Abstract
Background:
Alteration in homeostasis of calcium, phosphate and parathyroid hormone (PTH) predispose to vascular calcification that increases the risk of cardiovascular morbidity and mortality. The data on this aspect are scarce in patients with sporadic idiopathic hypoparathyroidism (SIH).
Objective:
The aim was to assess the effect of altered calcium, phosphate and PTH homeostasis in patients with SIH on intima media thickness (IMT), a surrogate marker of increased vascular risk.
Methods:
In this case–control study, we measured carotid IMT (CIMT), aortic IMT (AIMT) and renal arteries IMT (RIMT) in 30 consecutive patients with SIH, and compared with healthy subjects. IMT was measured by ultrasound by a single operator blinded to subject's details.
Results:
CIMT, AIMT, RIMT values in patients with SIH were significantly more than healthy subjects (0.60 ± 0.08 mm vs. 0.52 ± 0.09 mm, P = 0.001; 0.73 ± 0.09 mm vs. 0.65 ± 0.10, P = 0.004; and 0.34 ± 0.04 mm vs. 0.30 ± 0.05, P = 0.003, respectively). Clinical or biochemical parameters did not correlate with CIMT, AIMT and RIMT in patients with SIH.
Conclusion:
The vascular risk is increased in patients with SIH as assessed by CIMT, AIMT, and RIMT.
Keywords: Aortic intima-media thickness, cardiovascular risk, carotid intima-media thickness, hypoparathyroidism, renal intima-media thickness
INTRODUCTION
Cardiovascular disease is now the leading cause of death worldwide.[1] Early disease detection continues to be the bedrock of most preventative strategies. Changes in calcium, phosphate and parathyroid hormone (PTH) homeostasis is a predisposing factor for vascular calcification and increases the risk of cardiovascular disease.[2,3] Plethora of literature is available for abnormal calcium homeostasis, relative hypoparathyroidism, increased vascular calcification and its correlation with increased cardiovascular events, but are mainly in relation to end-stage renal disease patients.[2,3,4,5,6,7,8] The same relationship in patients with sporadic idiopathic hypoparathyroidism (SIH) remains unexplored. An earlier pilot study by us had found an increase in carotid intima-media thickness (CIMT) (surrogate marker for increase cardiovascular risk) in patients with SIH.[9]
Since the publication of our previous study, there has been much renowned interest regarding calcium supplements and cardiovascular risk. European Prospective Investigation into Cancer and nutrition (EPIC) study reported increased risk of myocardial infarction with the use of calcium supplements.[10] Though, the relationship remains controversial, and is not universally agreed upon, there is still need of further studies to explore it.[11] The patients with SIH are on long-term calcium supplementations, understanding of this relationship in them may provide new insights in this field.
With this aim, we undertook this study evaluating patients with SIH with aortic IMT (AIMT) and renal IMT (RIMT) in addition to CIMT as surrogate markers of cardiovascular risk.
METHODS
Patient selection
The study comprised of 30 consecutive patients with SIH attending the endocrine clinic of a tertiary care hospital, from July 2011 to November 2013. Age and sex-matched equal number of healthy individuals were taken as control. Our study was approved by the institutional Ethics Committee. Written informed consent was taken from each subject.
The diagnosis of SIH was based on hypocalcemia and hyperphosphatemia associated with low or low-normal PTH level. Patients with a history of postoperative hypoparathyroidism or autoimmune polyendocrinopathy syndromes were excluded. Subjects with a history of smoking or any other chronic illness, including hypertension, diabetes, dyslipidemia, obesity, deranged renal function, any patient on antiepileptic drugs were excluded.
Office blood pressure was measured in sitting the posture after 15–20 min of rest in right arm twice and mean of two was taken for calculation. Blood samples were collected in fasting status prior to any medications between 800 and 900 h by venepuncture from the antecubital fossa (without tourniquet application) by technician. Blood samples for estimation of PTH were centrifuged immediately and processed, and stored at minus 20°C in cases of the anticipated delay in processing, till the time of analysis.
Laboratory methods
Serum PTH (reference range [RR], 15–65 pg/mL) was measured by chemiluminescence assay using commercially available kits (DiaSorin Inc., Stillwater, MN) and rest of biochemical parameters corrected serum calcium (RR, 8.6–10.2 mg/dL), inorganic phosphate (RR, 2.7–4.5 mg/dL), serum albumin (RR, 3.4–4.8 g/L), alkaline phosphatase (RR, 40–129 IU/L), and total cholesterol (RR, 150–200 mg/dL), triglyceride 50–200 mg/dL, high-density lipoprotein cholesterol (35–55 mg/dL), low-density lipoprotein cholesterol (30–100 mg/dL), were measured by auto analyzer (Roche diagnostics, Modular P 800).
Carotid, aortic, renal intima-media thickness measurement
Carotid intima-media thickness, AIMT and RIMT were measured by a single trained operator who was blinded to the clinical characteristics of the participants. The procedure was performed using Philips high definition imaging 11, system with a linear transducer of 3–12 MHz for all subjects.
Intima-media thickness is defined as the distance from the leading edge of first echogenic line to that of the second line. The first line represents the lumen-intima interface, and the second line represents the collagen-containing upper layer of the adventitia. The common carotid artery (CCA) was scanned on both sides with the participant lying supine, the head directed away from the side of interest, and the neck extended slightly. The transducer was manipulated so that the near and far walls of the CCA become parallel to the transducer footprint, and the lumen diameter is maximized in the longitudinal plane. Two measurements were obtained on each side at 0.5 cm intervals along the CCA moving proximally starting at 1.0 cm from the carotid bulb. The measurements were made on still images obtained during the sonographic evaluation. The CIMT was reported as the average IMT of right and left common carotid arteries [Figure 1a]. The carotid plaques were excluded while measuring CIMT. For AIMT, the abdominal aorta was scanned in transverse and longitudinally in the entire extent. Two measurements at 0.5 cm interval were obtained moving proximally [Figure 1b] and mean was calculated. IMT of renal arteries was measured just near their origin. The RIMT was reported as the average IMT of right and left renal arteries. The reproducibility of IMT measurement is reported from 0.89 to 0.97 (r value).
Figure 1.
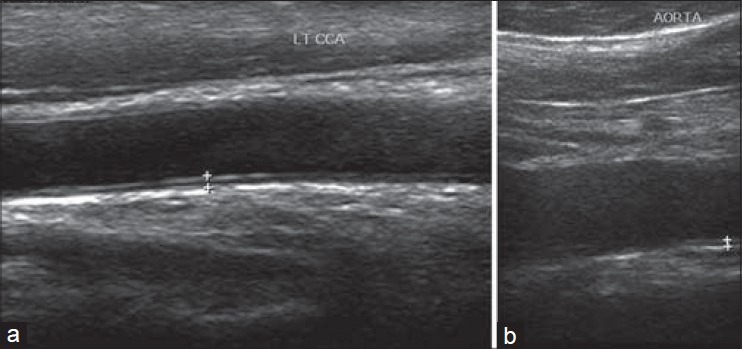
Longitudinal gray scale ultrasound images of carotid (a) and aorta (b) the method of measuring intima media thickening in the cases
Statistical analysis
Sample size
The sample size was calculated to detect a difference of 0.04 mm in the intima thickness with the standard deviation of 0.05. Twenty-five in each group were sufficient to detect this difference allowing an alpha error of 0.05. This number powers the study to 80%. Based upon the results obtained the minimum power of this study to detect a meaningful difference is 87% as the sample used is 30 in each group.
The statistical analysis was carried out using Statistical Package for the Social Sciences-16 (SPSS, Chicago, IL, USA). Normality of data was checked by Kolmogorov-Smirnov test. For normally distributed data means were compared using an independent t-test for two groups. For skewed data Mann–Whitney test was applied. Categorical variables were described as frequencies and proportions. Proportions were compared using Chi-square. Pearson's correlation was used to correlate clinical and biochemical variables with CIMT, AIMT and RIMT in ISH and control group. All statistical tests were two-sided and performed at a significance level of P < 0.05. The data are presented as mean ± standard deviation unless otherwise specified.
RESULTS
Baseline characteristic of study population
Thirty subjects (12 men) with SIH and an equal number of age and sex-matched healthy subjects were included in this study. The mean age of patients included in our study was 30.5 ± 5.8 years (range 20–40 years) whereas age of healthy controls was 31.3 ± 5.6 years (range 21–40 years). Baseline characteristic of two groups is shown in Table 1.
Table 1.
Baseline characteristic of the study participants
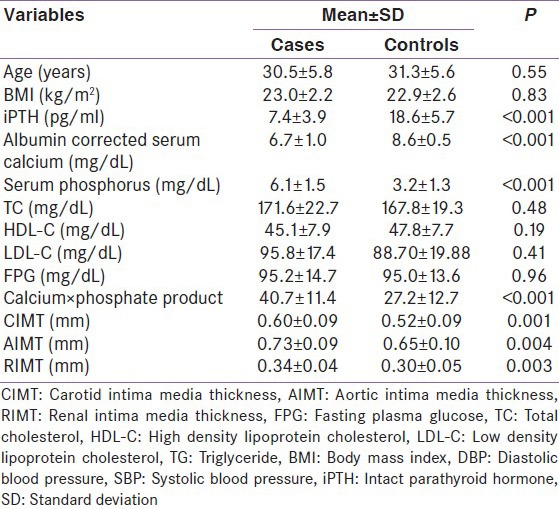
The mean total serum calcium, Intact PTH (iPTH) levels were significantly lower and value of serum phosphate levels significantly higher in patients with SIH than in controls [Table 1].
Carotid, aorta and renal intima-media thickness among study population
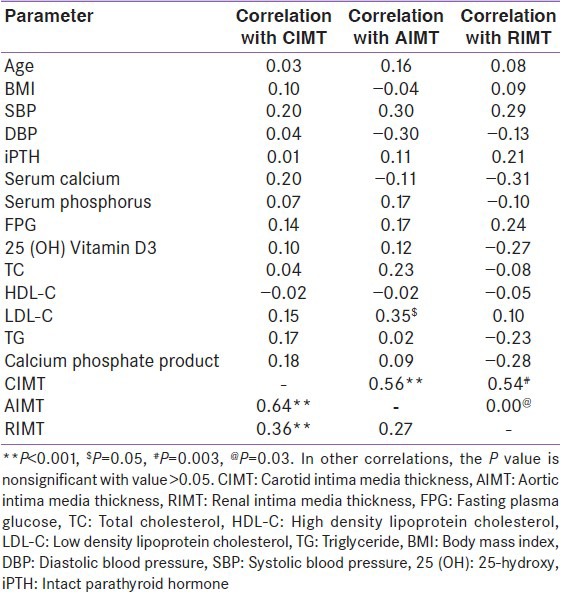
Carotid intima media thickness, AIMT and RIMT values were higher in patients with SIH than controls (CIMT-0.60 ± 0.08 mm vs. 0.52 ± 0.09, P = 0.001; AIMT-0.73 ± 0.09 vs. 0.65 ± 0.10, P = 0.004; RIMT-0.34 ± 0.04 vs. 0.30 + 0.05, P = 0.003) [Figure 2]. No significant correlation was observed between biochemical and IMT parameters [Table 2]. No significant correlation was observed with other cardiovascular risk factors [Table 2]. There was strong correlation of CIMT with RIMT and AIMT.
Figure 2.
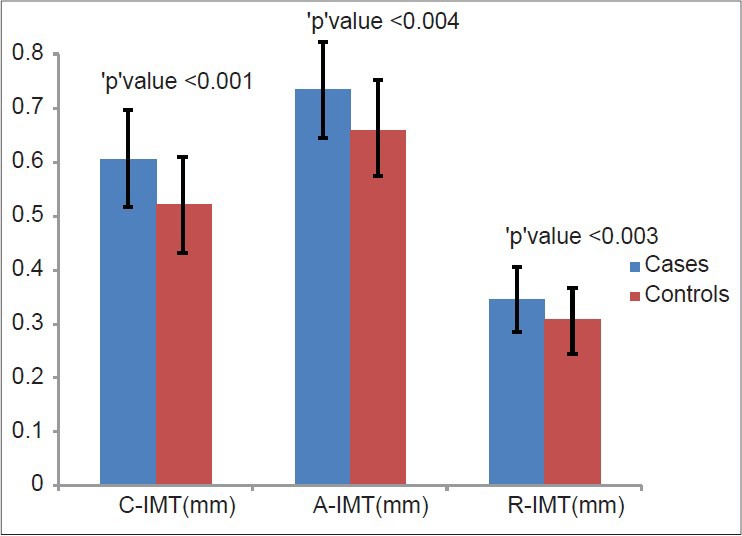
Distribution of mean carotid, aortic and renal intima-media thickness in the study participants
Table 2.
Correlations between intima media thickness measured at different sites with clinical, biochemical parameters and between them in cases
DISCUSSION
We found higher IMT, measured at carotid, aorta and renal arteries in patients with SIH compared to age and gender matched controls. Interesting, we did not found any correlation of biochemical parameters (serum calcium, phosphate, calcium-phosphate product and PTH) with any of the measured IMT.
There is no previous data for cardiovascular risk in patients with SIH, and it is difficult to put our findings in the context with others. The concept of relative hypoparathyroidism and increased cardiovascular risk has been previously explored in patients with renal failure.[12] In patients on dialysis, hyperphosphatemia is an independent risk factor for cardiovascular disease.[6] Contrarily, both high as well as low calcium and PTH levels have been associated with increased cardiovascular mortality.[2,12,13] However, the similar biochemical profile of high phosphate, low calcium level and low PTH levels did not account for increased cardiovascular risk in our study.
Another possible parameter that could account for this risk is calcium and vitamin D supplementation. The patients with SIH are long-term calcium and Vitamin D supplementation. In meta-analyses of three placebo-controlled trials, calcium and vitamin D increased the risk of myocardial infarction (relative risk 1.21 [95% confidence interval (CI) 1.01–1.44], P = 0.04), stroke (1.20 [1.00–1.43], P = 0.05), and the composite of myocardial infarction or stroke (1.16 [1.02–1.32], P = 0.02).[14] Thereafter EPIC-Heidelberg study reported data from 23,980 individuals aged 35–64 years and free of major cardiovascular disease events at recruitment. After an average follow-up time of 11 years, the authors observed that users of calcium supplements had a statistically significantly increased myocardial infarction risk (hazard ratio = 1.86; 95%CI: 1.17–2.96).[10] The study further concluded that increasing calcium intake from diet might not confer significant cardiovascular benefits, while calcium supplements, which might raise myocardial infarction risk. On the other side of it, the recent meta-analysis found that calcium supplementation with or without vitamin D increase coronary heart disease or all-cause mortality risk in elderly women. Five trials contributed coronary heart disease events with pooled relative RR of 1.02 (95% CI, 0.96–1.09; P = 0.51), which was not significant.[15] The hypothesis of calcium supplementation and increased cardiovascular risk is a debated one with little evidence existing for plausible biological mechanisms to link calcium supplement use with adverse cardiovascular outcomes.[16] More evidence will open the gates of better understanding.
Though, omission or decrease in calcium supplements may not be a viable option in patients with SIH, but with promising role of PTH in these patients, it may prove a suitable alternative,[17] in case strong evidence generated from future studies establishes the plausible mechanism between calcium supplementation and increased cardiovascular risk.
The strength of this study is that, it will help in expanding the horizon of understanding of altered mineral metabolism and its relationship with cardiovascular risk. SIH being a rare entity, this study gets weakened by the smaller number of recruited subjects. The utility of only IMT, in the absence of other biochemical or imaging markers of increased cardiovascular risk could be seen as another potential weakness of this study.
To conclude, this study conveys the message that IMT measurement at various sites is higher in patients with SIH. Hence, these patients should actually be followed up over a prolonged period whether this actually translates into increased cardiovascular events.
ACKNOWLEDGMENT
We acknowledge the help of Mr. Rakesh Mohinder and Dr Anand Srinivasan for statistical calculation of the manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Reddy KS, Hunter DJ. Noncommunicable diseases. N Engl J Med. 2013;369:2563. doi: 10.1056/NEJMc1313604. [DOI] [PubMed] [Google Scholar]
- 2.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–30. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Staude H, Jeske S, Schmitz K, Warncke G, Fischer DC. Cardiovascular risk and mineral bone disorder in patients with chronic kidney disease. Kidney Blood Press Res. 2013;37:68–83. doi: 10.1159/000343402. [DOI] [PubMed] [Google Scholar]
- 4.Kimata N, Albert JM, Akiba T, Yamazaki S, Kawaguchi T, Fukuhara S, et al. Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: The Japan dialysis outcomes and practice patterns study. Hemodial Int. 2007;11:340–8. doi: 10.1111/j.1542-4758.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 5.Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant. 2009;24:1506–23. doi: 10.1093/ndt/gfn613. [DOI] [PubMed] [Google Scholar]
- 6.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA. 2011;305:1119–27. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 7.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1,3, and 4 study. J Am Soc Nephrol. 2005;16:1788–93. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 8.Abe M, Okada K, Soma M. Mineral metabolic abnormalities and mortality in dialysis patients. Nutrients. 2013;5:1002–23. doi: 10.3390/nu5031002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta Y, Bhadada SK, Shah VN, Upreti V, Bhansali A, Jain S, et al. Carotid intima media thickness in patients with sporadic idiopathic hypoparathyroidism: A pilot study. Endocr J. 2012;59:555–9. doi: 10.1507/endocrj.ej11-0400. [DOI] [PubMed] [Google Scholar]
- 10.Li K, Kaaks R, Linseisen J, Rohrmann S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg) Heart. 2012;98:920–5. doi: 10.1136/heartjnl-2011-301345. [DOI] [PubMed] [Google Scholar]
- 11.Downing L, Islam MA. Influence of calcium supplements on the occurrence of cardiovascular events. Am J Health Syst Pharm. 2013;70:1132–9. doi: 10.2146/ajhp120421. [DOI] [PubMed] [Google Scholar]
- 12.Guh JY, Chen HC, Chuang HY, Huang SC, Chien LC, Lai YH. Risk factors and risk for mortality of mild hypoparathyroidism in hemodialysis patients. Am J Kidney Dis. 2002;39:1245–54. doi: 10.1053/ajkd.2002.33398. [DOI] [PubMed] [Google Scholar]
- 13.Foley RN, Parfrey PS, Harnett JD, Kent GM, Hu L, O’Dea R, et al. Hypocalcemia, morbidity, and mortality in end-stage renal disease. Am J Nephrol. 1996;16:386–93. doi: 10.1159/000169030. [DOI] [PubMed] [Google Scholar]
- 14.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: Reanalysis of the women's health initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis JR, Radavelli-Bagatini S, Rejnmark L, Chen JS, Simpson JM, Lappe JM, et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: A collaborative meta-analysis of randomized controlled trials. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2311. [DOI] [PubMed] [Google Scholar]
- 16.Spence LA, Weaver CM. Calcium intake, vascular calcification, and vascular disease. Nutr Rev. 2013;71:15–22. doi: 10.1111/nure.12002. [DOI] [PubMed] [Google Scholar]
- 17.Cusano NE, Rubin MR, McMahon DJ, Zhang C, Ives R, Tulley A, et al. Therapy of hypoparathyroidism with PTH (1-84): A prospective four-year investigation of efficacy and safety. J Clin Endocrinol Metab. 2013;98:137–44. doi: 10.1210/jc.2012-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]


