreactivity. No expression was observed in the glomerulus, proximal tubule, loop of Henle, or erythrocytes. The segmental distribution of RhCG in human kidney seems to be similar to the mouse and rat kidney (19,21,24).
Co-Localization with Calbindin-D28k
To confirm that the convoluted tubule segments that expressed RhCG were the DCT and CNT and not proximal tubule segments, we performed co-localization with calbindin-D28k, which labels DCT, CNT, and CCD but not the proximal tubule (27). Figure 3A shows representative findings in which calbindin- D28k–positive cells expressed RhCG, thereby confirming that RhCG is expressed by the DCT and CNT.
Figure 3.
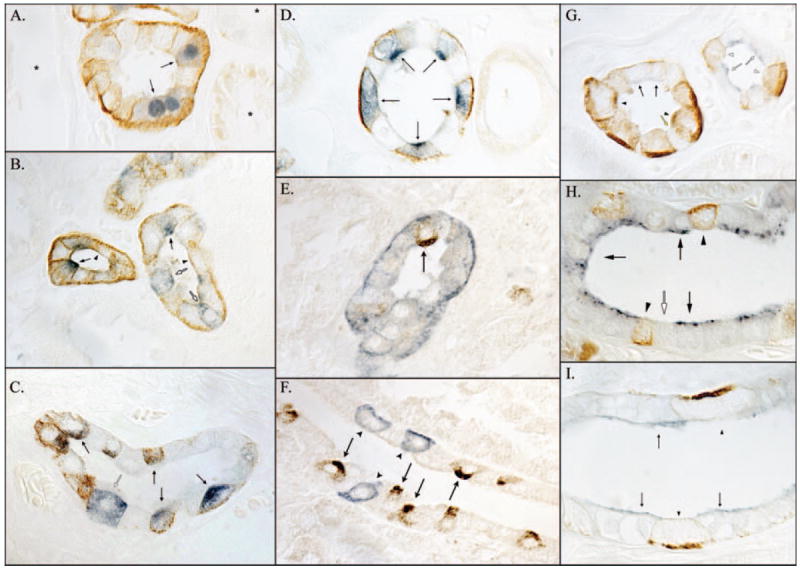
Co-localization of RhCG with calbindin-D28k, H+-ATPase, pendrin, and aquaporin-2 (AQP-2). (A) Co-localization of RhCG (brown) with the distal convoluted tubule (DCT)- and connecting segment (CNT)-specific marker calbindin-D28k (blue). Convoluted tubule segments that expressed calbindin-D28k (arrows) also expressed RhCG, whereas calbindin-D28k–negative cortical segments (*) did not. (B) Co-localization of H+-ATPase (blue) with RhCG (brown) in the CNT. Both cells with apical H+-ATPase immunoreactivity (arrows; the A-type intercalated cell and non-A, non-B cell) and cells without detectable H+-ATPase immunoreactivity (arrowheads; DCT and CNT cells) express apical and basolateral RhCG immunoreactivity. Cells with diffuse H+-ATPase immunoreactivity (B-type intercalated cells) do not (white arrows). (C) In the cortical collecting duct (CCD), cells with apical H+-ATPase immunoreactivity (blue) express RhCG (arrows), whereas cells with diffuse H+-ATPase immunoreactivity do not (white arrow). In contrast to the CNT, H+-ATPase–negative CCD principal cells do not express detectable RhCG immunoreactivity. (D) In the outer medullary collecting duct, H+-ATPase–positive cells (intercalated cells) express RhCG immunoreactivity (arrows), whereas H+-ATPase–negative cells (principal cells) do not. (E) In the CNT, both cells with apical (arrows) and without detectable apical pendrin (brown) express RhCG (blue). (F) CCD pendrin-positive cells (arrows) do not express detectable RhCG, and RhCG-positive cells do no express detectable pendrin (arrowheads). (G) Co-localization of AQP-2 (blue) with RhCG (brown) in the DCT (left tubule) and initial collecting tubule (ICT; right tubule). In the DCT, both apical AQP-2–positive (arrowhead) and AQP-2–negative (arrows) cells express RhCG, although AQP-2– negative cells, in general, express more intense RhCG immunoreactivity. Rare AQP-2–and RhCG-negative cells (yellow arrow) most likely are B-type intercalated cells. In the ICT (right tubule), AQP-2–positive cells (white arrows) do not exhibit significant RhCG immunoreactivity, whereas AQP-2–negative cells (white arrowheads) do. (H) A CCD in which AQP-2–positive principal cells (arrows) do not exhibit detectable RhCG immunoreactivity. AQP-2–negative cells (intercalated cells), in general, express intense RhCG immunoreactivity (arrowhead). Cells without either detectable AQP-2 or RhCG (white arrow) most likely are B-type intercalated cells. In the outer medulla (I), only AQP-2–negative cells (arrowheads) express detectable RhCG immunoreactivity; AQP-2–positive cells do not (arrows).
Co-Localization of RhCG with H+-ATPase
At least two fundamentally different cell types are present in the CNT and the collecting duct: The principal cell and the intercalated cell. Intercalated cells are involved in H+ secretion and are regulated by acid-base balance, are characterized by intense H+-ATPase expression, and in the mouse and rat kidneys are the sites of increased RhCG expression (19,21,24,25). Co-localization of RhCG with H+-ATPase in the human kidney showed increased RhCG expression in intercalated cells. CNT cells with apical H+-ATPase (A-type intercalated cells and non-A, non-B cells) expressed intense RhCG immunoreactivity (Figure 3B). Cells with diffuse H+-ATPase (B-type intercalated cells) did not express detectable RhCG immunoreactivity. H+-ATPase–negative cells expressed intermediate RhCG immunoreactivity. In the CCD, only intercalated cells with apical H+-ATPase immunoreactivity (A-type intercalated cells) expressed RhCG (Figure 3C). Cells with diffuse H+-ATPase immunoreactivity (B-type intercalated cells) did not express detectable RhCG immunoreactivity; neither was RhCG immunoreactivity identified in H+-ATPase–negative principal cells. In the OMCD, only H+-ATPase–positive A-type intercalated cells expressed RhCG; H+-ATPase–negative principal cells did not (Figure 3D).
Co-Localization of RhCG with Pendrin
These observations suggest that A-type and/or non-A, non-B intercalated cells but not B-type intercalated cells express RhCG. To confirm this, we co-localized RhCG with pendrin, an apical Cl−/HCO3− exchanger that is present in the B-type intercalated cell and the non-A, non-B cell but not in the A-type intercalated cell (28-30). In the CNT, where the majority of pendrin-positive cells are non-A, non-B cells, the majority of pendrin-positive cells expressed RhCG immunoreactivity (Figure 3E). This, combined with the observation that cells in the CNT with apical H+-ATPase immunoreactivity express RhCG, identifies that CNT non-A, non-B intercalated cells express RhCG.
In the CCD, where the majority of pendrin-positive intercalated cells are B-type intercalated cells, pendrin-positive cells did not express RhCG immunoreactivity (Figure 3F). This, combined with the observation that CCD intercalated cells with diffuse H+-ATPase immunoreactivity did not express RhCG, identifies that B-type intercalated cells do not express RhCG. We also observed that some CCD had many pendrin-positive cells, whereas other CCD had few, even in the same kidney. The explanation for this observation is not clear.
Co-Localization of RhCG with AQP-2
The observation that RhCG is not expressed by H+-ATPase–negative cells in the CCD, OMCD, and IMCD suggests that the principal cell and the IMCD cell do not express RhCG. To confirm this, we performed co-localization of RhCG with AQP-2, a principal cell and IMCD cell marker. AQP-2–positive cells in the CNT expressed RhCG (Figure 3G), whereas AQP- 2–positive cells in the CCD, OMCD, and IMCD did not (Figure 3, H and I). In the CNT, both principal cells and nonprincipal cells expressed RhCG. In contrast, CCD, OMCD principal cells, and IMCD cells did not express RhCG.
RhCG Expression in Renal Carcinomas
Because of the high level of RhCG expression in the kidney, we examined whether RhCG might be expressed in renal tumors. Both chromophobe renal cell carcinoma specimens (n = 2) and renal oncocytoma (n = 2) specimens expressed plasma membrane RhCG immunoreactivity (Figure 4, A and B). Apparent tubule-like profiles occasionally were evident in renal oncocytomas in which all cells expressed both apical and basolateral RhCG immunoreactivity (Figure 4C). Tubular profiles were not observed in chromophobe renal cell carcinomas. RhCG immunoreactivity intensity in both chromophobe renal cell carcinoma and renal oncocytoma was less intense than in RhCG-positive cells in adjacent portions of uninvolved renal parenchyma.
Figure 4.
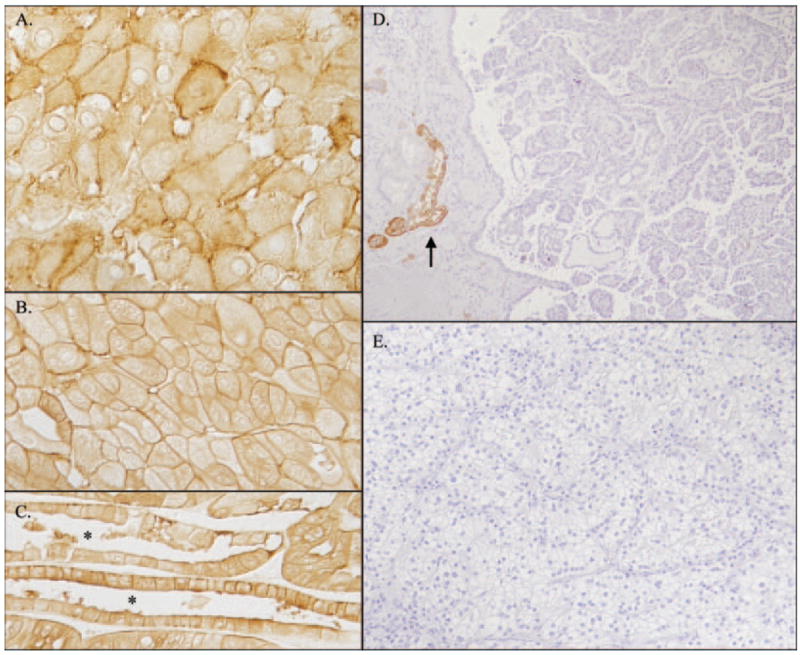
RhCG expression in renal carcinomas. (A) Rhcg expression in chromophobe renal cell carcinoma and plasma membrane RhCG immunoreactivity in this pleomorphic tumor. No immunoreactivity was seen in tumor sections that were stained with an unrelated antibody. (B and C) Rhcg immunoreactivity in human oncocytoma. Occasional tubular profiles (*) demonstrated RhCG immunoreactivity in all cells, consistent with origin from collecting duct A-type intercalated cells (C), and expressed both apical and basolateral Rhcg immunoreactivity. RhCG immunoreactivity in both chromophobe renal cell carcinoma and in oncocytoma was less intense than in adjacent normal renal parenchyma (data not shown). (D and E) Immunohistochemical examination of RhCG immunoreactivity in papillary renal cell carcinoma (D) and clear cell renal cell carcinoma (E). No detectable RhCG immunoreactivity was observed in either tumor type. RhCG immunoreactivity in adjacent uninvolved renal parenchyma (arrow, D) was normal.
No detectable RhCG immunoreactivity was observed in either papillary cell carcinoma (n = 4) or clear cell renal cell carcinoma (n = 5). RhCG immunoreactivity was easily visible in adjacent normal renal structures, thereby providing internal validation that confirms efficacy of the labeling procedure in these studies (Figure 4, D and E). Thus, RhCG is expressed by chromophobe renal cell carcinoma and renal oncocytoma but not by either renal cell or clear cell renal cell carcinomas.
Discussion
These studies provide the first examination of the expression of the nonerythroid ammonia transporter family member, RhCG, in the normal and neoplastic human kidney. RhCG is present in the DCT, CNT, and ICT and throughout the collecting duct, and it exhibits both apical and basolateral expression. In the cortex, CNT cells, A-type intercalated cells, and non-A, non-B cells but not B-type intercalated cells express RhCG. In the CCD, OMCD, and IMCD, only A-type intercalated cells express RhCG; there was no detectable expression by either principal cells, B-type intercalated cells, or IMCD cells. This expression pattern has important differences from both the mouse and the rat kidney and has important implications for the mechanisms of ammonia secretion by the human kidney. Finally, chromophobe renal cell carcinoma and renal oncocytomas, but not papillary renal cell carcinoma or clear cell renal cell carcinomas express RhCG.
The first major finding in this study is that RhCG is expressed in the human kidney in distal nephron sites involved in ammonia secretion. Ammonia is produced in the proximal tubule through glutamine metabolism, reabsorbed in the thick ascending limb of the loop of Henle, and then secreted by the collecting duct (1-3). Under normal conditions, the collecting duct secretes approximately 70 to 80% of total urinary ammonia, and collecting duct ammonia secretion is substantially stimulated in response to chronic metabolic acidosis (1,4). Moreover, RhCG expression is stimulated by chronic metabolic acidosis, at least in the OMCD and the IMCD, consistent with RhCG’s mediating a critical role in ammonia secretion (24). The finding that the human kidney expresses RhCG in distal nephron sites that are involved in ammonia secretion is consistent with RhCG’s contributing to renal ammonia metabolism.
The observation that RhCG is expressed in collecting duct intercalated cells supports existing hypotheses regarding ammonia secretion. In animal models, luminal acidification, including the generation of a luminal disequilibrium pH, results from apical H+ secretion by H+-ATPase and H+-K+-ATPase and is both necessary for and regulates transepithelial ammonia secretion (31-36). Collecting duct ammonia transport seems to involve both diffusive and transporter-mediated components (26,37), and the transporter-mediated component of apical transport is stimulated by luminal H+ (37). This may be relevant to maximizing the efficiency of ammonia secretion. H+ secretion in the absence of luminal carbonic anhydrase activity generates a luminal disequilibrium pH. This accelerates ammonia secretion to a greater degree than is explainable by the mean luminal pH (33,35,38). Because intercalated cells are the primary mechanism of collecting duct H+ secretion, the luminal disequilibrium pH is greatest in the region adjacent to the intercalated cell apical membrane. The close proximity of the greatest degree of luminal acidification with apical RhCG may increase apical membrane ammonia transport. Thus, the concomitant expression of both apical H+ and ammonia transport mechanisms in the same cells may enable synergistic H+ and ammonia secretion.
An important new observation is that the cellular expression of RhCG in the human kidney differs from that observed in rat and the mouse kidney. In the rat and mouse kidneys, principal cells in the CCD and OMCD express Rhcg (19,21,24), and principal cell Rhcg expression, at least in the OMCD, is stimulated by chronic metabolic acidosis (25). This contrasts with observations in the human kidney (our study) in which no detectable RhCG immunoreactivity was observed in these cells. This could reflect either fundamental differences in functional role of the CCD and OMCD principal cell in the rat and mouse as compared with the human kidney or RhCG expression in the human principal cells below the level of detectability. It is important to note that the H+-K+-ATPase isoforms HKα2a and HKα2c have been identified in rat and rabbit principal cells (39,40), that rabbit outer medullary collecting duct in the inner stripe (OMCDi) principal cells possess multiple physiologically regulated apical H+ transport mechanisms (41,42), and that chronic metabolic acidosis increases rat OMCDi principal cell RhCG expression (25). Thus, in the rat, mouse, and rabbit kidneys, principal cells seem to contribute to transporter-mediated trans-epithelial H+ and ammonia secretion. It is possible that differences in principal cell RhCG immunoreactivity between human and rodent kidneys could reflect principal cells contributing to acidbase and ammonia metabolism in the rodent kidney but not in the human kidney.
A second important observation is that human renal RhCG expression is predominantly basolateral, with a lesser degree of apical immunoreactivity. In the mouse kidney, only apical immunoreactivity has been observed (18,19). In the rat, one study found only apical RhCG immunoreactivity (18), whereas we observed, using both light and immunogold electron microscopy, that rat collecting duct cells express both apical and basolateral plasma membrane RhCG (24,25) and that basolateral plasma membrane Rhcg expression in the OMCDi exceeds apical plasma membrane expression under basal conditions (25). Chronic metabolic acidosis increases rat basolateral plasma membrane Rhcg expression, suggesting that basolateral Rhcg mediates a physiologically relevant role in increased ammonia secretion in physiologically relevant conditions (25). Therefore, it is possible that basolateral RhCG in the human kidney contributes to basolateral ammonia uptake and, thereby, to transepithelial ammonia secretion.
Differences in RhCG expression in the B-type intercalated cell and the non-A, non-B cell may provide important insights into the physiologic role of these cell types. The B-type intercalated cell generally is believed to mediate important roles in both bicarbonate secretion in response to metabolic alkalosis and chloride reabsorption in response to chloride depletion and/or mineralocorticoid stimulation (30,43-45). The absence of RhCG in this cell type is consistent with the lack of necessity for transcellular ammonia secretion in any of these conditions. In contrast, the non-A, non-B cell expresses apical H+-ATPase in conjunction with apical pendrin and apical RhCG, suggesting that this cell type may contribute to both proton secretion and ammonia secretion and to regulation of Cl− homeostasis.
Another observation from these studies is that RhCG is expressed by chromophobe renal cell carcinoma and renal oncocytomas but not by either papillary renal cell carcinoma or clear cell renal cell carcinomas. Renal oncocytomas are benign neoplasms with a granular eosinophilic cytoplasm that contains numerous mitochondria (46). Chromophobe renal cell carcinomas are low-grade malignant neoplasms with a mixture of eosinophilic cells and cells with clear cytoplasm and numerous microvesicles and variable numbers of mitochondria (46,47). Each tumor accounts for up to 5% of renal neoplasms (48,49). Despite their different neoplastic potential, both express carbonic anhydrase II (CA II) and AE1 and lack immunoreactivity for high molecular weight cytokeratin, vimentin, CD10, and peanut lectin (50-53). RhCG expression by both of these tumors, as demonstrated in our study, in combination with previous evidence of CA II and AE1 expression, strongly suggests that they derive from collecting duct A-type intercalated cells. Because DCT and CNT cells, which express RhCG, do not express CA II or AE1, chromophobe renal cell carcinomas and renal oncocytomas are unlikely to originate from either DCT or CNT cells. Because differentiating chromophobe renal cell carcinoma from renal cell carcinoma using standard histologic criteria sometimes is difficult, examining RhCG immunoreactivity may be helpful in selected cases. The lack of RhCG immunoreactivity in clear cell renal cell carcinoma and papillary renal cell carcinoma is consistent with previous studies, suggesting that these tumors likely derive from proximal tubule cells (54,55).
Although RhCG originally was cloned as a tumor-related cDNA (56), RhCG immunoreactivity in chromophobe renal cell carcinoma and renal oncocytoma was less intense than in adjacent uninvolved renal parenchyma. Decreased RhCG expression has been observed in other epithelial cancers (57). Therefore, decreased, not increased, RhCG overexpression may be characteristic of human tumors. It is interesting that the level of RhCG downregulation may have prognostic significance in the response of some tumors to treatment (58). Whether similar correlations between RhCG expression and treatment response will be possible for chromophobe renal cell carcinoma and renal oncocytoma is unknown at present.
It is important to recognize that RhCG has not been shown definitively to contribute in vivo to ammonia transport. Extensive in vitro evidence shows that all mammalian Rh glycoproteins, RhAG/Rhag, RhBG/Rhbg, and RhCG/Rhcg, can transport ammonia (8-16,59) and that collecting duct ammonia transport exhibits characteristics that are consistent with transport by Rh glycoproteins (26,37). However, disruption of the Rhbg gene does not detectably alter basal or acidosis-stimulated renal ammonia metabolism (60). Therefore, either Rhbg is not involved in renal ammonia transport, or compensatory mechanisms exist in its absence. An alternative role for Rh glycoproteins is CO2 transport, particularly in view of evidence in the green algae, Chlamydomonas reinhardtii, that the Rh glycoprotein Rh1 may be involved in CO2 transport (61,62).
Another potential limitation of these studies is that they use tissue from kidneys with coexisting carcinoma. Because patients with renal carcinomas generally have normal acid-base balance and normal renal function, at least before surgical nephrectomy, systemic pH alterations or impaired GFR are unlikely to have altered RhCG expression in the tissues studied. It is possible but unlikely that there might be alterations at the molecular or cellular level of uninvolved kidney that predisposed to the development of renal carcinoma that could alter RhCG expression. Against this possibility, however, is that different abnormalities probably are present in differing renal tumors and the similarity of RhCG expression that we observed in kidneys with multiple different types of renal carcinomas.
Conclusion
Our study identifies several important features of RhCG expression in the normal and neoplastic human kidney. Similar to the mouse and rat kidney, human renal RhCG is found in the DCT, CNT, ICT, and the collecting duct, sites that are responsible for secreting the majority of renal ammonia secretion. In contrast to the mouse and rat, however, in the collecting duct, RhCG is found only in intercalated cells that express apical H+-ATPase, the A-type intercalated cell, and the non-A, non-B cell, suggesting that coordinated proton and ammonia transport and generation of a luminal disequilibrium pH are critically important for transepithelial ammonia secretion. RhCG is not found in the B-type intercalated cell, consistent with this cell type having a primary role in the human kidney in bicarbonate excretion and chloride reabsorption and not in urine acidification or ammonia secretion. Finally, both chromophobe renal cell carcinoma and renal oncocytoma but not clear cell renal cell carcinoma or papillary renal cell carcinomas express RhCG, consistent with the former two but not the latter two originating from collecting duct intercalated cells.
Acknowledgments
These studies were supported by funds from the National Institutes of Health (DK45788 and NS47624 to I.D.W.), the Department of Veterans Affairs Merit Review Program (to I.D.W.), and the Korea Research Foundation (grant KRF-2005-003-E00006 to K.-H.H.) and by an International Society of Nephrology Fellowship Award (to H.Y.K.).
Portions of this work were published in abstract form previously (J Am Soc Nephrol 16: 344A, 2005).
We thank Gina Cowsert for secretarial assistance.
References
- 1.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol. 1987;253:F595–F605. doi: 10.1152/ajprenal.1987.253.4.F595. [DOI] [PubMed] [Google Scholar]
- 2.DuBose TD, Jr, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: New physiological concepts and their clinical implications. J Am Soc Nephrol. 1991;1:1193–1203. doi: 10.1681/ASN.V1111193. [DOI] [PubMed] [Google Scholar]
- 3.Good DW, Knepper MA. Ammonia transport in the mammalian kidney. Am J Physiol. 1985;248:F459–F471. doi: 10.1152/ajprenal.1985.248.4.F459. [DOI] [PubMed] [Google Scholar]
- 4.Sajo IM, Goldstein MB, Sonnenberg H, Stinebaugh BJ, Wilson DR, Halperin ML. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int. 1982;20:353–358. doi: 10.1038/ki.1981.146. [DOI] [PubMed] [Google Scholar]
- 5.Nakhoul NL, Hamm LL. Non-erythroid Rh glycoproteins: A putative new family of mammalian ammonium transporters. Pflugers Arch. 2004;447:807–812. doi: 10.1007/s00424-003-1142-8. [DOI] [PubMed] [Google Scholar]
- 6.Weiner ID. The Rh gene family and renal ammonium transport. Curr Opin Nephrol Hypertens. 2004;13:533–540. doi: 10.1097/00041552-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Weiner ID, Verlander JW. Renal and hepatic expression of the ammonium transporter proteins, Rh B glycoprotein and Rh C glycoprotein. Acta Physiol Scand. 2003;179:331–338. doi: 10.1046/j.0001-6772.2003.01210.x. [DOI] [PubMed] [Google Scholar]
- 8.Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet. 2000;26:341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- 9.Ripoche P, Bertrand O, Gane P, Birkenmeier C, Colin Y, Cartron JP. Human Rhesus-associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc Natl Acad Sci U S A. 2004;101:17222–17227. doi: 10.1073/pnas.0403704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westhoff CM, Siegel DL, Burd CG, Foskett JK. Mechanism of genetic complementation of ammonium transport in yeast by human erythrocyte Rh-associated glycoprotein (RhAG) J Biol Chem. 2004;279:17443–17448. doi: 10.1074/jbc.M311853200. [DOI] [PubMed] [Google Scholar]
- 11.Westhoff CM, Ferreri-Jacobia M, Mak DD, Foskett JK. Identification of the erythrocyte Rh-blood group glycoprotein as a mammalian ammonium transporter. J Biol Chem. 2002;277:12499–12502. doi: 10.1074/jbc.C200060200. [DOI] [PubMed] [Google Scholar]
- 12.Mak DD, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol. 2006;290:F297–F305. doi: 10.1152/ajprenal.00147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zidi-Yahiaoui N, Mouro-Chanteloup I, D’Ambrosio AM, Lopez C, Gane P, Le van Kim C, Cartron JP, Colin Y, Ripoche P. Human Rhesus B and Rhesus C glycoproteins: Properties of facilitated ammonium transport in recombinant kidney cells. Biochem J. 2005;391:33–40. doi: 10.1042/BJ20050657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, Cherif-Zahar B, Planelles G. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem. 2004;279:15975–15983. doi: 10.1074/jbc.M308528200. [DOI] [PubMed] [Google Scholar]
- 15.Nakhoul NL, DeJong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol. 2004;288:F170–F181. doi: 10.1152/ajprenal.00419.2003. [DOI] [PubMed] [Google Scholar]
- 16.Ludewig U. Electroneutral ammonium transport by basolateral Rhesus B glycoprotein. J Physiol. 2004;559:751–759. doi: 10.1113/jphysiol.2004.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins, Rh B glycoprotein and Rh C glycoprotein, in the intestinal tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1036–G1047. doi: 10.1152/ajpgi.00418.2004. [DOI] [PubMed] [Google Scholar]
- 18.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol. 2003;14:545–554. doi: 10.1097/01.asn.0000050413.43662.55. [DOI] [PubMed] [Google Scholar]
- 19.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B Glycoprotein and Rh C glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol. 2003;284:F323–F337. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- 20.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B glycoprotein and Rh C glycoprotein in the mouse liver. Gastroenterology. 2003;124:1432–1440. doi: 10.1016/s0016-5085(03)00277-4. [DOI] [PubMed] [Google Scholar]
- 21.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol. 2002;13:1999–2008. doi: 10.1097/01.asn.0000025280.02386.9d. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Peng J, Mo R, Hui CC, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem. 2001;276:1424–1433. doi: 10.1074/jbc.M007528200. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Chen Y, Mo R, Hui Cc, Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem. 2000;275:25641–25651. doi: 10.1074/jbc.M003353200. [DOI] [PubMed] [Google Scholar]
- 24.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol. 2006;290:F397–F408. doi: 10.1152/ajprenal.00162.2005. [DOI] [PubMed] [Google Scholar]
- 25.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol. 2006;290:F1443–F1452. doi: 10.1152/ajprenal.00459.2005. [DOI] [PubMed] [Google Scholar]
- 26.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3) Am J Physiol Renal Physiol. 2004;287:F628–F638. doi: 10.1152/ajprenal.00363.2003. [DOI] [PubMed] [Google Scholar]
- 27.Biner HL, Arpin-Bott MP, Loffing J, Wang X, Knepper M, Hebert SC, Kaissling B. Human cortical distal nephron: Distribution of electrolyte and water transport pathways. J Am Soc Nephrol. 2002;13:836–847. doi: 10.1681/ASN.V134836. [DOI] [PubMed] [Google Scholar]
- 28.Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol. 2003;284:F229–F241. doi: 10.1152/ajprenal.00147.2002. [DOI] [PubMed] [Google Scholar]
- 29.Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunohistochemical and immunoelectron microscopic localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol. 2002;283:F744–F754. doi: 10.1152/ajprenal.00037.2002. [DOI] [PubMed] [Google Scholar]
- 30.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flessner MF, Knepper MA. Ammonium transport in collecting ducts. Miner Electrolyte Metab. 1990;16:299–307. [PubMed] [Google Scholar]
- 32.Star RA, Burg MB, Knepper MA. Luminal disequilibrium pH and ammonia transport in outer medullary collecting duct. Am J Physiol. 1987;252:F1148–F1157. doi: 10.1152/ajprenal.1987.252.6.F1148. [DOI] [PubMed] [Google Scholar]
- 33.Star RA, Kurtz I, Mejia R, Burg MB, Knepper MA. Disequilibrium pH and ammonia transport in isolated perfused cortical collecting ducts. Am J Physiol. 1987;253:F1232–F1242. doi: 10.1152/ajprenal.1987.253.6.F1232. [DOI] [PubMed] [Google Scholar]
- 34.Hamm LL, Trigg D, Martin D, Gillespie C, Buerkert J. Transport of ammonia in the rabbit cortical collecting tubule. J Clin Invest. 1985;75:478–485. doi: 10.1172/JCI111723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knepper MA, Good DW, Burg MB. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol. 1985;249:F870–F877. doi: 10.1152/ajprenal.1985.249.6.F870. [DOI] [PubMed] [Google Scholar]
- 36.Knepper MA, Good DW, Burg MB. Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am J Physiol. 1984;247:F729–F738. doi: 10.1152/ajprenal.1984.247.5.F729. [DOI] [PubMed] [Google Scholar]
- 37.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3) Am J Physiol Renal Physiol. 2005;289:F347–F358. doi: 10.1152/ajprenal.00253.2004. [DOI] [PubMed] [Google Scholar]
- 38.Flessner MF, Wall SM, Knepper MA. Ammonium and bicarbonate transport in rat outer medullary collecting ducts. Am J Physiol. 1992;262:F1–F7. doi: 10.1152/ajprenal.1992.262.1.F1. [DOI] [PubMed] [Google Scholar]
- 39.Verlander JW, Moudy RM, Campbell WG, Cain BD, Wingo CS. Immunohistochemical localization of H-K-ATPase alpha(2c)-subunit in rabbit kidney. Am J Physiol Renal Physiol. 2001;281:F357–F365. doi: 10.1152/ajprenal.2001.281.2.F357. [DOI] [PubMed] [Google Scholar]
- 40.Ahn KY, Kone BC. Expression and cellular localization of mRNA encoding the “gastric” isoform of the H+-K+ subunit alpha subunit in rat kidney. Am J Physiol. 1995;268:F99–F109. doi: 10.1152/ajprenal.1995.268.1.F99. [DOI] [PubMed] [Google Scholar]
- 41.Weiner ID, Frank AE, Wingo CS. Apical proton secretion by the inner stripe of the outer medullary collecting duct. Am J Physiol Renal Physiol. 1999;276:F606–F613. doi: 10.1152/ajprenal.1999.276.4.F606. [DOI] [PubMed] [Google Scholar]
- 42.Weiner ID, Wingo CS, Hamm LL. Regulation of intracellular pH in two cell populations of the inner stripe of the rabbit outer medullary collecting duct. Am J Physiol. 1993;265:F406–F415. doi: 10.1152/ajprenal.1993.265.3.F406. [DOI] [PubMed] [Google Scholar]
- 43.Quentin F, Chambrey R, Trinh-Trang-Tan MM, Fysekidis M, Cambillau M, Paillard M, Aronson PS, Eladari D. The Cl-/HCO3- exchanger pendrin in the rat kidney is regulated in response to chronic alterations in chloride balance. Am J Physiol Renal Physiol. 2004;287:F1179–F1188. doi: 10.1152/ajprenal.00211.2004. [DOI] [PubMed] [Google Scholar]
- 44.Wall SM. Recent advances in our understanding of intercalated cells. Curr Opin Nephrol Hypertens. 2005;14:480–484. doi: 10.1097/01.mnh.0000168390.04520.06. [DOI] [PubMed] [Google Scholar]
- 45.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: Role in Clconservation. Hypertension. 2004;44:982–987. doi: 10.1161/01.HYP.0000145863.96091.89. [DOI] [PubMed] [Google Scholar]
- 46.Krishnan B, Truong LD. Renal epithelial neoplasms: The diagnostic implications of electron microscopic study in 55 cases. Hum Pathol. 2002;33:68–79. doi: 10.1053/hupa.2002.30210. [DOI] [PubMed] [Google Scholar]
- 47.Crotty TB, Farrow GM, Lieber MM. Chromophobe cell renal carcinoma: Clinicopathological features of 50 cases. J Urol. 1995;154:964–967. doi: 10.1016/s0022-5347(01)66944-1. [DOI] [PubMed] [Google Scholar]
- 48.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Alanen KA, Ekfors TO, Lipasti JA, Nurmi MJ. Renal oncocytoma: The incidence of 18 surgical and 12 autopsy cases. Histopathology. 1984;8:731–737. doi: 10.1111/j.1365-2559.1984.tb02389.x. [DOI] [PubMed] [Google Scholar]
- 50.Bonsib SM, Bromley C. Immunocytochemical analysis of band 3 protein in renal cell carcinoma, nephroblastoma, and oncocytoma. Arch Pathol Lab Med. 1994;118:702–704. [PubMed] [Google Scholar]
- 51.Kollert-Jons A, Wagner S, Hubner S, Appelhans H, Drenckhahn D. Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am J Physiol. 1993;265:F813–F821. doi: 10.1152/ajprenal.1993.265.6.F813. [DOI] [PubMed] [Google Scholar]
- 52.Storkel S, Steart PV, Drenckhahn D, Thoenes W. The human chromophobe cell renal carcinoma: Its probable relation to intercalated cells of the collecting duct. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;56:237–245. doi: 10.1007/BF02890022. [DOI] [PubMed] [Google Scholar]
- 53.Storkel S, Pannen B, Thoenes W, Steart PV, Wagner S, Drenckhahn D. Intercalated cells as a probable source for the development of renal oncocytoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56:185–189. doi: 10.1007/BF02890016. [DOI] [PubMed] [Google Scholar]
- 54.Vogelzang NJ, Stadler WM. Kidney cancer. Lancet. 1998;352:1691–1696. doi: 10.1016/S0140-6736(98)01041-1. [DOI] [PubMed] [Google Scholar]
- 55.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, Lubensky I, Duan DR, Florence C, Pozzatti R, Walther MM, Bander NH, Grossman HB, Brauch H, Pomer S, Brooks JD, Isaacs WB, Lerman MI, Zbar B, Linehan WM. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 56.Cloning and characterization of a novel cDNA (DRC2) associated with human cancer. [November 14, 2005]; Available: http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=protein&val=5911316.
- 57.Chen B, Xu Z, Xu X, Cai Y, Han Y, Wang J, Xia S, Hu H, Wei F, Wu M, Wang M. RhCG is downregulated in oesophageal squamous cell carcinomas, but expressed in multiple squamous epithelia. Eur J Cancer. 2002;38:1927. doi: 10.1016/s0959-8049(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 58.Luthra R, Wu TT, Luthra MG, Izzo J, Lopez-Alvarez E, Zhang L, Bailey J, Lee JH, Bresalier R, Rashid A, Swisher SG, Ajani JA. Gene expression profiling of localized esophageal carcinomas: Association with pathologic response to preoperative chemoradiation. J Clin Oncol. 2006;24:259–267. doi: 10.1200/JCO.2005.03.3688. [DOI] [PubMed] [Google Scholar]
- 59.Benjelloun F, Bakouh N, Fritsch J, Hulin P, Lipecka J, Edelman A, Planelles G, Thomas SR, Cherif-Zahar B. Expression of the human erythroid Rh glycoprotein (RhAG) enhances both NH3 and NH4+ transport in HeLa cells. Pflugers Arch. 2005;450:155–167. doi: 10.1007/s00424-005-1381-y. [DOI] [PubMed] [Google Scholar]
- 60.Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol. 2005;289:F1281–F1290. doi: 10.1152/ajprenal.00172.2005. [DOI] [PubMed] [Google Scholar]
- 61.Soupene E, Inwood W, Kustu S. Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc Natl Acad Sci U S A. 2004;101:7787–7792. doi: 10.1073/pnas.0401809101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soupene E, King N, Feild E, Liu P, Niyogi KK, Huang CH, Kustu S. Rhesus expression in a green alga is regulated by CO(2) Proc Natl Acad Sci U S A. 2002;99:7769–7773. doi: 10.1073/pnas.112225599. [DOI] [PMC free article] [PubMed] [Google Scholar]


