Abstract
Background:
Thyroiditis involves thyroid gland inflammation due to a wide variety of causes. The common varieties are subacute, silent and postpartum thyroiditis.
Aims and Objectives:
To retrospectively collect demographic data of thyroiditis from Bangalore over the past 5 years.
Materials and Methods:
Data were collected from three major nuclear medicine centers in Bangalore of the patients who came for technetium (Tc) 99m pertechnetate scan of the thyroid. The diagnosis was based on the Tc 99 scan evidence of thyroiditis in these patients and biochemical evidence of thyrotoxicosis.
Results:
The total number of cases recorded were 2513. The females were more commonly affected compared with males with sex distribution of 1698 females and 815 females (2:1). The mean age of females was 32.5 ± 11.3 years whereas the mean age of males was 37.2 ± 12.4 years. The highest numbers of cases were recorded in the months of June and August.
Conclusions:
The females developed thyroiditis frequently and at an earlier age when compared with males. This data could give us an insight into the demographic pattern of thyroiditis in our country and may help in planning future preventive strategies.
Keywords: Scan, season, thyroiditis
INTRODUCTION
The term thyroiditis encompasses a diverse group of disorders characterized by some form of thyroid inflammation Thyroiditis are very similar in terms of clinical course, although most likely have different etiologies. Thyroiditis may be categorized as acute (suppurative), subacute (granulomatous or lymphocytic) or chronic (invasive fibrous or lymphocytic).[1]
Acute suppurative thyroiditis is typically caused by a bacterial infection and resolves with appropriate antibiotic treatment. There is some controversy concerning the nomenclature used to categorize the different forms of subacute thyroiditis (SAT). The term SAT applies specifically to subacute granulomatous thyroiditis. Other names for this disorder are subacute nonsuppurative thyroiditis, giant cell thyroiditis, painful thyroiditis, and de Quervain's thyroiditis. It is an uncommon cause of thyrotoxicosis and affects women more often than men (3–5:1).[2,3] The etiology is probably viral and clinical features of SAT are well-known, which include thyroid pain with symptoms of thyrotoxicosis, suppressed levels of thyroid-stimulating hormone (TSH), low thyroid uptake of radioactive iodine, and elevated erythrocyte sedimentation rate. Diagnosis is based on both clinical and laboratory data. Tissue diagnosis is rarely needed. Symptomatic relief is achieved with anti-inflammatory treatment, but in severe cases corticosteroid therapy may be needed.[4,5,6] Rare reports of thyroid storm are described in the literature secondary to SAT.[7] Although the clinical features and outcome have been described in many nonpopulation-based studies community and incidence cohort studies are scarce.
The term “SAT” is not usually applied to silent, painless thyroiditis with lymphocytic pathological features or to postpartum thyroiditis.[8] Subacute lymphocytic thyroiditis is typically painless and often occurs in the postpartum period. Invasive fibrous thyroiditis (Riedel's struma) is exceedingly rare, often mimics carcinoma and is associated with extracervical foci of fibrosclerosis. Chronic lymphocytic (Hashimoto's) thyroiditis, an organ-specific autoimmune disease, occurs in at least 2% of women.[9]
A technetium (Tc) 99 pertechnetate scan is commonly ordered in cases with thyroiditis especially in cases where differentiation from graves is not possible. Any therapy does not prevent early and late-onset thyroid dysfunction.
The demographic studies about the prevalence of thyroiditis have been published in other countries like China, which have shown a maximum prevalence in the months of June and July. The present study was done with the objective to find out the seasonal prevalence of thyroiditis and to find out whether there is any gender predominance in our country.[10]
MATERIALS AND METHODS
We collected retrospective data of Tc 99 scan performed on patients from different nuclear medicine centers in Bangalore who came with history suggestive of thyrotoxicosis and had biochemical evidence confirming thyrotoxicosis in the form of suppressed TSH and increase T4 and or T3 levels. These patients came to the hospital/endocrine clinic for complaints suggestive of thyrotoxicosis. The etiology of thyrotoxicosis was varied in these patients, but all these patients had confirmed biochemical thyrotoxicosis at presentation.
RESULTS
The total number of cases recorded was 2513. The females were more commonly affected compared with males with sex distribution of 1698 females versus 815 males (2:1). The mean age of females was 32.5 ± 11.3 years while the mean age of males was 37.2 ± 12.4 years. The highest numbers of cases were recorded in the month of June and August [Figure 1]. Age range in men was 12–91 years and women was 12–85 years. The youngest patient was 12 years of age, and oldest were 85 and 91 years among females and males respectively.
Figure 1.
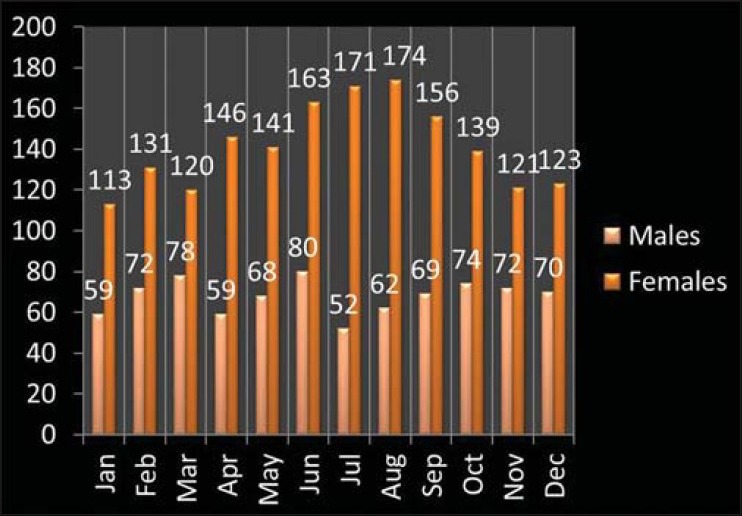
Seasonal distribution of thyroiditis in males and females (P = 0.443 for difference in the seasonal distribution)
DISCUSSION
The different varieties of thyroiditis have varied etiologies and in our study all patients who had reduced Tc 99 uptake were included irrespective of the cause of thyroiditis.
The females developed thyroiditis frequently and at an earlier age as compared to males. Thus, the females showed more predisposition to develop thyroiditis which is in concordance with the other studies. The maximum number of cases were seen in the months of June and August and studies in the literature from different countries also show almost similar seasonal predilection. Nishihara et al.[10] showed in their study that SAT was common in female patients aged 40–50 years, with significant seasonal clustering during summer to early autumn. The rates of any virus infections and diseases did not differ in their study from those in the general population, but recurrent episodes of SAT at intervals of 13.6 ± 5.6 years accounted for 1.6% of all cases. Sex distribution showed a higher incidence in females (F/M 3.2/1), with a mean age of 44 years in a study done by Martino et al.[5] in Italy. In the majority of patients (51/80 = 66%) the onset of the disease was between June and September (46% in July and August). The remaining cases were distributed in the other months without a clear monthly prevalence. They hypothesized that summer enteroviruses may be responsible for most of the cases of thyroiditis. De Bruin et al.[4] reported atypical thyroiditis in 12 cases in the months of July, August and September. Thyroid Tc99 scan showed reduced uptake in 10 out of 11 tested cases.
The epidemiology study of SAT from 1960 to 1967 in Olmsted County in Minnesota showed that of the 160 patients 46 were seen in the spring, 45 in the fall, 36 in the summer, and 33 in the winter. In spring and summer, more patients were seen in May and August than in any other 2 months. Although there was a trend towards more cases in fall and spring, it did not show statistical significance (P = 0.26). There was no obvious geographic clustering within the county, no apparent familial aggregation of cases and no involvement of more than one member in the household.[2]
When compared to other studies on the demographic data our population is prone to develop thyroiditis at an earlier age and the gender predilection is the same as shown in studies from other countries [Table 1].
Table 1.
Comparison with other studies
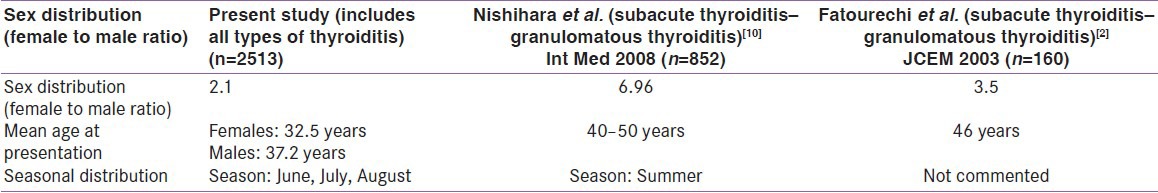
Our study is the first of its kind from India to show the demographic pattern and seasonal distribution of thyroiditis in our country. The main limitation of our study is that we have included all the cases of thyroiditis irrespective of the etiology, and there is no segregation into different varieties of thyroiditis.
CONCLUSIONS
This data can give us an insight into the demographic pattern of thyroiditis in our country and help us in planning future preventive strategies and also help us in planning further research into the etiology of thyroiditis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348:2646–55. doi: 10.1056/NEJMra021194. Review. Erratum in: N Engl J Med 2003;349:620. [DOI] [PubMed] [Google Scholar]
- 2.Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88:2100–5. doi: 10.1210/jc.2002-021799. [DOI] [PubMed] [Google Scholar]
- 3.Qari FA, Maimani AA. Subacute thyroiditis in Western Saudi Arabia. Saudi Med J. 2005;26:630–3. [PubMed] [Google Scholar]
- 4.de Bruin TW, Riekhoff FP, de Boer JJ. An outbreak of thyrotoxicosis due to atypical subacute thyroiditis. J Clin Endocrinol Metab. 1990;70:396–402. doi: 10.1210/jcem-70-2-396. [DOI] [PubMed] [Google Scholar]
- 5.Martino E, Buratti L, Bartalena L, Mariotti S, Cupini C, Aghini-Lombardi F, et al. High prevalence of subacute thyroiditis during summer season in Italy. J Endocrinol Invest. 1987;10:321–3. doi: 10.1007/BF03348138. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann P, Cannie M. Blood tests and imaging in thyroid pathology. Rev Med Brux. 2012;33:246–53. [PubMed] [Google Scholar]
- 7.Sherman SI, Ladenson PW. Subacute thyroiditis causing thyroid storm. Thyroid. 2007;17:283. doi: 10.1089/thy.2007.0070. [DOI] [PubMed] [Google Scholar]
- 8.Stagnaro-Green A. Approach to the patient with postpartum thyroiditis. J Clin Endocrinol Metab. 2012;97:334–42. doi: 10.1210/jc.2011-2576. [DOI] [PubMed] [Google Scholar]
- 9.Mönig H, Harbeck B. Thyroiditis. Dtsch Med Wochenschr. 2008;133:301–4. doi: 10.1055/s-2008-1046710. [DOI] [PubMed] [Google Scholar]
- 10.Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kudo T, et al. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med. 2008;47:725–9. doi: 10.2169/internalmedicine.47.0740. [DOI] [PubMed] [Google Scholar]


