Abstract
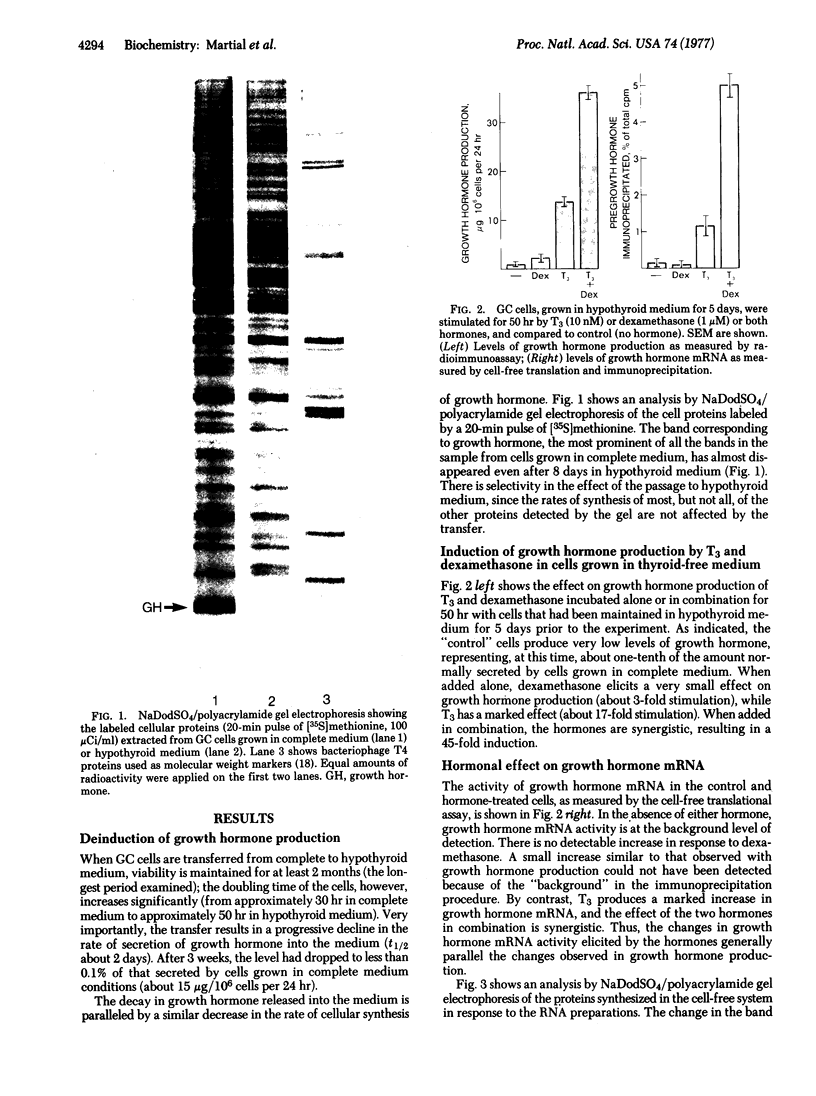
Cultured rat pituitary cells (GC) respond to thyroid and glucocorticoid hormones by increases in growth hormone production and growth hormone mRNA. When these cells are transferred from medium containing normal animal serum (with 1.8 mug of thyroxine per dl) to a medium containing serum from a thyroidectomized calf, "hypothyroid medium" (with no detectable thyroid hormone), growth hormone production decreases markedly. In cells maintained for 5 days in hypothyroid medium, triiodothyronine induces within 50 hr a 17-fold increase in growth hormone production whereas glucocorticoids, during the same time, produce a negligible (3-fold or less) stimulation. In combination, the two hormones promote a 45-fold stimulation. In all instances the changes in growth hormone production are paralleled by changes in the levels of growth hormone mRNA as measured by cell-free translation. The transfer to hypothyroid medium and the hormonal induction do not affect the relative activities of other mRNAs whose products are detectable on polyacrylamide gels. These studies indicate that thyroid hormone can be an activator of the expression of the growth hormone gene. The results also show that triiodothyronine controls the magnitude of the effect of glucocorticoids on growth hormone mRNA, and provide a model for "permissive" triiodothyronine action. The synergistic effect of these two classes of hormone suggests that they increase levels of growth hormone mRNA by different mechanisms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brookeman V. A., Williams C. M. Evaluation of the resin strip technique for determining serum T3 binding capacity and serum thyroxine. J Nucl Med. 1971 Feb;12(2):55–60. [PubMed] [Google Scholar]
- Chopra I. J., Ho R. S., Lam R. An improved radioimmunoassay of triiodothyronine in serum: its application to clinical and physiological studies. J Lab Clin Med. 1972 Nov;80(5):729–739. [PubMed] [Google Scholar]
- Kurtz D. T., Sippel A. E., Feigelson P. Effect of thyroid hormones on the level of the hepatic mRNA for alpha2u globulin. Biochemistry. 1976 Mar 9;15(5):1031–1036. doi: 10.1021/bi00650a013. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Higgins S. J., Tomkins G. M. Steroid-induced nuclear binding of glucocorticoid receptors in intact hepatoma cells. J Mol Biol. 1973 Sep 25;79(3):539–554. doi: 10.1016/0022-2836(73)90405-1. [DOI] [PubMed] [Google Scholar]
- Roy A. K., Schiop M. J., Dowbenko D. J. The role of thyroxine in the regulation of translatable messenger RNA for alpha2u globulin in rat liver. FEBS Lett. 1976 May 1;64(2):396–399. doi: 10.1016/0014-5793(76)80335-3. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Cintron R. Thyroid hormone action: a cell-culture system responsive to physiological concentrations of thyroid hormones. Science. 1973 Sep 28;181(4106):1253–1256. doi: 10.1126/science.181.4106.1253. [DOI] [PubMed] [Google Scholar]
- Schalch D. S., Reichlin S. Plasma growth hormone concentration in the rat determined by radioimmunoassay: influence of sex, pregnancy, lactation, anesthesia, hypophysectomy and extrasellar pituitary transplants. Endocrinology. 1966 Aug;79(2):275–280. doi: 10.1210/endo-79-2-275. [DOI] [PubMed] [Google Scholar]
- Schutz G., Beato M., Feigelson P. Messenger RNA for hepatic tryptophan oxygenase: its partial purification, its translation in a heterologous cell-free system, and its control by glucocorticoid hormones. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1218–1221. doi: 10.1073/pnas.70.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler B. J., MacLeod K. M., Ring J., Baxter J. D. Thyroid hormone receptors. Binding characteristics and lack of hormonal dependency for nuclear localization. J Biol Chem. 1975 Jun 10;250(11):4113–4119. [PubMed] [Google Scholar]