Abstract
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance occurring first time during pregnancy. Its prevalence is simultaneously increasing with the global rise of diabesity. GDM commonly develops, when maternal glucose metabolism is unable to compensate for the progressive development of insulin resistance, arising primarily from the consistently rising diabetogenic placental hormones. It classically develops during the second or third trimester. Theoretically, insulin sensitizers should have been the ideal agent in its treatment, given the insulin resistance, the major culprit in its pathogenesis. Fortunately, majority of women can be treated satisfactorily with lifestyle modification, and approximately 20% requires more intensive treatment. For several decades, insulin has been the most reliable treatment strategy and the gold standard in GDM. Metformin is effective insulin sensitizing agent and an established first line drug in type 2 diabetes currently. As it crosses the placenta, a safety issue remains an obstacle and, therefore, metformin is currently not recommended in the treatment of GDM. Nevertheless, given the emerging clinically equivalent safety and efficacy data of metformin compared to insulin, it appears that it may perhaps open a rather new door in managing GDM. The aim of this review is to critically analyze, the safety and efficacy data of metformin regarding its use in GDM and pregnant mothers with polycystic ovarian disease, which has emerged in past decades.
Keywords: Gestational diabetes, maternal outcome, metformin, neonatal outcome
INTRODUCTION
The incidence of gestational diabetes mellitus (GDM) is rising, partly due to surreptitious increase in diabesity epidemic. Currently, 1–14% of all pregnancies are complicated by GDM, depending upon the population studied and the diagnostic tests employed to define it.[1] It is increasingly clear now that GDM is associated with several maternal and neonatal adverse outcomes. GDM can cause increased preeclampsia, higher cesarean section rate and development of type 2 diabetes (T2DM) after pregnancy in mothers. It can cause higher neonatal death, stillbirth, congenital defects, macrosomia, shoulder dystocia and hypoglycemia in neonates as well. The Australian Carbohydrate Intolerance Study in Pregnant Women, was a randomized controlled trial (n = 1000), which convincingly suggested, that the treatment of GDM can significantly reduce serious perinatal morbidity, and can increase quality of life of pregnant women.[2] This outcome was further supported by yet another randomized controlled trial (n = 958), which also suggested that treating even milder GDM, can improve several maternal and neonatal outcomes.[3]
Currently, the mainstay of GDM management continues to be Maternal Nutritional Therapy and human insulin, a US FDA category B drug. Some of newer insulin analogs, like insulin lispro, aspart and detemir also have been upgraded to FDA category B during last 5 years; however, their practical implications and real benefits, remains to be established. Although, insulin therapy has been the gold standard, and universally accepted mode of treatment over several decade, its short-term use during GDM, remains to be labor-intensive, especially in the context to developing countries, where limited resources, poor logistics and poor literacy rate is still a hard core reality. Moreover, needle phobia, multiple daily injections, potential for hypoglycemia, weight gain and psycho-social stigma, including gender discrimination, perhaps, makes injectable therapy, somehow cumbersome for many pregnant patients.[4]
It is worth to mention that metformin, exerts its clinical effect both by increasing insulin sensitivity and by reducing hepatic glucose production, without any purported risk of hypoglycemia and weight gain. It has also been associated with a significant cardiovascular (CV) risk reduction in obese T2DM, as evident from UKPDS trial. These characteristics taken together, have established metformin as an ideal first-line treatment of T2DM. Presumably, metformin would have been an attractive drug for use in GDM, had it not cross the placenta. However, as metformin cross placenta, concern of adverse effects on both mother and the fetus, theoretically exists and therefore has limited its potential to be used in GDM.
Interestingly, with the emerging efficacy and safety data with metformin in a recent decade, it appears that the isolated domain of insulin could perhaps be challenged. The relative ease of administration, low cost, better acceptance among patients, better follow up, and ease with the treatment, can make this oral agent a preferable alternative both for patients and to the physicians. The ease with the use of this oral drug can also be stretched to the remotest areas with poor literacy, and where facilities as well as storage of insulin may pose hindrances in the treatment of GDM. However, because of the paucity of adequate data, use of these drugs in GDM is currently restricted only to USA, although it is increasingly being used in Europe and South Africa off late. It should also be noted that metformin has been historically used during 1970s, in South Africa.[5]
This review will critically analyze, the available efficacy and safety data of metformin in GDM including its head-to-head studies with insulin and glibenclamide. The review will also discuss its safety and efficacy in pregnant polycystic ovary syndrome (PCOS) mothers.
METFORMIN IN GESTATIONAL DIABETES MELLITUS
Metformin is currently listed as a FDA category B drug for use during pregnancy, thereby suggesting that animal studies have failed to demonstrate any risk to the fetus, but there are no adequate and well-controlled studies in pregnant women.
Placental and breast milk transfer
Historically, Denno and Sadler, in an experimental animal study, suggested that phenformin were associated with embryo toxicity and metformin too caused decrease in yolk sac protein as well as delayed neural tube closure.[6] Since then, teratogenicity with metformin has been a subject of speculation, primarily because metformin crosses placenta. Nevertheless, a study by Brigg in humans, found no teratogenicity with metformin even when the doses exceeded as high as 600 mg/kg.[7] Moreover, a meta-analysis done by Gilbert and Koren involving 8 studies (n = 496) of metformin used during first trimester, found significantly lesser malformed babies in metformin exposed group (1.7%), compared to control (7.2%). This roughly corresponds to 57% lowering of malformed babies in metformin treated mothers, compared to control and could be hypothesized as “protective effect” of metformin.[8,9] It is biologically possible that by reversing insulin resistance, metformin may protect against malformations. Nonetheless, this protective effect still remains challenging, as it is difficult to clarify whether it has appeared subsequent to better glycemic control or direct actions of the drug itself.
However, as it crosses the placenta, its use in pregnancy has raised some concern. Some data suggest that the umbilical cord concentration of metformin could be at least half the maternal concentration and sometime it may exceed, nevertheless, infant exposure to metformin through breast milk, has been found to be pretty low. As obvious, pregnant state can alter the metabolism of any drugs and metformin clearance also increases during pregnancy, owing to enhanced renal elimination. Therefore, increase in dose of metformin may also be required by at least 20% during pregnancy, to maintain comparable therapeutic effect to non-pregnant state.[10]
Clinical data comparing metformin with insulin
Historically, the earliest clinical uses of metformin from South Africa, dates back to 1970s, where it had been used both in pre-existing diabetes as well as GDM. Interestingly, while the perinatal mortality was equal to those shifted to insulin and significantly lower than untreated GDM mothers, it was still higher than that observed in the general obstetric population.[11,12]
Randomized controlled trials
Only two small randomized trials, using metformin during GDM have been reported before the MIG (Metformin versus Insulin for the treatment of Gestational Diabetes) trial [2008], by Rowan et al. published. Both Hague et al. and Moore et al. showed favorable results of metformin in GDM and finds no difference in any maternal and neonatal outcome compared to insulin.[13,14] Interestingly, metformin dose of 500 mg twice daily was found to be equivalent to insulin, in achieving glycemic control in 84% of cases in this study by Moore et al.[14] Meanwhile, an observational study by Hellmuth et al., conducted in mixed population of GDM and pre-existing type 2 diabetes (PGDM), hinted at significantly higher pre-eclampsia (P < 0.001) and higher perinatal mortality (P < 0.02) in metformin treated mothers.[15] Another, observational retrospective study by Ekpebegh et al., conducted in PGDM showed lesser perinatal mortality (P = 0.003) in insulin treated mothers compared to glibenclamide and metformin.[16] Both these observational studies created a needle of suspicion in clinician minds. However, both these observational studies had severe limitations. There were mixed population of patients including PGDM and GDM, with no comparator control arm in the former and exclusive PGDM included in the latter studies.[15,16]
To date, the largest (n = 733), prospective randomized trial, evaluating the use of metformin (maximum doses 2000 mg) was, Metformin versus Insulin for the treatment of Gestational Diabetes (MIG) trial. MIG trial was primarily conducted, to rule out 33% increase in composite of perinatal complications of infants of women taking metformin. The primary outcome of this study was neonatal outcome, and the secondary outcomes being maternal outcome as well as neonatal body composition.[17] The results clearly suggested, no significant increase in composite measures of neonatal complications in metformin treated mother. Interestingly, severe hypoglycemic episodes occurred significantly less in infants of mother taking metformin (P = 0.008) although, both arms had similar rates of neonatal hypoglycemia. Moreover, study ended with a positive note that metformin treatment was more acceptable than insulin (76% vs. 27%, P < 0.001). However, the frequency of pre-term birth (before 37 gestational weeks), was higher in the metformin group compared to insulin (P = 0.04). It was observed that the increase in preterm birth was related to greater frequency of spontaneous causes, rather than iatrogenic, and it was not associated with a higher rate of any neonatal complications. This might also suggest that preterm birth could have happened either due to chance or to an unrecognized effect of metformin on labor process. Nonetheless, 46% of patients on metformin required supplemental insulin therapy, to achieve optimal control. Those who required supplemental insulin had significantly higher body mass index, history of GDM and higher fasting glucose at enrollment. Nonetheless, women taking metformin, who required insulin had lesser total insulin dose (median dose 42 unit), and gained less weight, compared to those taking insulin alone (median dose 50 unit).[17] Taken together, it is clearly evident that this landmark study hinted at similar clinical equivalency (safety and efficacy) of metformin compared to insulin.
Subsequently, several other RCTs, albeit smaller in number than MIG trial, were also conducted by Ijaas et al., Niromanesh et al., Hasan et al., Tertti et al., and Spaulonci et al. All of these RCTs, suggested a comparable clinical equivalency of metformin compared to insulin.[18,19,20,21,22] Additionally, significantly lesser maternal weight gain were observed in the metformin group in the study by Niromanesh et al. and Spaulonci et al. (P = 0.0002).[19,22] Macrosomia was significantly lesser in metformin treated mother, in the study by Hasan et al. (10.65% vs. 18.67% in the insulin group, P < 0.05).[20] Furthermore, neonatal hypoglycemia were significantly lesser in metformin treated group, in the study by Hasan et al. and Spaulonci et al. (P = 0.032) [Table 1].[20,22]
Table 1.
RCTs comparing metformin to insulin
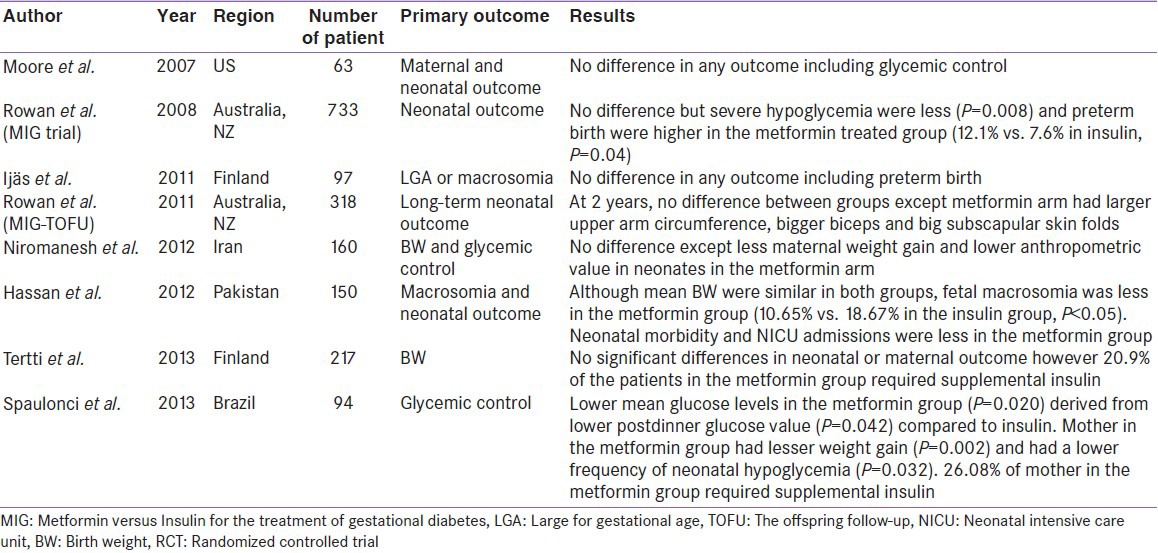
Observational studies
Similarly, several observational studies conducted in the past decade, also hinted at comparable equivalency with metformin.[23,24,25,26,27] Surprisingly, an observational study by Balani et al., suggested significantly higher preterm birth in insulin treated mothers (10% vs. 0% in the metformin; P = 0.01).[24] Furthermore, one study by Goh et al. hinted at significantly lesser preterm birth in metformin treated mothers (12.5% vs. 19.2% in insulin; P = 0.005).[26] It should be recalled here, that preterm birth were significantly higher in metformin treated arm in RCT conducted by Rowan et al. However, no increased risk of preterm birth seen in these two observational studies appeared reassuring for metformin. Perhaps, this may also suggest that higher preterm birth in the study by Rowan et al. could be a chance finding. Meanwhile, a retrospective observational study by Gandhi et al., comparing metformin plus lifestyle modification (LSM) to LSM alone, revealed significantly lower incidence of macrosomia (8.2% vs. 14.3%; OR = 0.56; 95% CI = 0.33–0.99), and lower birth weight (BW) (>90th centile 14.8% vs. 23.7%; OR = 0.56; 95% CI = 0.37–0.85) in metformin treated mothers.[27] Furthermore, majority of these observational studies, also hinted at less maternal weight gain and lesser neonatal hypoglycemia in metformin treated mothers compared to insulin. Intriguingly, two observational studies by Rai et al. and Goh et al., suggested better glucose control in the metformin arm compared to insulin [Table 2].[25,26]
Table 2.
Observational studies (prospective and retrospective) comparing metformin to insulin
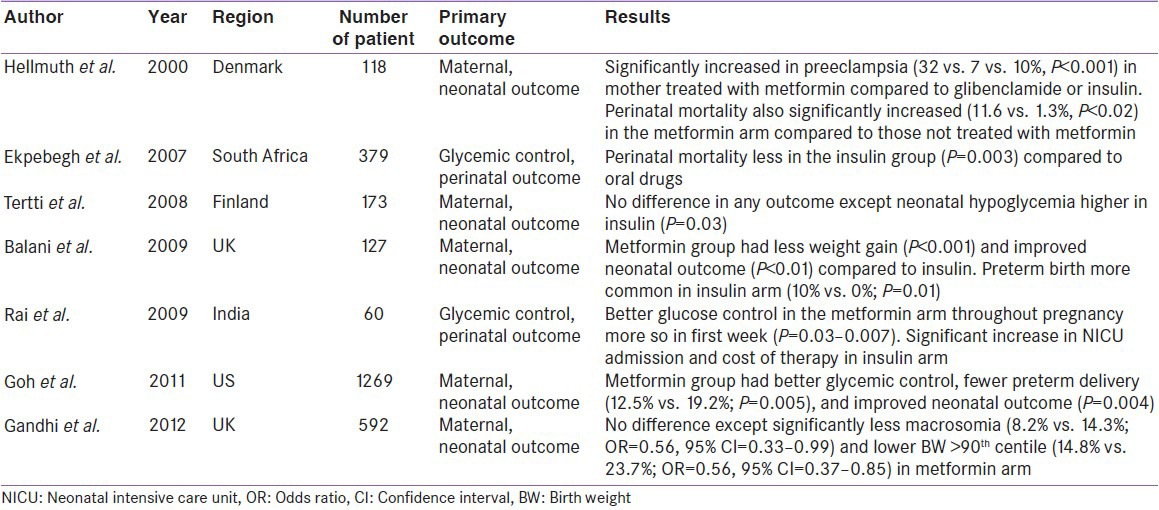
It is worthwhile to mention that these studies are limited with several methodological issues, which must be taken in to account while interpreting the results. Many of these studies were retrospective in nature, had a small number of participants, had varied definitions of outcomes, had extremely variable diagnostic criteria for GDM and many of them possess extreme heterogeneity.[28]
Meta-analysis
Two recent meta-analysis conducted by Guie et al. (involving five RCTs), and Su et al. (involving six RCTs) [2014], suggested a significantly better maternal outcome (less weight gain, lesser pregnancy induced hypertension and pre-eclampsia) in metformin treated mothers compared to insulin. Neonatal outcomes (significantly lesser neonatal hypoglycemia), were also better in metformin treated mothers compared to insulin.[29,30] However, these meta-analysis hinted at significantly higher preterm birth in the metformin arm, compared to insulin [Table 3]. Nonetheless, this only negative outcome appears to have driven, primarily from the inclusion of Rowan et al. (MIG trial) study in both meta-analyses.
Table 3.
Meta-analysis of RCT: Metformin versus insulin
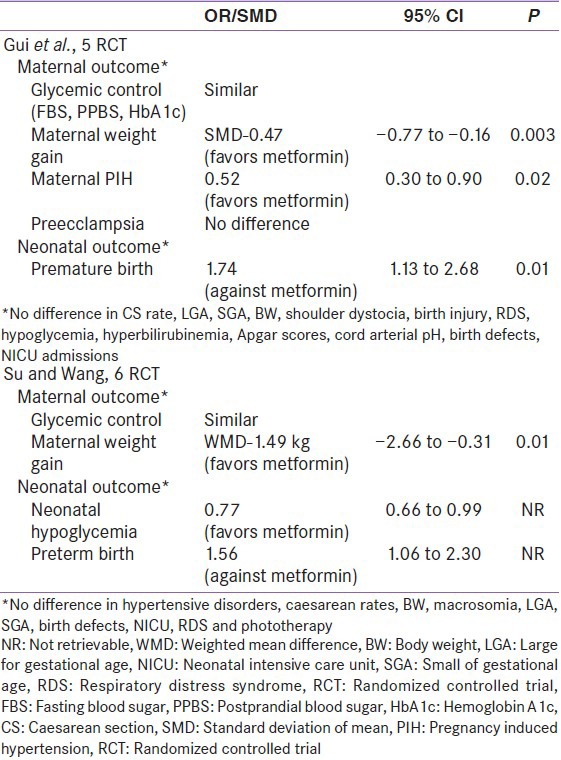
Few meta-analysis also exists in literature, where oral anti-diabetic drugs (OAD) together (metformin, glibenclamide and acarbose), have been compared to insulin. Nicholson et al., conducted a meta-analysis (involving 4 RCTs) and found no difference in maternal and neonatal outcome, although neonatal hypoglycemia were higher in insulin arm (8.1% vs. 3.3%; P = 0.008), when compared to OAD.[31] Dhulkotia et al., conducted a meta-analysis (involving 6 RCTs) and found no difference in any outcomes, except higher maternal hypoglycemia observed in insulin treated mothers.[32] Recently, Nicholson et al., conducted another meta-analysis (involving 8 RCTs) and suggested no difference in any of the maternal or neonatal outcomes [Table 4].[33]
Table 4.
Meta-analysis: OAD versus insulin
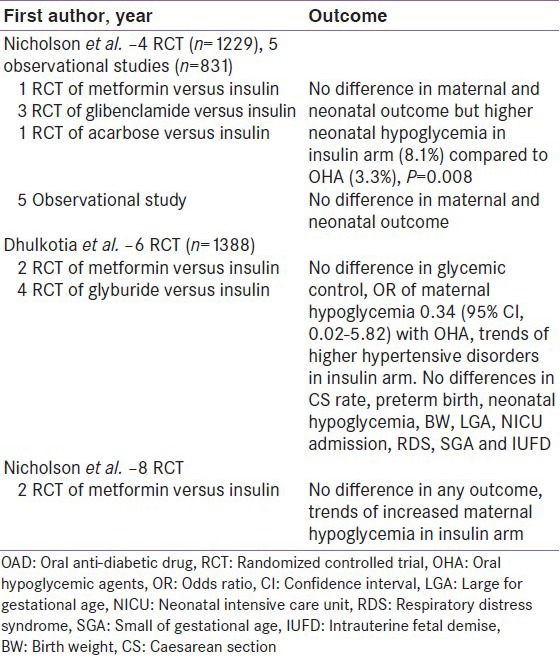
Clinical data comparing metformin to glibenclamide in gestational diabetes mellitus
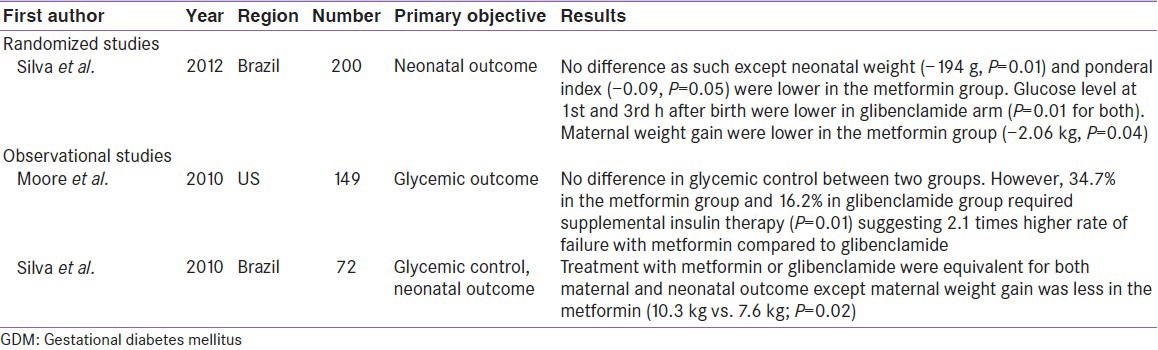
Only RCT conducted by Silva et al., compared glibenclamide to metformin (n = 200), and suggested no significant difference between two groups. However, significantly lesser maternal (−2.06 Kg, P = 0.04) and neonatal weight gain (−194 g, P = 0.01) observed in metformin treated mothers, compared to glibenclamide.[34] Interestingly, earlier observational studies by Moore et al. and Silva et al. also found same results.[35,36] Nevertheless, Moore et al. suggested significantly (2.1 times) higher failure rate of metformin in achieving optimal glycemic control compared to glibenclamide (P = 0.01) [Table 5].[35]
Table 5.
Metformin versus glibenclamide in GDM
Limitations of metformin
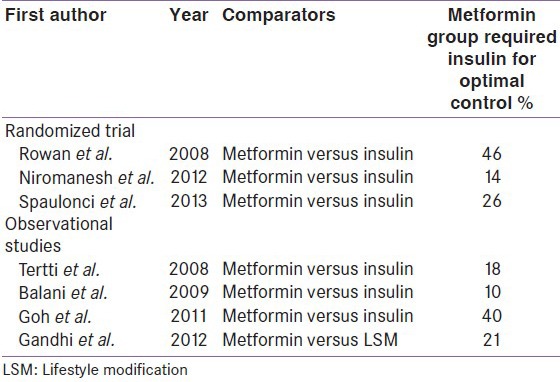
The biggest limitation with metformin is failure to achieve optimal glycemic control. As low as 10% (Balani et al.), to as high as 46% (Rowan et al.) required supplementary insulin [Table 6]. Moreover, the supplemental insulin requirement with metformin appears to be 11.5 fold higher (46% in Rowan et al. study), compared to glibenclamide (4% in Langer et al. study). Theoretically, this time lag until the start of insulin may appear hazardous for the fetus with a period of unintended hyperglycemia and, therefore, glibenclamide may be considered ahead of metformin. However, the supplementation of insulin in the metformin group did not impact the reduction in the incidence of the primary or secondary outcomes compared with insulin alone. Furthermore, these differences in supplemental insulin requirement could also be attributable to several factors including the differences in the ethnicity, BMI, and baseline blood glucose across the trials.
Table 6.
Supplementary insulin requirement in metformin treated mothers
Follow-up data of metformin in offspring
Long-term effect of metformin on future maternal and neonatal outcomes is currently minimal; however, the 2 year follow-up study of MIG trial in offspring (MIG: TOFU), suggested no difference in central fat measures, total fat mass or percentage body fat. This study also hinted that, metformin group had larger upper arm circumferences, bigger biceps and larger sub-scapular skin folds, perhaps suggesting a favorable pattern of fat distribution with less visceral fat when compared to insulin treated mothers.[37] While, the results of the MIG-TOFU study are both encouraging and reassuring, the possible benefits in children and adolescents with in-utero exposure to metformin are intriguing.
Metformin in pregnant polycystic ovarian disease
Metformin is one of the most commonly prescribed drugs in polycystic ovarian disease (PCOD) to manage associated metabolic syndrome. It is also well known to facilitate conception in women with PCOD. Yet, it is unclear as to how long it should be continued once a PCOD patient gets pregnant.
Randomized controlled trials
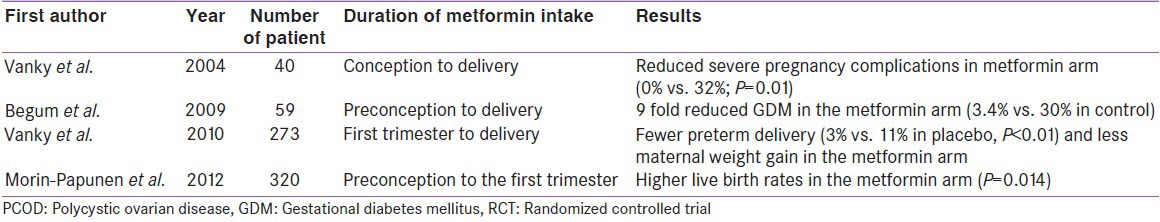
Vanky et al., conducted the first RCT of metformin in pregnant PCOD from conception till delivery. This study found significantly reduced severe pregnancy-related complications, compared to placebo (0% vs. 32%; P = 0.01).[38] Another RCT by Begum et al., using metformin from pre-conception till delivery, suggested a 9-fold lesser risk of developing GDM in PCOD mothers.[39] Subsequently, Vanky et al., conducted a large RCT (n = 273) applying metformin from first trimester till delivery, and found a significantly lesser preterm delivery in metformin arm (3% vs. 11% in placebo, P < 0.01), compared to placebo.[40] Most recent RCT (n = 320) by Morin Papunen et al., suggested higher live birth rate in metformin arm when it was continued from pre-conception till first trimester (P = 0.014) [Table 7].[41]
Table 7.
RCTs of metformin in pregnant PCOD
Observational studies
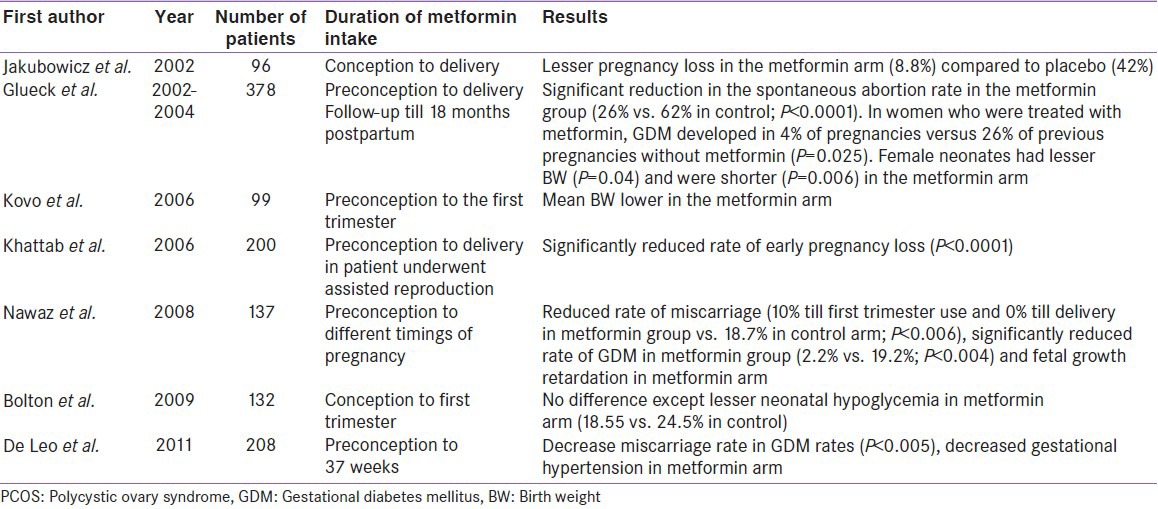
Several case control studies (prospective and retrospective) conducted so far, have largely supported the equivalent outcome as observed in RCTs. Some studies clearly hinted at few unique benefits in metformin treated mothers. This benefit includes significantly lesser early pregnancy loss as well as a significant reduction in GDM conversion rate, with metformin use in PCOD mothers [Table 8].[42,43,44,45,46,47,48,49]
Table 8.
Observational studies of metformin in pregnant PCOS
Taken together, the use of metformin during pregnancy in PCOS clearly appears to reduce the rates of early pregnancy loss and preterm labor. It also appeared to prevent fetal growth restriction. Most assuring to note that no teratogenic effects, intrauterine deaths or developmental delays have been reported with metformin use during PCOD pregnancy, even when used during the first trimester.[50]
Pragmatic use of metformin in gestational diabetes mellitus and pregnant polycystic ovary syndrome
Until now, both glibenclamide and metformin have not been recommended in the treatment of GDM, primarily owing to the fear of their potential teratogenicity and anticipated adverse fetal outcome. Although first landmark MIG trial, clearly suggested clinical equivalency of metformin compared to insulin, the international bodies are yet to recommend its use. The primary reason appears to be the remaining ounce of doubt regarding their safety.
World Health Organization (WHO), National Institute of Clinical Excellence UK (NICE 63) and Australian Obstetrics and Gynecological Society (ADS) currently recommends use of metformin and glibenclamide in the resource limited area where insulin administration has logistic issues; however, it is still recommended as a second line to insulin.[51,52,53] IDF[2009] guidelines recommends use of metformin in resource limited area when patients are least likely to comply with insulin.[54] Canadian guideline [2013], also recommends that metformin [level of evidence: Grade B, Level 2] may be used as alternative agents for glycemic control for women who are non-adherent to or who refuse insulin. However, use of oral agents in pregnancy is off-label and should be discussed with the patient [Grade D, Consensus].[55] The most recent, German Diabetes Association (DDG) and German Association of Gynecology and obstetrics (DGGG) guidelines [2014], does not recommend the use of any oral hypoglycemic agents during pregnancy.[56]
Nevertheless, the evidence in support of these recommendations is perhaps getting challenged. The evidence published thus far, supports metformin use in pregnancy with respect to the immediate pregnancy outcomes. As discussed earlier, several RCTs, meta-analysis and observational studies have largely supported the outcome with metformin. Ease of its use, acceptability by the patients, significantly less maternal weight gain and less maternal hypoglycemia, as evident from these studies, appears a lot encouraging. Moreover, two years follow up of offspring in MIG-TOFU, also reassures no anticipated harm and in fact suggests its beneficial effects in offspring. Positive outcomes in pregnant PCOD with metformin use even during the first trimester, almost rules out the remaining ounce of potential harm in terms of any potential teratogenic effect. While, no guidelines currently suggest as to how long should metformin be used during pregnancy in PCOS, the available data clearly suggests its benefit up to first trimester use. Nevertheless, duration of treatment with metformin in PCOS should be based on clinical judgment.
There are several parts in the world including India, where insulin may not be easily available, apart from various logistic and other psycho-socio-economic issues. These could be the situations, where metformin use can be desirable. At any point of time, adverse effects of untreated hyperglycemia will be worse than treating with metformin.
Future directions
With the global increase in the diabesity among reproductive age women, it is anticipated that the use of metformin will likely increase. As metformin reduces insulin resistance and has the potential to lower the risk of ensuing GDM in PCOS mothers, its role appears truly encouraging.
Future studies that will foster a large number of participants and adjust for all potentially confounding factors will likely shed some more light on these issues. Currently, some randomized trials are underway to examine the possible benefit of metformin in obese women with or without diabetes. The Metformin in Obese non-diabetic Pregnant women (MOP) a multicenter, randomized trial (n = 2178) in UK, is currently assessing the effects of metformin on BW percentile as a primary outcome.[57] Metformin in Women with type 2 diabetes in pregnancy (MiTY) is another randomized trial currently undergoing in Canada (n = 500), assessing the effects of metformin or placebo in addition to the usual regimen of insulin. Composite fetal outcome is the primary objective of MiTY trial.[58] The MiTy Kids Trial is a follow-up of MiTy Trial, which will determine whether treatment with metformin in pregnant type 2 diabetes will lead to a reduction in adiposity and insulin resistance in the offspring at 2 years of age.[59]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.American Diabetes Association. Standards of medical care in diabetes – 2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 3.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalra B, Gupta Y. Pragmatic use of metformin in pregnancy based on biopsychosocial model of health. Indian J Endocrinol Metab. 2013;17:1133–5. doi: 10.4103/2230-8210.122654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feig DS, Moses RG. Metformin therapy during pregnancy: Good for the goose and good for the gosling too? Diabetes Care. 2011;34:2329–30. doi: 10.2337/dc11-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denno KM, Sadler TW. Effects of the biguanide class of oral hypoglycemic agents on mouse embryogenesis. Teratology. 1994;49:260–6. doi: 10.1002/tera.1420490405. [DOI] [PubMed] [Google Scholar]
- 7.Briggs GG. Drug effects on the fetus and breast-fed infant. Clin Obstet Gynecol. 2002;45:6–21. doi: 10.1097/00003081-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert CJ, Koren G. Safety of metformin use during the first trimester. Can Fam Physician. 2005;51:1070–1073. [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metformin: A meta-analysis. Fertil Steril. 2006;86:658–63. doi: 10.1016/j.fertnstert.2006.02.098. [DOI] [PubMed] [Google Scholar]
- 10.Hughes RC, Gardiner SJ, Begg EJ, Zhang M. Effect of pregnancy on the pharmacokinetics of metformin. Diabet Med. 2006;23:323–6. doi: 10.1111/j.1464-5491.2005.01769.x. [DOI] [PubMed] [Google Scholar]
- 11.Coetzee EJ, Jackson WP. Metformin in management of pregnant insulin-independent diabetics. Diabetologia. 1979;16:241–5. doi: 10.1007/BF01221950. [DOI] [PubMed] [Google Scholar]
- 12.Coetzee EJ, Jackson WP. Pregnancy in established non-insulin-dependent diabetics. A five-and-a-half year study at Groote Schuur Hospital. S Afr Med J. 1980;58:795–802. [PubMed] [Google Scholar]
- 13.Hague WM, Davoren PM, Oliver J, Rowan J. Contraindications to use of metformin. Metformin may be useful in gestational diabetes. BMJ. 2003;326:762. [PubMed] [Google Scholar]
- 14.Moore LE, Briery CM, Clokey D, Martin RW, Williford NJ, Bofill JA, et al. Metformin and insulin in the management of gestational diabetes mellitus: Preliminary results of a comparison. J Reprod Med. 2007;52:1011–5. [PubMed] [Google Scholar]
- 15.Hellmuth E, Damm P, Mølsted-Pedersen L. Oral hypoglycaemic agents in 118 diabetic pregnancies. Diabet Med. 2000;17:507–11. doi: 10.1046/j.1464-5491.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- 16.Ekpebegh CO, Coetzee EJ, van der Merwe L, Levitt NS. A 10-year retrospective analysis of pregnancy outcome in pregestational type 2 diabetes: Comparison of insulin and oral glucose-lowering agents. Diabet Med. 2007;24:253–8. doi: 10.1111/j.1464-5491.2007.02053.x. [DOI] [PubMed] [Google Scholar]
- 17.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–15. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 18.Ijäs H, Vääräsmäki M, Morin-Papunen L, Keravuo R, Ebeling T, Saarela T, et al. Metformin should be considered in the treatment of gestational diabetes: A prospective randomised study. BJOG. 2011;118:880–5. doi: 10.1111/j.1471-0528.2010.02763.x. [DOI] [PubMed] [Google Scholar]
- 19.Niromanesh S, Alavi A, Sharbaf FR, Amjadi N, Moosavi S, Akbari S. Metformin compared with insulin in the management of gestational diabetes mellitus: A randomized clinical trial. Diabetes Res Clin Pract. 2012;98:422–9. doi: 10.1016/j.diabres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Hassan JA, Karim N, Sheikh Z. Metformin prevents macrosomia and neonatal morbidity in gestational diabetes. Pak J Med Sci. 2012;28:384–9. [Google Scholar]
- 21.Tertti K, Ekblad U, Koskinen P, Vahlberg T, Rönnemaa T. Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes Metab. 2013;15:246–51. doi: 10.1111/dom.12017. [DOI] [PubMed] [Google Scholar]
- 22.Spaulonci CP, Bernardes LS, Trindade TC, Zugaib M, Francisco RP. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol. 2013;209:34.e1–7. doi: 10.1016/j.ajog.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Tertti K, Ekblad U, Vahlberg T, Rönnemaa T. Comparison of metformin and insulin in the treatment of gestational diabetes: A retrospective, case-control study. Rev Diabet Stud. 2008;5:95–101. doi: 10.1900/RDS.2008.5.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balani J, Hyer SL, Rodin DA, Shehata H. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: A case-control study. Diabet Med. 2009;26:798–802. doi: 10.1111/j.1464-5491.2009.02780.x. [DOI] [PubMed] [Google Scholar]
- 25.Rai L, Meenakshi D, Kamath A. Metformin – A convenient alternative to insulin for Indian women with diabetes in pregnancy. Indian J Med Sci. 2009;63:491–7. [PubMed] [Google Scholar]
- 26.Goh JE, Sadler L, Rowan J. Metformin for gestational diabetes in routine clinical practice. Diabet Med. 2011;28:1082–7. doi: 10.1111/j.1464-5491.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi P, Bustani R, Madhuvrata P, Farrell T. Introduction of metformin for gestational diabetes mellitus in clinical practice: Has it had an impact? Eur J Obstet Gynecol Reprod Biol. 2012;160:147–50. doi: 10.1016/j.ejogrb.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Lautatzis ME, Goulis DG, Vrontakis M. Efficacy and safety of metformin during pregnancy in women with gestational diabetes mellitus or polycystic ovary syndrome: A systematic review. Metabolism. 2013;62:1522–34. doi: 10.1016/j.metabol.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: A meta-analysis. PLoS One. 2013;8:e64585. doi: 10.1371/journal.pone.0064585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su DF, Wang XY. Metformin vs insulin in the management of gestational diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;104:353–7. doi: 10.1016/j.diabres.2013.12.056. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson W, Bolen S, Witkop CT, Neale D, Wilson L, Bass E. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: A systematic review. Obstet Gynecol. 2009;113:193–205. doi: 10.1097/AOG.0b013e318190a459. [DOI] [PubMed] [Google Scholar]
- 32.Dhulkotia JS, Ola B, Fraser R, Farrell T. Oral hypoglycemic agents vs insulin in management of gestational diabetes: A systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:457.e1–9. doi: 10.1016/j.ajog.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson W, Baptiste-Roberts K. Oral hypoglycaemic agents during pregnancy: The evidence for effectiveness and safety. Best Pract Res Clin Obstet Gynaecol. 2011;25:51–63. doi: 10.1016/j.bpobgyn.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Silva JC, Fachin DR, Coral ML, Bertini AM. Perinatal impact of the use of metformin and glyburide for the treatment of gestational diabetes mellitus. J Perinat Med. 2012;40:225–8. doi: 10.1515/jpm-2011-0175. [DOI] [PubMed] [Google Scholar]
- 35.Moore LE, Clokey D, Rappaport VJ, Curet LB. Metformin compared with glyburide in gestational diabetes: A randomized controlled trial. Obstet Gynecol. 2010;115:55–9. doi: 10.1097/AOG.0b013e3181c52132. [DOI] [PubMed] [Google Scholar]
- 36.Silva JC, Pacheco C, Bizato J, de Souza BV, Ribeiro TE, Bertini AM. Metformin compared with glyburide for the management of gestational diabetes. Int J Gynaecol Obstet. 2010;111:37–40. doi: 10.1016/j.ijgo.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 37.Rowan JA, Rush EC, Obolonkin V, Battin M, Wouldes T, Hague WM. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): body composition at 2 years of age. Diabetes Care. 2011;34:2279–84. doi: 10.2337/dc11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: Results of a randomized study. Hum Reprod. 2004;19:1734–40. doi: 10.1093/humrep/deh347. [DOI] [PubMed] [Google Scholar]
- 39.Begum MR, Khanam NN, Quadir E, Ferdous J, Begum MS, Khan F, et al. Prevention of gestational diabetes mellitus by continuing metformin therapy throughout pregnancy in women with polycystic ovary syndrome. J Obstet Gynaecol Res. 2009;35:282–6. doi: 10.1111/j.1447-0756.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 40.Vanky E, Stridsklev S, Heimstad R, Romundstad P, Skogøy K, Kleggetveit O, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: A randomized, controlled multicenter study. J Clin Endocrinol Metab. 2010;95:E448–55. doi: 10.1210/jc.2010-0853. [DOI] [PubMed] [Google Scholar]
- 41.Morin-Papunen L, Rantala AS, Unkila-Kallio L, Tiitinen A, Hippeläinen M, Perheentupa A, et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): A multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab. 2012;97:1492–500. doi: 10.1210/jc.2011-3061. [DOI] [PubMed] [Google Scholar]
- 42.Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Roberts KA, Nestler JE. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:524–9. doi: 10.1210/jcem.87.2.8207. [DOI] [PubMed] [Google Scholar]
- 43.Glueck CJ, Wang P, Goldenberg N, Sieve-Smith L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod. 2002;17:2858–64. doi: 10.1093/humrep/17.11.2858. [DOI] [PubMed] [Google Scholar]
- 44.Glueck CJ, Goldenberg N, Pranikoff J, Loftspring M, Sieve L, Wang P. Height, weight, and motor-social development during the first 18 months of life in 126 infants born to 109 mothers with polycystic ovary syndrome who conceived on and continued metformin through pregnancy. Hum Reprod. 2004;19:1323–30. doi: 10.1093/humrep/deh263. [DOI] [PubMed] [Google Scholar]
- 45.Kovo M, Weissman A, Gur D, Levran D, Rotmensch S, Glezerman M. Neonatal outcome in polycystic ovarian syndrome patients treated with metformin during pregnancy. J Matern Fetal Neonatal Med. 2006;19:415–9. doi: 10.1080/14767050600682370. [DOI] [PubMed] [Google Scholar]
- 46.Khattab S, Mohsen IA, Foutouh IA, Ramadan A, Moaz M, Al-Inany H. Metformin reduces abortion in pregnant women with polycystic ovary syndrome. Gynecol Endocrinol. 2006;22:680–4. doi: 10.1080/09513590601010508. [DOI] [PubMed] [Google Scholar]
- 47.Nawaz FH, Khalid R, Naru T, Rizvi J. Does continuous use of metformin throughout pregnancy improve pregnancy outcomes in women with polycystic ovarian syndrome? J Obstet Gynaecol Res. 2008;34:832–7. doi: 10.1111/j.1447-0756.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 48.Bolton S, Cleary B, Walsh J, Dempsey E, Turner MJ. Continuation of metformin in the first trimester of women with polycystic ovarian syndrome is not associated with increased perinatal morbidity. Eur J Pediatr. 2009;168:203–6. doi: 10.1007/s00431-008-0737-7. [DOI] [PubMed] [Google Scholar]
- 49.De Leo V, Musacchio MC, Piomboni P, Di Sabatino A, Morgante G. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur J Obstet Gynecol Reprod Biol. 2011;157:63–6. doi: 10.1016/j.ejogrb.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 50.Glueck CJ, Goldenberg N, Pranikoff J, Khan Z, Padda J, Wang P. Effects of metformin-diet intervention before and throughout pregnancy on obstetric and neonatal outcomes in patients with polycystic ovary syndrome. Curr Med Res Opin. 2013;29:55–62. doi: 10.1185/03007995.2012.755121. [DOI] [PubMed] [Google Scholar]
- 51. [Last accessed on 2014 Sep 21]. Available from: http://www.whqlibdoc.who.int/publications/2006/924159084X_eng.pdf?ua = 1 .
- 52. [Last accessed on 2014 Sep 21]. Available from: http://www.nice.org.uk/guidance/cg63 .
- 53. [Last accessed on 2014 Sep 21]. Available from: http://www.adips.org/
- 54. [Last accessed on 2014 Sep 21]. Available from: http://www.idf.org/webdata/docs/Pregnancy_EN_RTP.pdf .
- 55. [Last accessed on 2014 Sep 21]. Available from: http://www.guidelines.diabetes.ca/Browse/Chapter 36 .
- 56.Kleinwechter H, Schäfer-Graf U, Bührer C, Hoesli I, Kainer F, Kautzky-Willer A, et al. Gestational diabetes mellitus (GDM) diagnosis, therapy and follow-up care: Practice Guideline of the German Diabetes Association (DDG) and the German Association for Gynaecologyand Obstetrics (DGGG) Exp Clin Endocrinol Diabetes. 2014;122:395–405. doi: 10.1055/s-0034-1366412. [DOI] [PubMed] [Google Scholar]
- 57.Metformin in Obese Non-diabetic Pregnant Women (MOP) [Last accessed on 2014 Sep 21]. Available from: http://www.clinicaltrials.gov/ct2/show/study/NCT01273584 .
- 58.Metformin in Women with Type 2 Diabetes in Pregnancy Trial (MiTy) [Last accessed on 2014 Sep 21]. Available from: http://www.clinicaltrials.gov/show/NCT01353391 .
- 59.MiTy Kids (Metformin in Women with Type 2 Diabetes in Pregnancy Kids Trial) [Last accessed on 2014 Sep 21]. Available from: http://www.clinicaltrials.gov/show/NCT01832181 .


