Abstract
The objective of the study was to compare disease characteristics over the first 5 years of disease in patients with RA, with disease onset in 1990s and 2000s, respectively.
Methods :
All 2235 patients with early RA (disease duration ≤12 months) were recruited from the BARFOT prospective observational study. These patients were divided into group 1 included 1992 to 1999 (N=1084, 66% women) and group 2 included 2000 to 2006 (N=1151, 69% women). Disease Activity Score (DAS28), VAS pain and Health Assessment Questionnaire (HAQ) were assessed during 5 years. Remission was defined as DAS28 <2.6.
Results :
At inclusion, both women and men in group 2 had higher mean DAS28 (SD) than group 1, 5.42 (1.22) vs 5.26 (1.19), p=0.004 and 5.28 (1.22) vs 5.00 (1.27), p=0.004, respectively, mainly dependant on pain and not on inflammatory related measures. Over time DAS28 decreased and was in both genders, from 6 months to the 5-year follow-up, significantly lower in group 2. At 5-year, both women and men in group 2 had higher rate of remission than women and men in group 1. However, despite reduction of VAS pain and HAQ there were no differences in pain and HAQ between groups at any time point.
Conclusion :
Patients included in the 2000s achieved higher frequency of remission at the 5 year follow-up compared with those included in the 1990s, suggested to reflect the more active medical treatment. Interestingly, however, improvement in pain and HAQ did not differ between the two patient cohorts.
Keywords: Disease activity, early RA, HAQ, pain, “cohort compare”, rheumatoid arthritis.
INTRODUCTION
Treatment strategies for patients with rheumatoid arthritis (RA) have changed over the last decades [1]. In the 1980s, anti-malaria drugs and drugs with gold and sulphasalazine (SSZ) were the most common medical agents in treatment of patients with RA in Sweden. In those days the medical treatment was pyramidal, and in addition to analgesics the initial treatment was with non-steroidal anti-rheumatic drugs (NSAIDs) and in a later stage disease modifying anti-rheumatic drugs (DMARDs) i.e. drugs with gold and SSZ [2, 3]. In the late 1980s methotrexate (MTX) became available as a drug for RA and became successively the first choice because of its positive clinical effects [4]. Additional studies showed that a combination therapy with MTX and low dose glucocorticoids (GC) was even more effective than MTX alone [5-7]. In Sweden the recommendation in the 1990s was initial DMARD monotherapy and early use of low-dose GC, and “step-up” combination therapy reserved for more severe disease. By the development of new drugs the therapy of RA
The intention of this study was to compare patients with disease onset in the 1990s and the 2000s, respectively, i.e. two periods with changing treatment strategy, as to disease activity, physical function and pain over the first 5 years of disease, with special focus on gender differences.
PATIENTS AND METHODS
Patients
All 2542 patients were recruited from the Better Anti-Rheumatic PharmacOTherapy (BARFOT) study which is a Swedish observational prospective multicentre study of patients with early RA with the intention to improve outcome. The patients were included consecutively between 1992 and 2006 when they were diagnosed with RA. They had at inclusion a disease duration of ≤12 months and fulfilled the ACR 1987 classification criteria [9] and gave their consent to participate. The patients were assessed according to a structured protocol at inclusion, 3 and 6 months, at 1, 2, 5, 8 and 15 years, a protocol not changed over time. In this study, data for the first 5 years follow-up are presented. The patients were treated with DMARDs in accordance with the recommended treatment strategy in Sweden as described in the introduction. Current treatment with GC and DMARDs were recorded at each visit.
Clinical Disease Assessments
Disease activity was measured by the composite index Disease Activity Score, calculated on 28 joints (DAS28) [10]. This composite index includes number of swollen joints (SJC28), number of tender joints (TJC28), patient’s global assessment of disease activity (PatGA) measured on a visual analogue scale (VAS, range 0-100 mm, best to worse) and erythrocyte sedimentation rate (ESR, mm/h). DAS28 was calculated by the formula ((0.56*sqrt(TJC)+0.28* sqrt(SJC)+0.70*Ln(ESR))+0.014*PatGA. As a comparison disease activity was also calculated as DAS28-3, where PatGA was excluded ((0.56*sqrt(TJC)+0.28*sqrt(SJC)+ 0.70*Ln(ESR))*1.08+0.16).
Remission was defined as DAS28<2 .6 i.e. the European League Against Rheumatism (EULAR) criteria [11]. EULAR response was calculated as described by van Gestel et al. [12], in three groups: no response, moderate response or good response.
Pain was measured by VAS (0-100 mm, best to worse). Functional disability was assessed using the Swedish version of Stanford Health Assessment Questionnaire (HAQ) (0-3 best to worse) [13]. Radiographic changes at inclusion were assessed according to ACR 1987 criteria for classification of RA, yes/no [9]. ESR was analysed by the Westergren method and rheumatoid factor (RF) was measured according to the current laboratory standards at the hospitals.
Statistical Methods
Statistical analyses were performed using SPSS Statistics 19 software. All significance tests were two tailed and conducted at the 0.05 significance level. A Bonferroni correction with significance level of 0.005, would not have change the interpretation of the study. To test differences between groups Chi-square was used for proportions and Kruskal-Wallis with post hoc pairwise analysis, when comparing more than two groups, or Mann-Whitney, when comparing two groups, for continuous variables, because some of the variables were not normally distributed. For over time analysis, with-in group comparisons Wilcoxon’s test was used. Correlations were tested with bivariate Spearman correlation analysis. Multivariate logistic regression analyses were performed to assess if inclusion in 1990s (group 1) v.s. 2000s (group 2), was associated with being in remission. The multivariate logistic regression analyses were controlled for gender, age, disease duration, smoking habits, RF and DAS28, pain or HAQ at inclusion. DAS28, pain and HAQ were included in separate models due to high correlation.
Ethical Approval
Ethical approval was obtained from the Regional ethical review board at Lund University, Gothenburg University, Karolinska Institutet and Linköping University at study start (LU 398-01; Gbg Ö 282-01; LI 01-263; KI 02-075). The study followed the guidelines of the Helsinki Declaration. Written consent from the participants was obtained.
RESULTS
Characteristics at Inclusion
Of the 2542 patients, 307 patients lost to follow-up (172 included 1992-1999 and 135 included 2000-2006); 178 died, 15 moved, 19 rejected participation and 95 for reasons unknown. In both cohorts the patients lost to follow-up were older at inclusion, had more inflammation, worse physical function and were more often not treated with DMARD and/or GC than those accessible for follow-up.
The patients were divided into two groups, those included 1992 to 1999, group 1 (N=1084, 66% women) and those included 2000 to 2006, group 2 (N=1151, 69% women). There was no difference in gender distribution between the groups, p=0.217.
At inclusion, group 2 was older compared to group 1, mean age (SD) 59 (15) vs 57 (15), p<0.001, and had shorter mean disease duration, months (SD) 5.7 (3.0) vs 6.1 (3.1), p<0.001. In group 2 more patients were RF positive 62% vs 58%, p=0.021, had higher DAS28, mean (SD) 5.37 (1.23) vs 5.18 (1.25), p<0.001, higher HAQ, mean (SD) 1.06 (0.66) vs 1.00 (0.65), p=0.011, scored higher on VAS pain, mean (SD) 48.1 (24.5) vs 44.9 (24.4), p<0.001 and PatGA, mean (SD) 47.4 (25.7) vs 44.0 (25.4), p=0.001. There was no difference in ESR between the two groups.
When dividing the groups by gender women in group 2 were older, had shorter disease duration and were more often RF positive than women in group 1. Furthermore, they were more often previous smokers, had higher DAS28, scored higher in PatGA and pain (Table 1). In women DAS28-3 showed no difference between the groups.
Table 1.
Demographic and clinical characteristics of the RA patients, mean (95% CI) values at inclusion (incl.)
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Group 1 Mean (95% CI) |
Group 2 Mean (95% CI) |
P-Value | Group 1 Mean (95% CI) |
Group 2 Mean (95% CI) |
P-Value | |
| N | 720 (66%) | 793 (69%) | 0.217 | 364 | 358 | |
| Age, years | 54 (52-55) | 57 (56-58) | <0.001 | 60 (58-61) | 61 (59-62) | 0.271 |
| Disease duration, months | 6.3 (6.1-6.5) | 5.8 (5.6-6.0) | 0.002 | 5.9 (5.6-6.2) | 5.6 (5.3-5.9) | 0.168 |
| Never smoker (%) | 49 | 44 | 0.033 | 31 | 27 | 0.189 |
| Present Smoker (%) | 27 | 26 | 30 | 26 | ||
| Previous smoker (%) | 24 | 30 | 39 | 47 | ||
| RF pos (%) | 57 | 64 | 0.011 | 61 | 59 | 0.614 |
| Radiographic changes incl (%) | 23 | 24 | 0.600 | 25 | 25 | 0.890 |
| DAS28 incl. (0-10) | 5.26 (5.17-5.35) | 5.42 (5.34-5.51) | 0.004 | 5.00 (4.86-5.12) | 5.28 (5.15-5.41) | 0.004 |
| DAS28-3 incl. (0-10) | 5.21 (5.12-5.30) | 5.26 (5.18-5.34) | 0.337 | 4.96 (4.81-5.10) | 5.18 (5.06-5.30) | 0.045 |
| PatGA incl. (0-100 mm) | 46.1 (44.2-48.0) | 49.7 (47.9-51.5) | 0.004 | 39.4 (36.9-42.0) | 43.6 (40.9-46.4) | 0.040 |
| Swollen joints incl. (28) | 10.3 (9.8-10.7) | 10.3 (9.9-10.7) | 0.704 | 10.6 (9.9-11.3) | 11.4 (10.8-12.0) | 0.096 |
| Tender joints incl. (28) | 8.5 (8.0-9.0) | 8.7 (8.3-9.2) | 0.454 | 6.6 (5.9-7.3) | 8.2 (7.6-8.9) | 0.001 |
| ESR incl. (mm/h) | 36.0 (34.1-37.9) | 36.7 (35.0-38.5) | 0.305 | 35.9 (33.4-38.5) | 35.1 (32.5-37.6) | 0.699 |
| HAQ incl. (0-3) | 1.06 (1.01-1.10) | 1.12 (1.08-1.17) | 0.090 | 0.82 (0.76-0.88) | 0.88 (0.81-0.94) | 0.221 |
| VAS pain incl. (0-100 mm) | 46.3 (44.5-48.1) | 50.4 (48.7-52.1) | <0.001 | 41.8 (39.2-44.3) | 44.8 (42.2-47.4) | 0.139 |
RF; rheumatoid factor, DAS; disease activity score, PatGA; patient global assessment, ESR; erythrocyte sedimentation rate, HAQ; health assessment questionnaire, VAS visual analog scale.
P-values denotes the overall significance of differences between groups calculated by Kruskal-Wallis test if continuous or by Chi-square if proportions.
There were no differences in age or disease duration in men between the two groups, although DAS28, TJC28 and PatGA were higher in group 2 than in group 1. Also DAS28-3 was higher in group 2 than in group 1. Trend vise men in group 2 scored higher in pain but this did not reach statistical significance (Table 1).
Neither in women nor in men were there any statistically significant differences in SJC28, ESR or HAQ between the groups at inclusion. There was no statistical significant difference in HAQ at inclusion between patients with or without radiographic changes at inclusion, p=0.432.
Clinical Measures Over Time
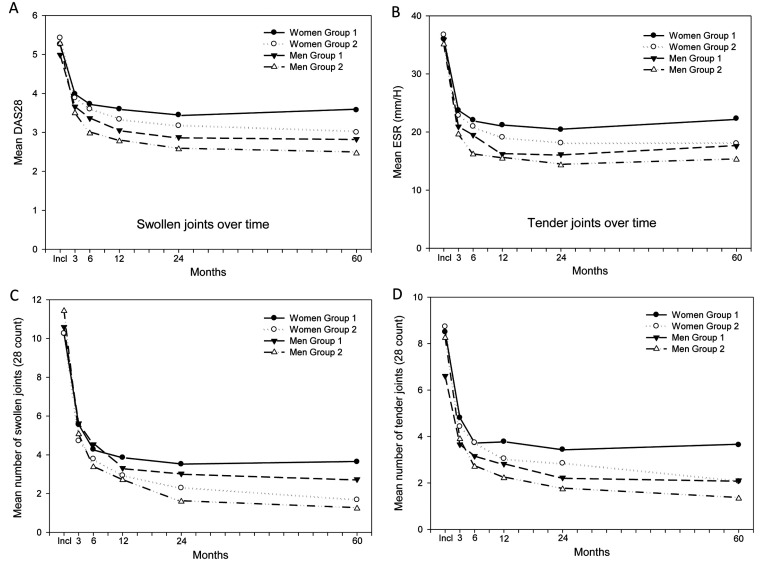
The change in DAS28 and its components, SJC28, TJC28 and ESR showed similar pattern over time, Fig. (1A-D). Thus, these variables in both groups 1 and 2 decreased statistically significant already after 3 months and leveled off after 6 months in DAS28, TJC28 and ESR and after 12 months in SJC28. However, in group 2 these variables improved more, both in women and men.
In comparisons with-in the groups, women in both groups leveled off in DAS28 and ESR at follow-up from 3 months (p≤0.001) and in SJC28 from 12 months (p<0.04) to a statistical significant higher level than men.
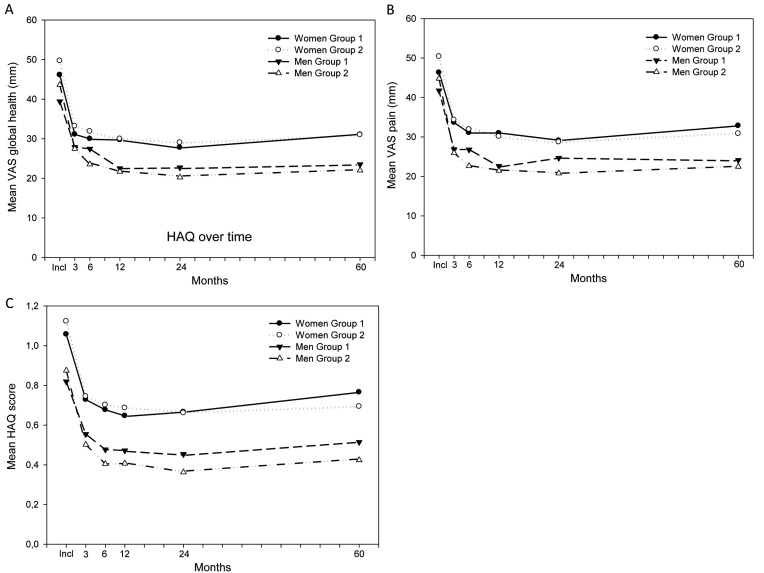
As shown in Fig. (2A-C), PatGA, pain and HAQ present a different pattern compared to the variables in Fig. (1A-D), with a decrease only over the first three (pain) to first six months (PatGA and HAQ), and women scored higher than men at all time points. However, there was no statistically significant difference between women in the two groups or between men in the two groups at any occasion.
EULAR Response and Remission at 5-Year Follow-Up
In group 1, 74% of the women and 81% of the men reached good or moderate EULAR response at the 5-year follow-up, the corresponding rates for group 2 were 87% and 91%, respectively (Table 2).
Table 2.
Demographic and clinical characteristics of the RA patients, mean (95% CI) values at five year follow-up (5 year).
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Group 1 Mean (95% CI) |
Group 2 Mean (95% CI) |
P-Value | Group 1 Mean (95% CI) |
Group 2 Mean (95% CI) |
P-Value | |
| DAS28 5 year (0-10) | 3.57 (3.46-3.68) | 3.00 (2.91-3.10) | <0.001 | 2.83 (2.67-2.98) | 2.46 (2.32-2.60) | 0.001 |
| PatGA 5 year (0-100 mm) | 31.1 (29.2-33.1) | 31.0 (29.0-32.8) | 0.899 | 23.5 (21.0-26.0) | 22.0 (19.4-24.6) | 0.310 |
| Swollen joints 5 year (28) | 3.6 (3.3-4.0) | 1.7 (1.5-1.9) | <0.001 | 2.7 (2.3-3.2) | 1.2 (1.0-1.5) | <0.001 |
| Tender joints 5 year (28) | 3.6 (3.3-4.0) | 2.1 (1.8-2.3) | <0.001 | 2.1 (1.7-2.5) | 1.3 (1.0-1.6) | 0.003 |
| ESR 5 year (mm/h) | 22.3 (20.9-23.7) | 18.0 (17.0-19.1) | <0.001 | 17.6 (15.6-19.6) | 15.3 (13.5-17.0) | 0.185 |
| HAQ 5 year (0-3) | 0.77 (0.71-0.82) | 0.69 (0.65-0.74) | 0.071 | 0.51 (0.45-0.58) | 0.42 (0.36-0.48) | 0.080 |
| VAS pain (0-100 mm) | 32.8 (30.9-34.9) | 30.8 (29.0-32.7) | 0.239 | 24.1 (21.6-26.6) | 22.5 (19.9-25.1) | 0.284 |
| EULAR respons 5 year (good/moderate %) | 74 | 87 | <0.001 | 81 | 91 | <0.001 |
| Remission 5 year (%) | 29 | 42 | <0.001 | 52 | 64 | 0.001 |
RF; rheumatoid factor, DAS; disease activity score, PatGA; patient global assessment, ESR; erythrocyte sedimentation rate, HAQ; health assessment questionnaire, VAS visual analog scale.
P-values denotes the overall significance of differences between groups calculated by Kruskal-Wallis test if continuous or by Chi-square if proportions.
A higher proportion of patients in group 2 was in remission, group 2 vs group 1, in women 42% vs 29%, p<0.001, and in men 64% vs 52%, p<0.001 (Table 2). Within group there was a statistical significant difference between women and men being in remission, with a higher rate of men in remission, p<0.001 in both groups. At the 5-year follow-up the rate of patients not in remission reporting clinically significant pain (VAS pain >40) [14] were 44% among women in group 1 vs 48% in group 2, p=0.225, and in men 38% vs 44%, p=0.301. Of those in remission at 5 year follow-up, 10% of the women in both groups reported pain >40, p=0.866, and in men 10% vs 9% reported pain >40, p=0.632.
In a multivariate logistic regression analysis, there was a positive association between group 2 and being in remission at 5 year follow-up, OR 1.756 (95% CI 1.414-2.180, p<0.001), compared to group 1. The following variables were included in the regression analyses: gender, age, smoking habits, disease duration, RF and DAS28 at inclusion (Table 3). There was a positive association for male gender OR 2.652 (95% CI 2.107-3.339, p<0.001) and negative associations for older age OR 0.988 (95% CI 0.981-0.995, p=0.001), present smoker OR 0.739 (95% CI 0.567-0.964, p=0.026), longer disease duration OR 0.941 (95% CI 0.908-0.976, p=0.001), RF positivity OR 0.615 (95% CI 0.494-0.766, p<0.001) and a higher DAS28 at inclusion OR 0.658 (95% CI 0.601-0.721, p<0.001) (Table 3). Similar associations, except for present smoker, were found when adding HAQ at inclusion or pain at inclusion in to the model, separately (Table 3).
Table 3.
Odds ratios (95% CI) in multivariate analysis of associations of being in remission at the 5year follow-up and DAS28 at inclusion, HAQ at inclusion or pain at inclusion, controlled for group (group 1 – included in 1990s, group 2 – included in 2000s), gender, age, smoking habits, disease duration and RF. DAS28, HAQ and pain were analysed separate due to high correlation.
| DAS28 | HAQ | Pain | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Group | 1 | 1 | 1 | 1 | |||
| 2 | 1.756 (1.414-2.180) | <0.001 | 1.749 (1.408-2.173) | <0.001 | 1.705 (1.380-2.106) | <0.001 | |
| Gender | Women | 1 | 1 | 1 | |||
| Men | 2.652 (2.107-3.339) | <0.001 | 2.527 (2.000-3.191) | <0.001 | 2.630 (2.099-3.294) | <0.001 | |
| Age | 0.988 (0.981-0.995) | 0.001 | 0.987 (0.979-0.994) | <0.001 | 0.985 (0.978-0.996) | <0.001 | |
| Smoking | Non | 1 | 1 | 1 | |||
| Smoker | 0.739 (0.567-0.964) | 0.026 | 0.813 (0.623-1.061) | 0.127 | 0.777 (0.599-1.007) | 0.057 | |
| Previous | 0.946 (0.734-1.219) | 0.669 | 0.961 (0.744-1.240) | 0.757 | 0.947 (0.739-1.213) | 0.664 | |
| Duration | 0.941 (0.908-0.976) | 0.001 | 0.953 (0.919-0.98) | 0.009 | 0.956 (0.923-0.990) | 0.012 | |
| RF | negative | 1 | 1 | 1 | |||
| positive | 0.615 (0.494-0.766) | <0.001 | 0.593 (0.476-0.739) | <0.001 | 0.638 (0.515-0.790) | <0.001 | |
| DAS28 | 0.658 (0.601-0.721) | <0.001 | |||||
| HAQ | 0.593 (0.496-0.709) | <0.001 | |||||
| Pain | 0.991 (0.987-0.996) | <0.001 | |||||
Medical Treatment
Treatment at inclusion and 5-year are presented in Table 4. As expected the medical treatment differed between the groups. In group 1, one third of the patients (34% women, 32% men) were not treated with any DMARD at inclusion, although about half of those patients were treated with GC. In group 2 the corresponding rate was 11% in women and 14% in men, and of those about one third were treated with GC. In total, 46% (44% women, 50% men, p=0.039) in group 1 and 32% (30% women, 35% men, p=0.101) in group 2 were treated with GC (alone or in combination with DMARD) at inclusion. Patients in group 2 were at inclusion treated with methotrexate to higher extent than group 1.
Table 4.
Medical treatment at inclusion and at 5 year follow-up (%).
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Inclusion | 5 Year Follow-Up | Inclusion | 5 Year Follow-Up | |||||
| Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | |
| No Prednisolone/DMARD | 19 | 8 | 30 | 14 | 13 | 9 | 28 | 18 |
| GC no DMARD | 15 | 3 | 6 | 4 | 19 | 5 | 7 | 3 |
| MTX* | 26 | 62 | 32 | 39 | 26 | 63 | 33 | 45 |
| SSZ* | 29 | 18 | 6 | 5 | 32 | 18 | 9 | 6 |
| Other mono* | 10 | 5 | 10 | 6 | 9 | 2 | 10 | 2 |
| Combination* | 1 | 3 | 10 | 9 | 1 | 2 | 7 | 11 |
| Biologics* | 0 | 1 | 6 | 23 | 0 | 1 | 6 | 15 |
With or without prednisolone, GC-Glucocorticoids
DMARD; disease modifying anti-rheumatic drug, GC; glucocorticoids, MTX; methotrexate, SSZ; sulphasalazine.
From 3 months to 5 year follow-up there was no difference in GC treatment between women and men in any of the groups, though a higher rate of patients in group 1 was treated with GC at all follow-ups (p<0.001) (data not shown). Over time there were no differences in DMARD treatment between women and men in the two groups. In group 1 the treatment tended to switch from SSZ to MTX and there was an increasing rate of biologics (6% in both women and men). There was also a decreasing rate of patients treated with prednisolone over the 5 years (Table 4). In group 2, most patients (61% women, 63% men) were treated with MTX at inclusion and after 5-years follow-up an increasing amount of patients in group 2 were treated with biologics, 23% of the women and 15% of the men (Table 4).
DISCUSSION
This longitudinal observational study of patients with early RA focus on gender differences, comparing patients with disease onset in the 1990s and in the 2000s, respectively, two periods with different treatment strategies.
At inclusion, both women and men included in the 2000s had higher disease activity, assessed with DAS28, than those included in the 1990s, though in women there was no difference between the two groups when disease activity was assessed with DAS28-3. Obviously, the higher DAS28 in women was mainly dependent on the higher score of PatGA probably influenced by the higher pain score, as PatGA and pain has been shown to be highly correlated [15].
Also in men, the pain related measures are suggested to cause the higher DAS28 at inclusion in group 2. Thus not only PatGA but also TJC28 was higher for men in that group, and as TJC28 has a great weight in DAS28-3, it may explains that also DAS28-3 was higher. We thus suggest that the disease severity at presentation did not increase over the studied periods, which is in line with a recent report of early RA [16].
The shorter disease duration in patients included in the 2000s could be explained by the changed recommendations in 2000, which recommended an early treatment strategy for patients with RA. This recommendation was supported by several studies published in late 1990s, reporting that joint destruction starts early in disease course and that early treatment reduce clinical inflammation, improve physical function and diminished joint destruction [17-22].
The women included in the 2000s were older and more often previous smoker than women included in the 1990s. There are studies reporting a change towards higher age in RA patients at disease onset in recent years, which could support the results in this study [23, 24]. The higher rate of RF positive women included in the 2000s could be due to their higher age, since the rate of RF positive patients increases with age [25, 26] and could also be due to more smokers in that group [27]. These differences are not suggested to depend on differences in participation between the two periods as that was similar.
The medical treatment differed between the groups owing to the different treatment strategies in the 1990s and 2000s, though there were no differences between women and men in with-in group comparisons. Although there was no difference in treatment women in both groups had higher disease activity, more pain and, worse function over the first 5 years than men in the same group. In both groups fewer women were in remission and moderate/good EULAR response at the 5-year follow-up. Strikingly, this was also the case in the group included in the 2000s when women had been treated with biologics to a higher extent than women in the group included in the 1990s.
Over time there was a decrease in inflammation, assessed by ESR and SJC. The decrease was more pronounced in group 2, probably due to the intensified treatment strategy and the increased use of biologics. When it comes to pain and pain related measures such as PatGA and HAQ, there were also decreases over time, though with smaller amplitude especially in women but with no significant differences between the groups. PatGA and HAQ have been shown to be affected by pain i.e. have a rather high correlation with pain [15, 28, 29]. The treatment of RA has often been focused on inflammation, with the intension to reduce joint destruction. Patients, however, often rate pain as their main problem. About ten per cent of the patients in remission at the 5-year follow-up had clinically significant pain, which has also been reported in a study by Lee et al. [30], and about 40% of the patients not in remission. The pain at inclusion was probably an acute peripheral inflammatory pain, which was diminished by treating the inflammation. In most patients this treatment was enough to reduce the pain reported earlier [29, 31]. As discussed in some other studies, in some patients the inflammatory pain changes to a more non-inflammatory widespread pain [14, 29, 32]. Maybe this is an explanation to the long-term results of the pain related outcomes. The mechanism behind the alteration from acute inflammatory pain to chronic non-inflammatory pain is not fully understood, though animal models have shown TNF-α to play a role in the sensitization of the central nervous system [33, 34]. Studies evaluating the effect of anti-TNF-α treatment on pain in humans have reported divergent results [29, 35]. The incidence rate of developing chronic non-inflammatory pain is reported to be highest the first 12 months after diagnosis of RA [36], also described in acute back pain [37, 38].
The patients in the 2000s had access to a more intensified treatment strategy and biologics, effective to reduce the inflammation, resulting in more patients in DAS28 remission and moderate/good EULAR response at the 5-year follow-up. However, this strategy did not sufficiently reduce the pain or the pain associated physical function (HAQ).
The strength of this study is that it is a prospective, longitudinal study with over 2500 RA patients included consecutively, who are followed by structured protocol. Further the patients are representative for patients with early RA in Sweden at time for inclusion.
A limitation of the study is that there are some missing data, 4-8% at each follow-up from 3 months to the 5-year follow-up, which could have affected the outcome of the study. Another limitation is that patients could have changed medical treatment between the follow-ups.
CONCLUSION
At the 5-year follow-up the patients included in the 2000s achieved higher frequency of remission and good/moderate EULAR response than those included in the 1990s, suggested to reflect the more active medical treatment. Interestingly, however, improvement in pain and pain related outcomes as PatGA and HAQ did not differ between the two patient cohorts, why also other mechanisms than inflammation might be of importance.
Fig. (1).
Disease activity and inflammatory measures during the first 5 years of follow-up. Group 1 is patients included between 1992 and 1999, group 2 included between 2000 and 2006. Data are reported separately for men and women. A presents the mean DAS28 in the separate groups over time, B presents the mean ESR in the separate groups over time. C and D presents mean SJC28 and mean TJC28 in the separate groups over time. DAS28; 28 joints-Disease Activity Score, ESR; Erythrocyte sedimentation rate, SJC28; Swollen Joint Count 28, TJC28; Tender Joint Count 28.
Fig. (2).
(A-C) PatGA, pain and, HAQ during the first 5 years of follow-up. Group 1 is patients included between 1992 and 1999, group 2 included between 2000 and 2006. Data are reported separate for men and women, A presents the mean PatGA in the separate groups over time, B presents the mean VAS pain in the separate groups over. C presents mean HAQ in the separate groups over time. PatGA; Patient Global Assessment, HAQ; Health Assessment Questionnaire.
ACKNOWLEDGEMENTS
The authors thank the other members of the BARFOT Study Group especially Maria Söderlin for valuable comments and further Sofia Ajeganova, Valentina Bala, Björn Svensson, Stefan Bergman, Ann Bremander, Åsa Häggström, Catharina Keller, Ido Leden, Annika Teleman, Jan Theander, and Anneli Östenson.
The County Council of Halland research fund, The Regional board of Southern Sweden research fund and the Swedish Rheumatism Association, The Thelma Zoegas foundation in Helsingborg, and Stiftelsen för Rörelsehindrade i Skåne for supporting by grants.
AUTHORS CONTRIBUTIONS
MA participated in planning the study design, gathered the data from the database, performed the statistical analyses, and drafted the manuscript. KF participated in planning the study design, data interpretation and helped draft the manuscript. IH participated in planning the study design, data interpretation and critically revised the manuscript. All authors read and approved the final manuscript.
ABBREVIATIONS
- ACR
= American College of Rheumatology
- BARFOT
= Better Anti-Rheumatic Pharmacotherapy
- DAS28
= 28 joints-Disease Activity Score
- DMARD
= Disease-modifying Anti-Rheumatic Drug
- ESR
= Erythrocyte sedimentation rate
- EULAR
= European League Against Rheumatism
- GC
= Glucocorticoids
- HAQ
= Health Assessment Questionnaire
- MTX
= Methotrexate
- NSAID
= Non-Steroidal Anti-rheumatic Drug
- PatGA
= Patient Global Assessment
- RA
= Rheumatoid Arthritis
- RF
= Rheumatoid Factor
- SSZ
= Sulphasalazine
- SJC28
= Swollen Joint Count 28
- TJC28
= Tender Joint Count 28
- TNFα
= Tumour Necrosis Factor alpha
- VAS
= Visual Analog Scale
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Upchurch KS, Kay J. Evolution of treatment for rheumatoid arthritis. Rheumatology (Oxford). 2012;51(suppl 6: ): vi28–36. doi: 10.1093/rheumatology/kes278. [DOI] [PubMed] [Google Scholar]
- 2.Healey LA, Wilske KR. Reforming the pyramid.A plan for treating rheumatoid arthritis in the. Rheum Dis Clin North Am. . 1989;15:615–9. [PubMed] [Google Scholar]
- 3.Wilske KR, Healey LA. Remodeling the pyramid-a concept whose time has come. J Rheumatol. 1989;16:565–7. [PubMed] [Google Scholar]
- 4.Kremer JM, Lee JK. The safety and efficacy of the use of methotrexate in long-term therapy for rheumatoid arthritis. Arthritis Rheum. 1986;29:822–31. doi: 10.1002/art.1780290702. [DOI] [PubMed] [Google Scholar]
- 5.Harris ED , Jr, Emkey RD, Nichols JE, Newberg A. Low dose prednisone therapy in rheumatoid arthritis a double blind study. J Rheumatol. 1983;10:713–21. [PubMed] [Google Scholar]
- 6.Kirwan Jr. The effect of glucocorticoids on joint destruction in rheumatoid arthritis.The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. . N Engl J Med. 1995;333:142–6. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- 7.Svensson B, Boonen A, Albertsson K , et al. Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate a two-year randomized trial. Arthritis Rheum. 2005;52:3360–70. doi: 10.1002/art.21298. [DOI] [PubMed] [Google Scholar]
- 8.Moreland LW. Inhibitors of tumor necrosis factor new treatment options for rheumatoid arthritis. Cleve Clin J Med. 1999;66:367–74. doi: 10.3949/ccjm.66.6.367. [DOI] [PubMed] [Google Scholar]
- 9.Arnett FC, Edworthy SM, Bloch DA , et al. The American Rheumatism Association 1987, revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 10.Prevoo ML, van 't Hof MA, Kuper HH , et al. Modified disease activity scores that include twenty-eight-joint counts.Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. . Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 11.Fransen J, Creemers MC, van Riel PL. Remission in rheumatoid arthritis agreement of the disease activity score (DAS28):with the ARA preliminary remission criteria. Rheumatology (Oxford). 2004;43:1252–5. doi: 10.1093/rheumatology/keh297. [DOI] [PubMed] [Google Scholar]
- 12.van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–50. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Ekdahl C, Eberhardt K, Andersson SI, Svensson B. Assessing disability in patients with rheumatoid arthritis.Use of a Swedish version of the Stanford Health Assessment Questionnaire. Scand J Rheumatol. 1988;17:263–71. doi: 10.3109/03009748809098795. [DOI] [PubMed] [Google Scholar]
- 14.Lee YC. Effect and treatment of chronic pain in inflammatory arthritis. Curr Rheumatol Rep. 2013;15:300. doi: 10.1007/s11926-012-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson ML, Svensson B, Bergman S. Chronic widespread pain in patients with rheumatoid arthritis and the relation between pain and disease activity measures over the first 5 years. J Rheumatol. 2013;40:1977–85. doi: 10.3899/jrheum.130493. [DOI] [PubMed] [Google Scholar]
- 16.Diffin JG, Lunt M, Marshall T , et al. Has the severity of rheumatoid arthritis at presentation diminished over timeκ. J Rheumatol. 2014;41:1590–9. doi: 10.3899/jrheum.131136. [DOI] [PubMed] [Google Scholar]
- 17.van de Putte LB, van Gestel AM, van Riel PL. Early treatment of rheumatoid arthritis rationale, evidence, and implications. Ann Rheum Dis. 1998;57:511–2. doi: 10.1136/ard.57.9.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Heide A, Jacobs JW, Bijlsma JW , et al. The effectiveness of early treatment with "second-line" antirheumatic drugs.A randoized controlled trial. Ann Intern Med. 1996; 124:699–707. doi: 10.7326/0003-4819-124-8-199604150-00001. [DOI] [PubMed] [Google Scholar]
- 19.van der Heijde DM. Joint erosions and patients with early rheumatoid arthritis. Br J Rheumatol. 1995;34(suppl 2: ): 74–8. [PubMed] [Google Scholar]
- 20.van der Heijde DM, van Leeuwen MA, van Riel PL, van de Putte LB. Radiographic progression on radiographs of hands and feet during the first 3 years of rheumatoid arthritis measured according to Sharp's method (van der Heijde modification). J Rheumatol. 1995;22:1792–6. [PubMed] [Google Scholar]
- 21.Stenger AA, van Leeuwen MA, Houtman PM , et al. Early effective suppression of inflammation in rheumatoid arthritis reduces radiographic progression. Br J Rheumatol. 1998;37:1157–63. doi: 10.1093/rheumatology/37.11.1157. [DOI] [PubMed] [Google Scholar]
- 22.Weinblatt ME. Rheumatoid arthritis treat now, not later. Ann Intern Med. 1996;124:773–4. doi: 10.7326/0003-4819-124-8-199604150-00012. [DOI] [PubMed] [Google Scholar]
- 23.Kaipiainen-Seppanen O, Aho K, Isomaki H, Laakso M. Shift in the incidence of rheumatoid arthritis toward elderly patients in Finland during 1975,-1990,. Clin Exp Rheumatol. 1996;14:537–42. [PubMed] [Google Scholar]
- 24.Imanaka T, Shichikawa K, Inoue K , et al. Increase in age at onset of rheumatoid arthritis in Japan over a 30 year period. Ann Rheum Dis. 1997;56:313–6. doi: 10.1136/ard.56.5.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cammarata RJ, Rodnan GP, Fennell RH. Serum anti-gamma-globulin and antinuclear factors in the aged. JAMA. 1967;199:455–8. [PubMed] [Google Scholar]
- 26.Litwin SD, Singer JM. Studies of the Incidence and Significance of Anti-Gamma Globulin Factors in the Aging. Arthritis Rheum. 1965;8:538–50. doi: 10.1002/art.1780080408. [DOI] [PubMed] [Google Scholar]
- 27.Masdottir B, Jonsson T, Manfredsdottir V , et al. Smoking, rheumatoid factor isotypes and severity of rheumatoid arthritis. Rheumatology (Oxford). 2000;39:1202–5. doi: 10.1093/rheumatology/39.11.1202. [DOI] [PubMed] [Google Scholar]
- 28.Ranzolin A, Brenol JC, Bredemeier M , et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and short form 36 scores in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61:794–800. doi: 10.1002/art.24430. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe F, Michaud K. Assessment of pain in rheumatoid arthritis minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J Rheumatol. 2007;34:1674–83. [PubMed] [Google Scholar]
- 30.Lee YC, Cui J, Lu B , et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission a longitudinal observational study. Arthritis Res Ther. 2011;13:R83. doi: 10.1186/ar3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strand V, Burmester GR, Ogale S , et al. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors results from the 24-week randomized controlled RADIATE study. Rheumatology (Oxford). 2012;51:1860–9. doi: 10.1093/rheumatology/kes131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13:211. doi: 10.1186/ar3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boettger MK, Hensellek S, Richter F , et al. Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis evidence of a neuronal target. Arthritis Rheum. 2008;58:2368–78. doi: 10.1002/art.23608. [DOI] [PubMed] [Google Scholar]
- 34.Hess A, Axmann R, Rech J , et al. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci U S A. 2011;108:3731–6. doi: 10.1073/pnas.1011774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lomholt JJ, Thastum M, Herlin T. Pain experience in children with juvenile idiopathic arthritis treated with anti-TNF agents compared to non-biologic standard treatment. Pediatr Rheumatol Online J. 2013;11:21. doi: 10.1186/1546-0096-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YC, Lu B, Boire G , et al. Incidence and predictors of secondary fibromyalgia in an early arthritis cohort. Ann Rheum Dis. 2013;72:949–54. doi: 10.1136/annrheumdis-2012-201506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klazen CA, Verhaar HJ, Lohle PN , et al. Clinical course of pain in acute osteoporotic vertebral compression fractures. J Vasc Interv Radiol. 2010;21:1405–9. doi: 10.1016/j.jvir.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Shaw WS, Means-Christensen AJ, Slater MA , et al. Psychiatric disorders and risk of transition to chronicity in men with first onset low back pain. Pain Med. 2010;11:1391–400. doi: 10.1111/j.1526-4637.2010.00934.x. [DOI] [PubMed] [Google Scholar]