Abstract
Context:
Thyroid dysfunction has been reported in human immunodeficiency virus (HIV)-infected individuals including children. Some studies have reported that thyroid dysfunction may be a marker of severity or progression of HIV.
Aims:
The aim was to study thyroid function in HIV-infected children with and without highly active anti-retroviral therapy (HAART).
Settings and Design:
Cross-sectional study carried out at a teaching hospital with Anti-Retroviral Therapy Centre (Centre of Excellence) of National AIDS Control Organization.
Subjects and Methods:
Thyroid stimulating hormone (TSH), total thyroxine (T4), and total tri-iodothyronine (T3) were analyzed in 60 pediatric HIV cases: 30 on HAART and 30 HAART naive. Correlation of T3, T4, and TSH with CD4 count was assessed.
Statistical Analysis Used:
Data reported as mean ± standard deviation and as the number of cases and percentages. Comparison between groups was done by independent sample t-test and χ2-test. Spearman's correlation coefficient is used to assess the association between thyroid dysfunction and CD4 count.
Results:
Thyroid function abnormality was seen in five out of 30 patients in both patients on HAART or without HAART therapy. Among patients on HAART, three had hypothyroidism, and two had biochemical feature of sick euthyroid syndrome. Among the HAART naive group, sub-clinical hypothyroisim was seen in four, and one had biochemical feature of sick euthyroid syndrome. None of the patients had clinical features of thyroid dysfunction. There is a highly significant correlation (P = 0.01) between TSH and CD4 count.
Conclusions:
Thyroid dysfunction is quite common among pediatric HIV cases. An inverse correlation is seen between TSH and CD4 count indicating trend for hypothyroidism as HIV disease progress.
Keywords: Highly active anti-retroviral therapy, hypothyroidism, sick-euthyroid, sub-clinical hypothyroidism
INTRODUCTION
Human immunodeficiency virus (HIV) infection can involve the endocrine system.[1] Abnormal thyroid function tests are commoner in HIV patients than the general population.[2,3] These include sick euthyroid state,[4] subclinical hypothyroidism,[5,6] hypothyroidism,[2,6,7,8] Grave's disease, and thyroiditis.[3] Subclinical hypothyroidism is seen especially in those on highly active anti-retroviral therapy (HAART).[2,6,7,9] Stavudine has been implicated in some studies.[5,10] However, another study refute this.[11]
Elevated levels of thyroxine-binding globulin (TBG)[12,13,14] and changes in serum T3 parallel the progression of infection.[14,15]
Thyroid dysfunction has also been reported in HIV children.[16,17,18,19,20] It may contribute to growth failure. Therapy with L-thyroxine results in improvement of height.[21]
Though there are similar studies from India in adults,[22,23] there is so far no study in Indian children. Hence, the present study has undertaken to study thyroid functions on HIV children.
SUBJECTS AND METHODS
This cross-sectional study was carried out in Department of Paediatrics of a Teaching Hospital in collaboration with an Anti-Retroviral Therapy Centre (Centre of Excellence) of National AIDS Control Organization (NACO), Ministry of Health and Family Welfare, Government of India. The study was approved by the Institutional Ethical Committee. Informed consent was taken from parents or guardians.
Inclusion criteria
Sixty confirmed cases of HIV-infected children as per NACO guidelines of 2006[24] (i) 30 HIV Children with a minimum of 6 months with HAART, and (ii) 30 HIV Children without HAART.
Exclusion criteria
Known cases of chronic renal failure, liver failure, chronic hepatitis B and C infection, and acute systemic infection likely to affect thyroid function, those who refused to give inform consent and children above 13 years of age.
For data collection, a semi-structural interview schedule was developed for the purpose and accordingly used. It includes (i) socio-demographic profile (ii) clinical features where details history of clinical information including the patients age, gender, weight, height, undercurrent illness, intravenous drug user, blood transfusion, parental HIV status, the duration of HIV infection, type and duration of HAART, presence of hepatitis B and C infection noted. The clinical features of thyroid dysfunction (hypothyroidism and hyperthyroidism) noted.
All 60 HIV children confirmed by NACO guidelines (2006) with or without HAART were done routine investigations including complete blood count, urine, liver function test, kidney function test, chest X-ray, CD4 count.
Thyroid stimulating hormone (TSH), total thyroxine (T4), and total tri-iodothyronine (T3) were analyzed by chemiluminescence assay using ADVIA Centaur manufactured by SIEMENS, New York, USA. The intra- and inter-assay coefficient of variation was 0.1 (10%). The upper and lower limit of the manufacturer range are; TSH: 0.35–5.50 mIU/L; TT4: 4.5–12.6 μg/dl; TT3: 60–181 ng/dl. Various thyroid function abnormalities were defined in the study were defined as follows according to the cut-off of the manufacturer as there are no normative data for children belonging to the mongoloid population of India:
Hypothyroid: TSH >5.5 mu/L total T4 < 4.5 μg/dl
Hyperthyroid: TSH <0.35 mu/L total T4 >12.6 μg/dl
Subclinical hypothyroid: TSH >5.5 mu/L and normal T3 T4
Sub-clinical hyperthyroid: TSH <0.35 mu/L and normal T3 T4
Sick-euthyroid: TSH normal and low T3.
CD4+ count estimation was carried out by FACS Count Machine manufactured by Becton Dickinson Biosciences, USA.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences, 21 version (Armonk, New York: IBM Corp). Numerical/continuous variables are reported as mean ± standard deviation and for qualitative/categorical variables are again described as the number of cases and percentages. The group's means are compared by Independent Sample test (t-test) and χ2-test is applied for categorical variables. Spearman's correlation coefficient is used as a nonparametric test to assess the association between thyroid dysfunction and CD4 count among the HIV children with HAART therapy. All comparisons are two-sided, and the P < 0.05 treated as the cut-off values for significance.
RESULTS
There are 34 males and 26 females in the study. Thirty each of patients were with and without HAART therapy. In the HAART group, 52.9% were males compared to 47.1% males in those without HAART.
Those children who received HAART are significantly taller and heavier significantly than those who did not receive HAART (29.87 ± 5.94 vs. 22.23 ± 3.74, P < 0.001). However, there is no difference in body mass index of children between the groups (P = 0.226).
Of the 30 patients on HAART, 70% are on zidovudine, lamivudine, and nevirapine, while 20% are on lamivudine, nevirapine and stavudine; and 10% are zidovudine, lamivudine, and efavirenz.
Thyroid function abnormality was seen in five out of 30 patients in both patients on HAART or without HAART therapy. The pattern of thyroid dysfunction differs between the groups as shown in Table 1. The percentage distribution of abnormalities in T3, T4, and TSH is shown in Figure 1 and Table 2.
Table 1.
Thyroid dysfunction according to treatment group

Figure 1.

Showing distribution of thyroid dysfunction among subjects with or without HAART. Blue bar – represent patients on HAART, Red bar - represent patients without HAART, T3 – tri-iodothyroinine, T4 - thyroxine, TSH – thyroid stimulating hormone, HAART – highly active antiretroviral therapy
Table 2.
Mean values of various thyroid function parameters according to treatment group
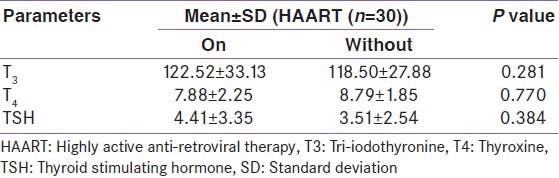
There is a highly significant positive correlation (P = 0.01) between TSH and CD4 count using Spearman's rank correlation coefficient. There is no significant correlation between CD4 count between and T3 and T4.
DISCUSSION
In the present study, evaluating thyroid function abnormalities in pediatric HIV patients with or without HAART, there was no difference in the overall prevalence of thyroid dysfunction between those with or without HAART treatment. But, there was an inverse correlation between serum TSH and CD4 count.
Clinical studies in HIV-infected children have shown a variable profile of thyroid indices. In the present study, 83.3% of both the groups of patients had normal thyroid function test. In those HAART, sub-clinical hypothyroidism was seen 4 (13.3%) and sick-euthyroid in 1 (3.33%). In the HAART group, there were 3 (10%) cases of frank hypothyroid and 2 (6.66%) cases of sick-euthyroid. However, none of the HIV children exhibited clinical features of thyroid dysfunction. In a study from Thailand, 16% of the HIV-infected had abnormal thyroid function test. And similar to the present study, none had clinical features of thyroid hormone dysfunction.[25]
Quirino et al., also reported a similar prevalence of sub-clinical hypothyroidism in both naïve and HAART treated subjects.[26] But Bongiovanni et al., found subclinical hypothyroidism 10.6% which was increased to 19% after 24 months of HAART suggesting a positive acute effect of ART on thyroid function.[27] Hoffmann et al., also described that sub-clinical hypothyroidism is more common during HAART than without HAART probably the effect triggered by immune reconstitution.[8]
Meena et al., found abnormal thyroid function in 40.66% (30% sub-clinical hypothyroidism, 10.66% primary hypothyroidism). However, all the patients were asymptomatic. These changes could be due to thyroiditis and are higher than our findings. This could be due to the more patients with lower CD4 count in their study. They suggested that if these findings are supported by a large longitudinal study a routine screening of thyroid function may be advocated at least in patients of HIV with advanced immune suppression.[23]
In the present study, there was a highly significant positive correlation between CD4 count and TSH which means that the probability of thyroid dysfunction increases as CD4 decreases or as the disease progresses. In another Indian study, there was a direct correlation between CD4 count and free T3 (FT3) and FT4 values (r = 0.357 with P < 0.05; r = 0.650 with P < 0.05, respectively). An inverse correlation of CD4 counts with serum TSH levels was also noted (r = −0.470 with P < 0.050).[22]
However, a study from Iran found no association between hypothyroidism in HIV-infected patients and CD4-cell count or use of HAART.[28]
LoPresti et al., correlated low T3 levels with severity of critical illness and mortality among hospitalized patients with AIDS.[15] Chiarelli et al., also found T3, T4, FT4, and TBG were significantly reduced in HIV children compared with controls whereas TSH, TBG were increased and suggested that thyroid dysfunction correlates with disease severity and can be observed early in the course of perinatal HIV infection and worsen over time.[18]
In a study by Panamonta et al., 14% of Thai children with HIV had low serum T3 and normal TSH, and FT4 levels consistent with sick-euthyroid. All were clinically euthyroid.[20]
Nelson et al., also found a higher than expected incidence of over hypothyroidism in patients receiving HAART and recommended universal screening of thyroid function test on HAART patients.[29]
The cause of thyroid dysfunction is unclear, but hypotheses include autoimmune disease, concurrent infections, destruction by opportunistic infections, and drug reactions.[19] Thyroid abnormalities are associated with disease progression, including severe immunosuppression and high viral load.[17,18] Serum levels of TBG progressively increase with the progression of the disease.[14,15] The reason for the increase in TBG is unknown but seems unrelated to serum estrogen levels or clearance of the protein.[30]
Stress of advanced disease or concomitant morbidities may manifest as the classic sick euthyroid syndrome probably due to hypothalamic-pituitary deficit related to the progress of immunodeficiency and cachexia.[31] Stavudine used for treatment of HIV infection may impair thyroid function but as the number of patients on this drug is limited this association cannot be established in the present study.
CONCLUSION
The present study shows that the biochemical abnormality of thyroid function is quite common among pediatric patients with HIV. An inverse correlation was seen between TSH and CD4 count indicating trend for hypothyroidism as HIV disease progress. However, it will require further longitudinal study with larger number of patients of different groups of CD4 counts and with more thyroid function parameters like free T4, free T3, TBG to confirm the need of regular thyroid function study in the management of HIV children.
LIMITATIONS OF THE STUDY
Small sample size may limit the applicability of the result in a larger population of HIV patients. Also in the present study, only total T3, Total T4, and TSH were evaluated. Free T3, T4, and TBG were not estimated in the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hofbauer LC, Heufelder AE. Endocrine implications of human immunodeficiency virus infection. Medicine (Baltimore) 1996;75:262–78. doi: 10.1097/00005792-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Beltran S, Lescure FX, Desailloud R, Douadi Y, Smail A, El Esper I, et al. Increased prevalence of hypothyroidism among human immunodeficiency virus-infected patients: A need for screening. Clin Infect Dis. 2003;37:579–83. doi: 10.1086/376626. [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Day SL, Metcalfe RA, Sethi G, Kapembwa MS, Brook MG, et al. Characteristics of autoimmune thyroid disease occurring as a late complication of immune reconstitution in patients with advanced human immunodeficiency virus (HIV) disease. Medicine (Baltimore) 2005;84:98–106. doi: 10.1097/01.md.0000159082.45703.90. [DOI] [PubMed] [Google Scholar]
- 4.Olivieri A, Sorcini M, Battisti P, Fazzini C, Gilardi E, Sun Y, et al. Thyroid hypofunction related with the progression of human immunodeficiency virus infection. J Endocrinol Invest. 1993;16:407–13. doi: 10.1007/BF03348867. [DOI] [PubMed] [Google Scholar]
- 5.Grappin M, Piroth L, Verges B, Sgro C, Mack G, Buisson M, et al. Increased prevalence of subclinical hypothyroidism in HIV patients treated with highly active antiretroviral therapy. AIDS. 2000;14:1070–2. doi: 10.1097/00002030-200005260-00026. [DOI] [PubMed] [Google Scholar]
- 6.Calza L, Manfredi R, Chiodo F. Subclinical hypothyroidism in HIV-infected patients receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31:361–3. doi: 10.1097/00126334-200211010-00014. [DOI] [PubMed] [Google Scholar]
- 7.Madeddu G, Spanu A, Chessa F, Calia GM, Lovigu C, Solinas P, et al. Thyroid function in human immunodeficiency virus patients treated with highly active antiretroviral therapy (HAART): A longitudinal study. Clin Endocrinol (Oxf) 2006;64:375–83. doi: 10.1111/j.1365-2265.2006.02472.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann CJ, Brown TT. Thyroid function abnormalities in HIV-infected patients. Clin Infect Dis. 2007;45:488–94. doi: 10.1086/519978. [DOI] [PubMed] [Google Scholar]
- 9.Collazos J, Ibarra S, Mayo J. Thyroid hormones in HIV-infected patients in the highly active antiretroviral therapy era: Evidence of an interrelation between the thyroid axis and the immune system. AIDS. 2003;17:763–5. doi: 10.1097/00002030-200303280-00019. [DOI] [PubMed] [Google Scholar]
- 10.Beltran S, Lescure FX, El Esper I, Schmit JL, Desailloud R. Subclinical hypothyroidism in HIV-infected patients is not an autoimmune disease. Horm Res. 2006;66:21–6. doi: 10.1159/000093228. [DOI] [PubMed] [Google Scholar]
- 11.Madge S, Smith CJ, Lampe FC, Thomas M, Johnson MA, Youle M, et al. No association between HIV disease and its treatment and thyroid function. HIV Med. 2007;8:22–7. doi: 10.1111/j.1468-1293.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- 12.Bourdoux PP, De Wit SA, Servais GM, Clumeck N, Bonnyns MA. Biochemical thyroid profile in patients infected with the human immunodeficiency virus. Thyroid. 1991;1:147–9. doi: 10.1089/thy.1991.1.147. [DOI] [PubMed] [Google Scholar]
- 13.Feldt-Rasmussen U, Sestoft L, Berg H. Thyroid function tests in patients with acquired immune deficiency syndrome and healthy HIV1-positive out-patients. Eur J Clin Invest. 1991;21:59–63. doi: 10.1111/j.1365-2362.1991.tb01359.x. [DOI] [PubMed] [Google Scholar]
- 14.Lambert M. Thyroid dysfunction in HIV infection. Baillieres Clin Endocrinol Metab. 1994;8:825–35. doi: 10.1016/s0950-351x(05)80303-9. [DOI] [PubMed] [Google Scholar]
- 15.LoPresti JS, Fried JC, Spencer CA, Nicoloff JT. Unique alterations of thyroid hormone indices in the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1989;110:970–5. doi: 10.7326/0003-4819-110-12-970. [DOI] [PubMed] [Google Scholar]
- 16.Blethen SL, Nachman S, Chasalow FI. Thyroid function in children with perinatally acquired antibodies to human immunodeficiency virus. J Pediatr Endocrinol. 1994;7:201–4. doi: 10.1515/jpem.1994.7.3.201. [DOI] [PubMed] [Google Scholar]
- 17.Hirschfeld S, Laue L, Cutler GB, Jr, Pizzo PA. Thyroid abnormalities in children infected with human immunodeficiency virus. J Pediatr. 1996;128:70–4. doi: 10.1016/s0022-3476(96)70429-8. [DOI] [PubMed] [Google Scholar]
- 18.Chiarelli F, Galli L, Verrotti A, di Ricco L, Vierucci A, de Martino M. Thyroid function in children with perinatal human immunodeficiency virus type 1 infection. Thyroid. 2000;10:499–505. doi: 10.1089/thy.2000.10.499. [DOI] [PubMed] [Google Scholar]
- 19.Viganò A, Riboni S, Bianchi R, Cafarelli L, Vago T, Manzoni P, et al. Thyroid dysfunction in antiretroviral treated children. Pediatr Infect Dis J. 2004;23:235–9. doi: 10.1097/01.inf.0000114903.05472.e4. [DOI] [PubMed] [Google Scholar]
- 20.Panamonta O, Kosalaraksa P, Thinkhamrop B, Kirdpon W, Ingchanin C, Lumbiganon P. Endocrine function in thai children infected with human immunodeficiency virus. J Pediatr Endocrinol Metab. 2004;17:33–40. doi: 10.1515/jpem.2004.17.1.33. [DOI] [PubMed] [Google Scholar]
- 21.Rana S, Nunlee-Bland G, Valyasevi R, Iqbal M. Thyroid dysfunction in HIV-infected children: Is L-thyroxine therapy beneficial? Pediatr AIDS HIV Infect. 1996;7:424–8. [PubMed] [Google Scholar]
- 22.Jain G, Devpura G, Gupta BS. Abnormalities in the thyroid function tests as surrogate marker of advancing HIV infection in infected adults. J Assoc Physicians India. 2009;57:508–10. [PubMed] [Google Scholar]
- 23.Meena LP, Rai M, Singh SK, Chakravarty J, Singh A, Goel R, et al. Endocrine changes in male HIV patients. (371).J Assoc Physicians India. 2011;59:365–6. [PubMed] [Google Scholar]
- 24.National AIDS Control Organisation. India: NACO; 2006. Indian Academic of Paediatrics: Guidelines for HIV Care and Treatment in Infants and Children; pp. 1–2. [Google Scholar]
- 25.Ketsamathi C, Jongjaroenprasert W, Chailurkit LO, Udomsubpayakul U, Kiertiburanakul S. Prevalence of thyroid dysfunction in Thai HIV-infected patients. Curr HIV Res. 2006;4:463–7. doi: 10.2174/157016206778560036. [DOI] [PubMed] [Google Scholar]
- 26.Quirino T, Bongiovanni M, Ricci E, Chebat E, Carradori S, Martinelli C, et al. Hypothyroidism in HIV-infected patients who have or have not received HAART. Clin Infect Dis. 2004;38:596–7. doi: 10.1086/381442. [DOI] [PubMed] [Google Scholar]
- 27.Bongiovanni M, Adorni F, Casana M, Tordato F, Tincati C, Cicconi P, et al. Subclinical hypothyroidism in HIV-infected subjects. J Antimicrob Chemother. 2006;58:1086–9. doi: 10.1093/jac/dkl360. [DOI] [PubMed] [Google Scholar]
- 28.Afhami S, Haghpanah V, Heshmat R, Rasoulinejad M, Izadi M, Lashkari A, et al. Assessment of the factors involving in the development of hypothyroidism in HIV-infected patients: A case-control study. Infection. 2007;35:334–8. doi: 10.1007/s15010-007-6163-3. [DOI] [PubMed] [Google Scholar]
- 29.Nelson M, Powles T, Zeitlin A, Sen P, Scourfield A, Bower M, et al. Thyroid dysfunction and relationship to antiretroviral therapy in HIV-positive individuals in the HAART era. J Acquir Immune Defic Syndr. 2009;50:113–4. doi: 10.1097/QAI.0b013e31818ce835. [DOI] [PubMed] [Google Scholar]
- 30.Weetman AP. Thyroid abnormalities. Endocrinol Metab Clin North Am. 2014;43:781–90. doi: 10.1016/j.ecl.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Tang WW, Kaptein EM. Thyroid hormone levels in the acquired immunodeficiency syndrome (AIDS) or AIDS-related complex. West J Med. 1989;151:627–31. [PMC free article] [PubMed] [Google Scholar]


