Abstract
We demonstrate that the cardiac and respiratory system exhibit three distinct forms of coupling that are independent from each other, respond differently to key physiologic parameters, and act on different time scales. We find that all three forms of coupling undergo pronounced phase transitions across sleep stages characterized by different stratification patterns, indicating markedly different response to changes in neuroautonomic control. Our analyses show that all three forms of cardio-respiratory interaction are not of constant strength but are of transient and intermittent nature with “on” and “off” periods, and that these forms of coupling, representing different aspects of physiologic regulation, can simultaneously coexist.
1. Introduction
It is a challenge to determine interactions between complex systems where their coupling is not a-priory known, and where the only available information is contained in the output signals of the systems. For integrated physiological systems, such as the cardiac and respiratory system, this problem is further complicated by transient and nonlinear characteristics, and continuous fluctuations in the output signals. Both systems are under neuroautonomic control that regulates their complex dynamics and further influences their coupling through intrinsic feedback mechanisms at different time scales. The nature of cardio-respiratory interaction, its physiological and clinical relevance is not well understood.
Utilizing concepts and methods from nonlinear dynamics and statistical physics, we investigate dynamical aspects of the cardio-respiratory coupling. We focus on whether and how cardio-respiratory interaction changes in response to transitions across physiologic states. We hypothesize that cardio-respiratory interaction is carried not only by one form of coupling but instead is mediated through multiple forms acting at different time scales. Further, we hypothesize that different forms of coupling may simultaneously coexist, representing different aspects of neural regulation. Specifically, we hypothesize that at any given moment the cardiac and respiratory system interact through complementary forms of coupling with intermittent “on” and “off” periods, different strength and different stratification patterns across physiologic states.
2. Subject data
To test our hypotheses, we analyze recordings from a group of 189 healthy subjects (99 female and 90 male, ages ranging from 20–95 years) during night-time sleep (average record duration is 7.8 h), tracking changes in cardio-respiratory coupling across different sleep stages. We focus on physiological dynamics during sleep, as sleep stages are well-defined physiological states and external influences due to physical activity or sensory inputs are reduced during sleep. We consider standard ECG (removed artifacts and ectopic beats) and respiratory signals (resampled to 4 Hz to eliminate high-frequency noise) [1].
3. Methods
Respiratory sinus arrhythmia (RSA)
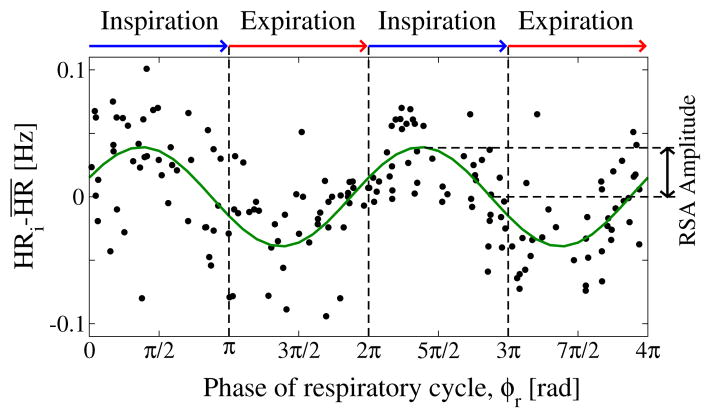
In physiological studies, cardio-respiratory coupling is traditionally identified through the respiratory sinus arrhythmia (RSA), which accounts for the periodic variation of the heart rate within a breathing cycle [2]. Typically, for normal breathing rates in the range 7–12 breaths per minute, RSA is characterized by the increase of heart rate during inspiration and decrease during expiration, and is quantified by the amplitude of this heart rate modulation. To obtain the RSA amplitude, we first estimate the instantaneous heart rate (inverse heartbeat RR interval) associated with each heartbeat within a breathing cycle, and from each instantaneous heart rate we subtract the average heart rate for this breathing cycle. We consider artifact-free segments of consecutive normal heartbeats with duration ≥300 sec. We plot the instantaneous heart rates (normalized to the mean) for each pair of consecutive breathing cycles within each artifact-free data segment, and we define the RSA amplitude as one half of the peak-through difference in the least-square-fit sinusoid line to the data points. This fit line represents the respiratory modulation of the heart rate (Fig. 1).
Figure 1. Respiratory sinus arrhythmia (RSA), classic form of cardio-respiratory coupling.
characterized by a periodic variation of the heart rate (HR) within each breathing cycle. The strength of the coupling is defined by the amplitude of the HR variation measured relative to the mean heart rate (RSA amplitude). Data points represent instantaneous HR, normalized to the mean within each breathing cycle, for a period of 200 sec over pairs of consecutive breathing cycles. RSA is highlighted by a sinusoidal least-square-fit line to the data points. Data are recorded from a healthy subject during deep sleep.
Cardio-respiratory phase synchronization (CRPS)
Phase-synchronization analysis was recently developed to identify interrelations between the output signals of coupled nonlinear oscillatory systems even when their output signals are not cross-correlated [3, 4]. Thus, phase-synchronization analysis can quantify the degree of coupling between nonlinear systems when other conventional methods can not. To study cardio-respiratory phase synchronization (Fig. 2), we developed an automated algorithm based on cardio-respiratory synchrograms [5].
Figure 2. Coexisting forms of cardio-respiratory coupling: RSA and phase synchronization (CRPS) represent different and independent forms of coupling.
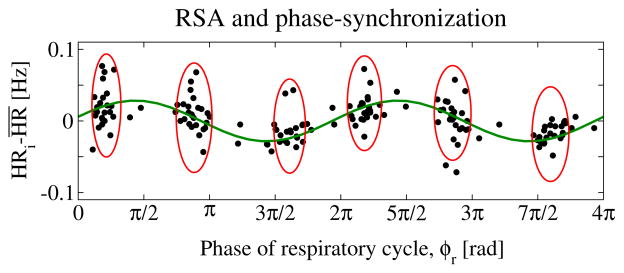
While RSA leads to periodic modulation of the heart rate within each breathing cycle (sinusoidal line), CRPS leads to clustering of heartbeats (ovals) at certain phases ϕr of the breathing cycle. Shown are consecutive heartbeats over a period of 200 sec. The x-axis indicates the phases ϕr of the breathing cycle where heartbeats occur. Data are selected from the same healthy subject during the same deep sleep episode as in Fig. 1, but represent a different segment of the episode where both RSA and CRPS coexist.
Time delay stability analysis (TDS)
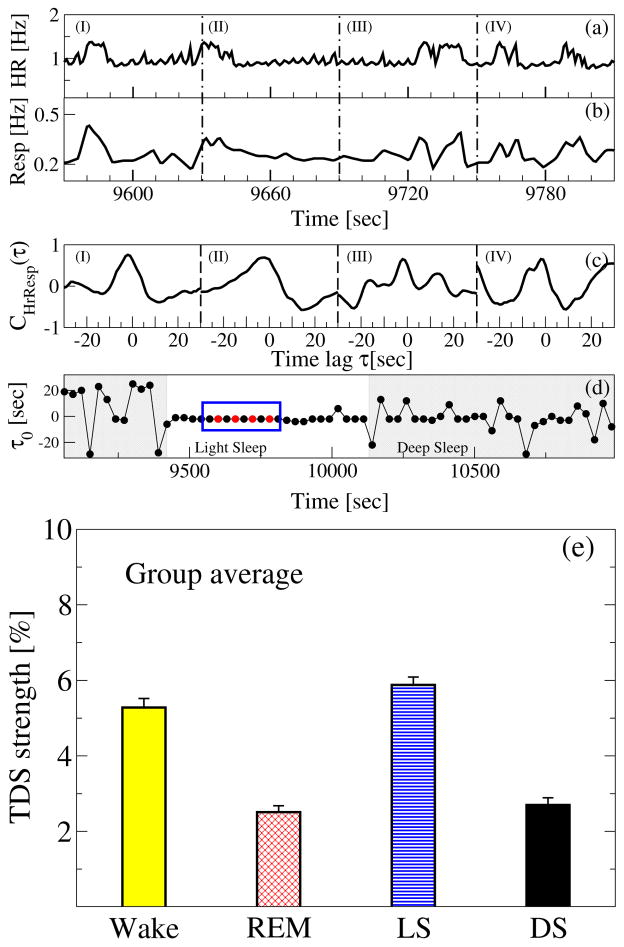
Integrated physiologic systems are coupled by feedback and/or feed forward loops with a broad range of time delays. To probe physiologic coupling we propose an approach based on the concept of time delay stability [6, 7]: in the presence of strong stable interactions between two systems, transient modulations (e.g., bursts) in the output signal of one system lead to corresponding changes that occur with a stable time lag in the output signal of another coupled system. Long periods of constant time delay indicate strong TDS coupling. We apply the TDS method to probe cardio-respiratory interaction, considering the time-delay interrelation between bursts in the heart rate and in the respiratory rate (Fig. 7).
Figure 7. Third form of cardio-respiratory coupling: time delay stability (TDS).
Segments of (a) heart rate (HR) and (b) respiratory rate (Resp) in 60 sec time windows (I), (II), (III) and (IV). Synchronous bursts in HR and Resp (signals normalized to zero mean and unit standard deviation) lead to pronounced cross-correlation (c) within each time window in (a) and (b), and stable time delay characterized by segments of constant τ0 as shown in (d) — four red dots highlighted by a blue box in panel (d) represents the time delay for the 4 time windows. Note the transition from strongly fluctuating behavior in τ0 to a stable time delay regime at the transition from deep sleep to light sleep at around 9400 sec (shaded area) in panel (d). The TDS analysis is performed on overlapping moving windows with a step of 30 sec. Long periods of constant τ0 indicate strong TDS coupling. (e) Note the pronounced stratification pattern in TDS: stronger coupling during wake and light sleep, and weaker during REM and deep sleep, which is very different from the stratification pattern of RSA and CRPS across sleep stages in Fig. 5.
4. Results and conclusion
We uncover that three distinct forms of coupling mediate cardio-respiratory interaction. In addition to the classic form of cardio-respiratory coupling, Respiratory sinus arrhythmia (RSA), we identified two other forms of nonlinear interaction, Cardio-respiratory phase synchronization (CRPS) and Time-delay stability (TDS). While RSA is a measure of amplitude modulation of the heart rate during the breathing cycle, CRPS and TDS characterize the temporal coordination between the cardiac and respiratory system. Specifically, the CRPS reflects the degree of clustering of heartbeats at specific relative phases within each breathing cycle (despite continuous fluctuations in heart rate and in breathing intervals), and the TDS quantifies the stability of the time-delay with which bursts in the activity in one system are consistently followed by corresponding bursts in the other system.
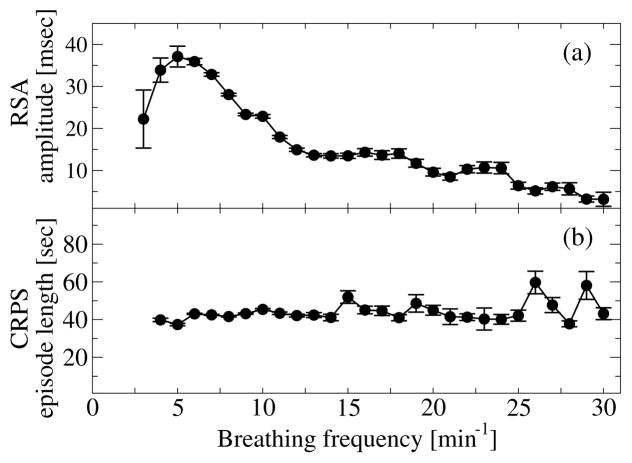
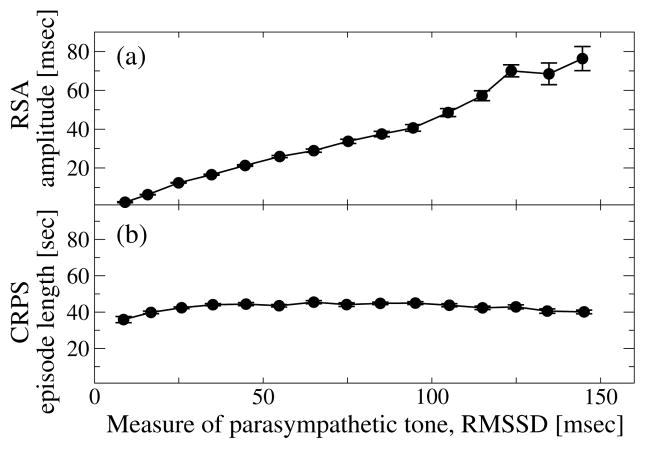
Our analyses demonstrate that RSA, CRPS and TDS are three distinct forms of cardio-respiratory coupling that correspond to different mechanisms of neural control, as they exhibit different dependences on key physiologic parameters such as breathing frequency (Fig. 3), level of parasympathetic tone (Fig. 4); and markedly different stratification patterns with transitions across sleep stages where the neuroautomic control is different (Figs. 5 and Fig. 7).
Figure 3. RSA and phase synchronization (CRPS) are distinct forms of cardio-respiratory coupling and their strength depend differently on breathing frequency.
(a) RSA nonlinearly increases (> 250%) with decreasing breathing frequency, and drops abruptly for very low breathing frequencies (Spearman’s rank order correlation: ρ = −0.42, p < 10−3). (b) In contrast to RSA, the strength of CRPS (length of CRPS episodes) is independent of the breathing frequency (Spearman ρ ≈ 0, p < 10−3). Error bars represent the standard error. Data are averaged over all subjects during the entire sleep period.
Figure 4. RSA and CRPS involve different mechanisms of cardio-respiratory coupling and respond differently to changes in autonomic parasympathetic control.
(a) RSA strongly increases with increasing RMSSD of heartbeat RR intervals, a measure of parasympathetic tone (Spearman ρ = 0.69, p < 10−3). (b) In contrast to RSA, the strength of CRPS does not significantly change with increasing RMSSD (Spearman ρ ≈ 0, p < 10−3). Error bars represent the standard error. Data are averaged over all subjects during the entire sleep period as in Fig. 3.
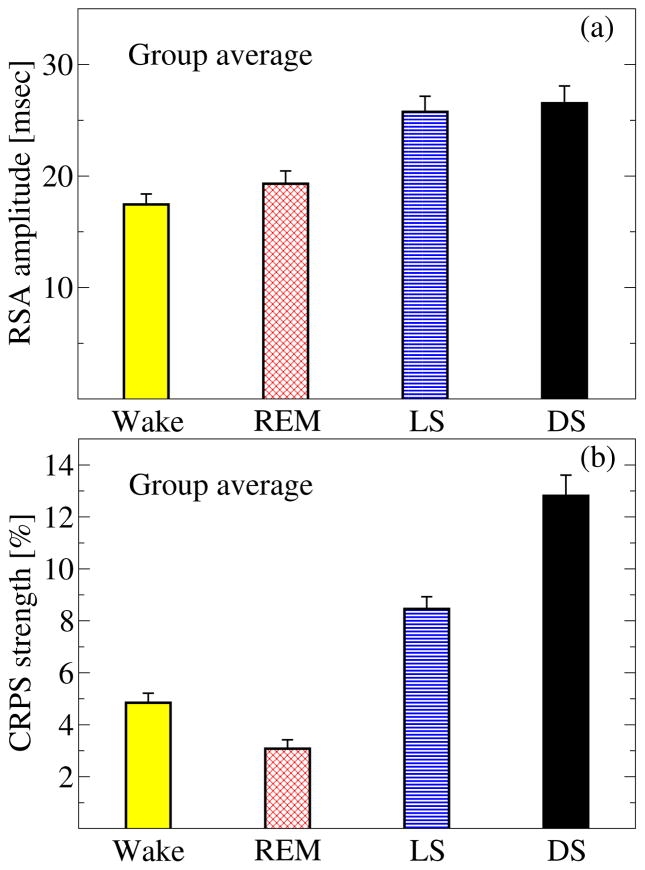
Figure 5. Phase transitions in the strength of two different forms of cardio-respiratory coupling across sleep stages.
(a) RSA and (b) CRPS exhibit very different stratification patterns across sleep stages, indicating (i) that each form of cardio-respiratory coupling undergoes a complex re-organization with sleep-stage transitions; and (ii) that RSA and CRPS are two independent forms of coupling, the streghten of which change differently during different sleep stages. Columns in each panel represent the group average of all subjects; error bars correspond to the standard error. CRPS is estimated as the percent of data segments in the entire recording for a given sleep stage where heart rate and respiratory rate are phase-synchronized.
Further, our empirical observations indicate that RSA, CRPS and TDS are three independent forms of cardio-respiratory interactions, since they exhibit different functional response to changes in key physiologic parameters, are characterized by different strength during the same sleep stage, and undergo different relative change in their strength across sleep stages (Figs. 5, 6 and 7). Moreover, RSA, CRPS and TDS act at different time scales, reflecting different temporal aspects of the communication between the cardiac and respiratory system (Fig. 8).
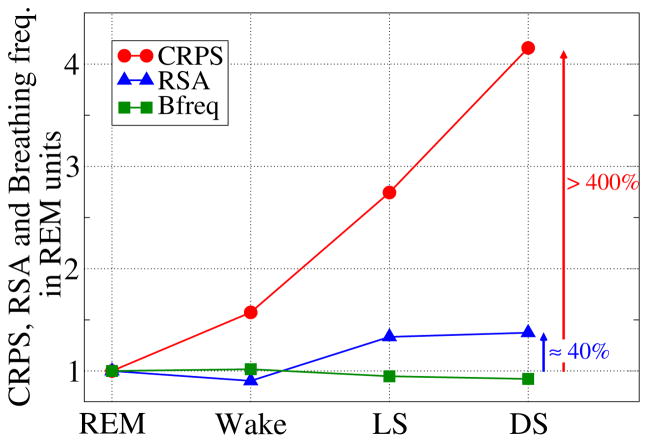
Figure 6. Change in the strength of CRPS, RSA and breathing frequency (Bfreq) across sleep stages.
Group average values for each sleep stage are normalized with respect to REM sleep. Notably, the sensitivity of CRPS to sleep-stage transitions is by a factor of 10 higher than RSA, indicating that sleep regulation and related changes in breathing frequency across sleep stages affect these two forms of cardio-respiratory coupling very differently.
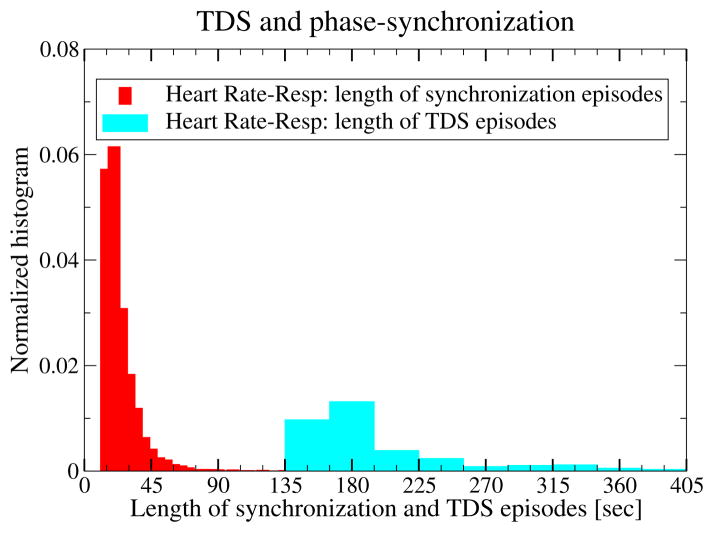
Figure 8. Coexisting forms of cardio-respiratory coupling, CRPS and TDS, function at different time scales.
Probability distributions of the length of CRPS epochs (red) and of TDS epochs (light-blue) indicate significant difference in the average epoch length. Thus, different forms of coupling acting on very different time scales are captured by these two measures. Both measures, CRPS and TDS, reveal a transient nature of the cardio-respiratory coupling, with “on” and “off” periods even within a single sleep stage. Results are obtained from all healthy subjects during the entire sleep period.
We find that these three distinct and independent forms of cardio-respiratory coupling are of transient nature, with nonlinear temporal organization of intermittent “on” and “off” periods, even during the same episode of any given physiologic state (sleep stage), and that these coupling forms can simultaneously coexist (Figs. 2 and 8).
Of relevance to the new field of network physiology [6, 7], our findings are a first demonstration that integrated physiological organ systems can communicate through multiple coexisting forms of interaction.
Acknowledgments
Support from NIH (Grant 1R01-HL098437), ONR (Grant 000141010078), BSF (Grant 2012219), EC-FP7 Marie Curie Fellowship (IIF 628159), NSF China (61271082, 61201029), NSF Jiangsu Province (BK2011759).
References
- 1.Schumann AY, Bartsch RP, Penzel T, Ivanov PCh, Kantelhardt JW. Aging effects on cardiac and respiratory dynamics in healthy subjects across sleep stages. Sleep. 2010;33:943–955. doi: 10.1093/sleep/33.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelone A, Coulter NA. Respiratory sinus arrhythmia: a frequency dependent phenomenon. J Appl Physiol. 1964;19:479–482. doi: 10.1152/jappl.1964.19.3.479. [DOI] [PubMed] [Google Scholar]
- 3.Pikovsky AS, Rosenblum MG, Kurths J. Synchronization: A Universal Concept in Nonlinear Sciences. Cambridge University Press; Cambridge: 2001. [Google Scholar]
- 4.Xu L, Chen Z, Hu K, Stanley HE, Ivanov PCh. Spurious detection of phase synchronization in coupled nonlinear oscillators. Phys Rev E. 2006;73:065201. doi: 10.1103/PhysRevE.73.065201. [DOI] [PubMed] [Google Scholar]
- 5.Bartsch R, Kantelhardt JW, Penzel T, Havlin S. Experimental evidence for phase synchronization transitions in the human cardiorespiratory system. Phys Rev Lett. 2007;98:054102. doi: 10.1103/PhysRevLett.98.054102. [DOI] [PubMed] [Google Scholar]
- 6.Bashan A, Bartsch RP, Kantelhardt JW, Havlin S, Ivanov PCh. Network physiology reveals relations between network topology and physiological function. Nat Commun. 2012;3:702. doi: 10.1038/ncomms1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivanov PCh, Bartsch RP. Network Physiology: Mapping Interactions Between Networks of Physiologic Networks. In: D’Agostino G, Scala A, editors. Networks of Networks: The Last Frontier of Complexity. Springer International Publishing; 2014. pp. 203–222. [Google Scholar]