Abstract
A titanium alkoxide-mediated convergent coupling between internal alkynes and allenes is described for the regio- and stereocontrolled synthesis of substituted acyclic 1,4-dienes.
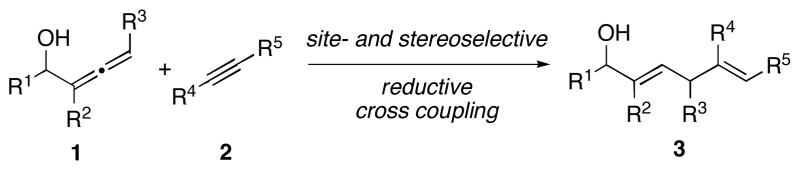
The direct cross coupling of unactivated π-systems is a powerful strategy for bimolecular C–C bond formation. Whereas palladium-catalyzed cross-coupling has become commonplace for bimolecular C–C bond formation,1 these catalytic transformations dictate the use of activated coupling partners, often prepared by multiple stoichiometric functionalization processes (i.e. unsaturated organic halides and functionalized organometallic reagents). The direct cross-coupling of unactivated π-systems provides an alternative, and perhaps more powerful, strategy for bimolecular C–C bond formation, as pre-activation of the reactive partners is not required. The reactivity of metal–π complexes is ideally suited for such bond construction. To date, numerous reports describe intramolecular C–C bond formation between unactivated π-systems via the intermediacy of metallacyclopentanes, however, bimolecular processes based on such reactivity are rare due to challenges associated with the control of regioselectivity, reactivity and stereoselectivity in the C–C bond forming event.2 Here, we describe a cross-coupling reaction between substituted allenes (1) and internal alkynes (2) that provides a convergent and highly stereoselective route to substituted 1,4-dienes of general structure 3 (Figure 1).
Figure 1.
Allene–alkyne cross coupling for 1,4-diene synthesis.
As part of our ongoing studies aimed at defining a suite of convergent coupling reactions between unactivated π-systems,3, 4 we began to explore a reaction for the stereoselective union of alkynes with allenes. Whereas alkyne–allene cross-coupling has been reported for the synthesis of 1,4-dienes,5 the bimolecular C–C bond forming event often proceeds with relatively modest levels of selectivity (ca. 2–3:1).5 In general, alkoxy- or silyl-substituted allenes are required for high levels of (Z)-selectivity. Here, we describe a new allene-alkyne cross-coupling for 1,4-diene synthesis that favors the formation of the stereoisomeric (E)-di- or trisubstituted olefin-containing product (represented by generic structure 3, Figure 1).
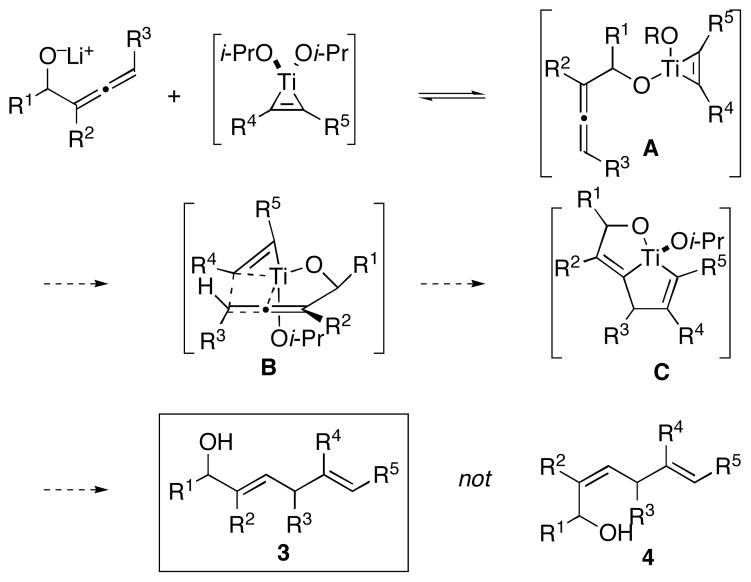
We anticipated that high regio- and stereoselection would result from the sequence of events proposed in Figure 2. Preformation of a titanium–alkyne complex, followed by exposure to an allenic alkoxide would result in rapid and reversible ligand exchange to provide the mixed titanate ester A. Intramolecular carbometalation via B would then deliver the fused bicyclic metallacyclopentene C. Selectivity in this process was anticipated to result from formation of a bicyclo[3.3.0]-ring system in preference to the regioisomeric bicyclo[3.2.0]-ring system that would result from C–C bond formation at the central position of the substituted allene (intermediate not shown). Finally, protonation of C would deliver 1,4-diene 3 in preference to the isomeric 1,4-diene 4.
Figure 2.
Proposed pathway for site- and stereoselective bimolecular coupling of allenes and alkynes.
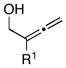
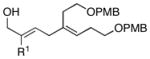
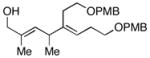
Initial studies were performed by exposing preformed allenic alkoxides to titanacyclopropenes (generated in situ from internal alkynes), followed by protonation of the presumed intermediate organometallic species. As depicted in entry 1 of Table 1, coupling of 2,3-butadiene-1-ol (5) with the symmetrical alkyne 6 leads to effective cross-coupling and furnishes the stereodefined 1,4-diene 7.6 Cross-coupling of the methyl-substituted allene 8 with alkyne 6 is similarly effective and provides 1,4-diene product 9 as a single isomer in 74% yield (entry 2). Coupling of allene 10, where the tethered hydroxyl is positioned further away from the cumulated diene, with alkyne 6 leads to the production of 1,4-diene products in good yield (64%), but modest stereoselection (E:Z = 3:1, entry 3). The observed product ratio may reflect a competition between directed and non-directed cross-coupling at the least substituted C=C of the allene. In this case, intramolecular carbometalation would proceed via a six-membered tether, as opposed to a five-membered (see B → C; Figure 2).
Table 1.

| ||||
|---|---|---|---|---|
| entrya | allene | alkyne | yield (%) | 1,4-dieneb |
|
|
|
||
| 1 | 5; R1 = H | 6 | 53 | 7c; R1 = H |
| 2 | 8; R1 = Me | 74 | 9; R1 = Me | |
| 3 |
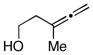 10 |
6 | 64 |
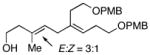 11 |
| 4 |
 12 |
6 | 87 |
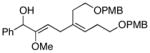 13 |
| 5 |
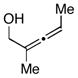 14 |
6 | 53 |
 15d |
| 6 | 12 |
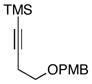 16 |
66 |
 17e |
| 7 | 8 |
 18 |
48 |
 19e |
| 8 | 8 |
 20 |
62 |
 21f |
Typical reaction conditions: alkyne (1.4 eq), Ti(Oi-Pr)4 (2.1 eq), c-C5H9MgCl (4.2 eq), PhMe (−78 to −30 °C), cool to −78 °C then add allenyl alkoxide (1 eq), (−78 to 0 °C).
Products formed as single olefin isomers unless otherwise noted.
Formed as a 3:1 mixture of products containing a minor cross-conjugated triene (see Supporting Information for details).
In addition, 31% of a cross-conjugated triene was isolated from this reaction.
rs = 4:1.
rr = 2:1.
Heteroatom substitution on the allene is tolerated in this coupling reaction, providing a convenient pathway to the synthesis of stereodefined enol ethers. For example, reaction of methoxy-substituted allene 12 with alkyne 6 affords the enol ether 13 in 87% yield (entry 4). In this transformation, no evidence was found for the production of stereoisomeric 1,4-diene products.
This cross-coupling reaction is similarly effective with more complex coupling partners. As illustrated in entry 5, reaction of the trisubstituted allene 14 with the internal alkyne 6 provides a convenient means of preparing 1,4-dienes bearing a central alkyl substituent. As observed in coupling reactions of less complex substrates, the substituted 1,4-diene 15 is produced as a single isomer.
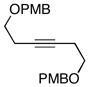
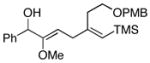
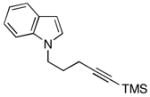
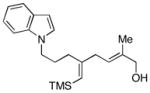
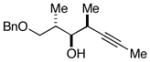
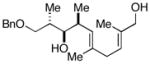
This allene–alkyne cross-coupling reaction can be extended to unsymmetrical alkynes. As depicted in entry 6, selective functionalization of two unsymmetrically substituted π-systems can be achieved. In this case, cross coupling of allene 12 with TMS-alkyne 16 provides the 1,4-diene 17 (rr = 4:1).7 Heteroaromatic-containing substrates are also suitable for cross-coupling. As illustrated in entry 7, cross-coupling of the indole-containing TMS-alkyne 18 with allene 8 provides the stereodefined 1,4-diene 19 in 48% yield (rr = 4:1). Finally, alkynes containing free hydroxyl functionality are viable substrates in this reaction. For example, coupling of allene 8 with the functionalized internal alkyne 20 furnishes the 1,4-diene-containing diol 21 in 62% yield, albeit with modest regioselection, and demonstrates the tolerance of this stereoselective bimolecular C–C bond forming reaction to free hydroxyls on both of the coupling partners (entry 8).
In summary, we document a bimolecular metal-mediated allene–alkyne cross-coupling reaction for the stereoselective synthesis of 1,4-dienes. Whereas coupling reactions of this class have previously been known to provide access to stereodefined 1,4-dienes with modest levels of stereoselection, the present contribution provides a highly stereoselective pathway for the formation of (E)-di- and trisubstituted olefins – in most cases, the 1,4-diene products are isolated as single olefin isomers. We have also demonstrated that this coupling process can be extended to unsymmetrical alkynes, where regio- and stereoselective functionalization of each π-component is accomplished. The utility of the present convergent coupling reaction in complex molecule synthesis, and the potential to transfer axial chirality in the generation of a C-3 stereodefined 1,4-diene are the topics of ongoing studies.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support of this work by the American Cancer Society (RSG-06-117-01), the American Chemical Society (PRF-45334-G1), the Arnold and Mabel Beckman Foundation, Boehringer Ingelheim, Bristol-Myers Squibb (for a graduate fellowship to H.L.S.), Eli Lilly & Co., and the National Institutes of Health – NIGMS (GM80266).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures and spectral data are included for all new compounds. See http://dx.doi.org/10.1039/b000000x/
Notes and references
- 1.For a recent review of palladium-catalyzed cross-coupling in total synthesis, see: Nicolaou KC, Bulger PG, Sarlah D. Angew Chem Int Ed. 2005;44:4442. doi: 10.1002/anie.200500368.
- 2.For recent reviews of metal-mediated C–C bond formation, with Group 4 metals, see: Marek I, editor. Titanium and Zirconium in Organic Synthesis. Wiley-VCH; Weinheim: 2002. p. 512.with Nickel, see: Montgomery J. Angew Chem Int Ed. 2004;43:3890. doi: 10.1002/anie.200300634.with Ruthenium, see: Trost BM, Toste FD, Pinkerton AB. Chem Rev. 2001;101:2067. doi: 10.1021/cr000666b.
- 3.Ryan J, Micalizio GC. J Am Chem Soc. 2006;128:2764. doi: 10.1021/ja057352w. [DOI] [PubMed] [Google Scholar]
- 4.Reichard HA, Micalizio GC. Angew Chem Int Ed. 2007;46:1440. doi: 10.1002/anie.200603515. [DOI] [PubMed] [Google Scholar]
- 5.For examles of metal-mediated cross-coupling of allenes and alkynes, see: Hideura D, Urabe H, Sato F. Chem Commun. 1998:271.Tanaka R, Sasaki M, Sato F, Urabe H. Tetrahedron Lett. 2005;46:329.For intramolecular allene–alkyne coupling, see: Kent JL, Wan H, Brummond KM. Tetrahedron Lett. 1995;36:2407.Hicks FA, Kablaoui NM, Buchwald SL. J Am Chem Soc. 1996;118:9450.Hicks FA, Kablaoui NM, Buchwald SL. J Am Chem Soc. 1999;121:5881.Urabe H, Takeda T, Hideura D, Sato F. J Am Chem Soc. 1997;119:11295.
- 6.The minor product in this cross-coupling reaction was a cross-conjugated triene – see Supporting Information for details. Cross-conjugated trienes have been observed in intramolecular reactions of alkynes with allenes bearing allenic leaving groups, see: Yamazaki T, Urabe H, Sato F. Tetrahedron Lett. 1998;39:7333.
- 7.For representative examples of regioselective functionalization of TMS-substituted alkynes via metallacyclopropenes, see: Sato F, Urabe H, Okamoto S. Chem Rev. 2000;100:2835. doi: 10.1021/cr990277l.and b) Ref 5(a).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.