Abstract
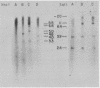
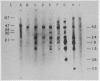
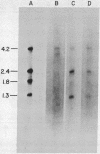
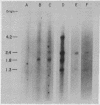
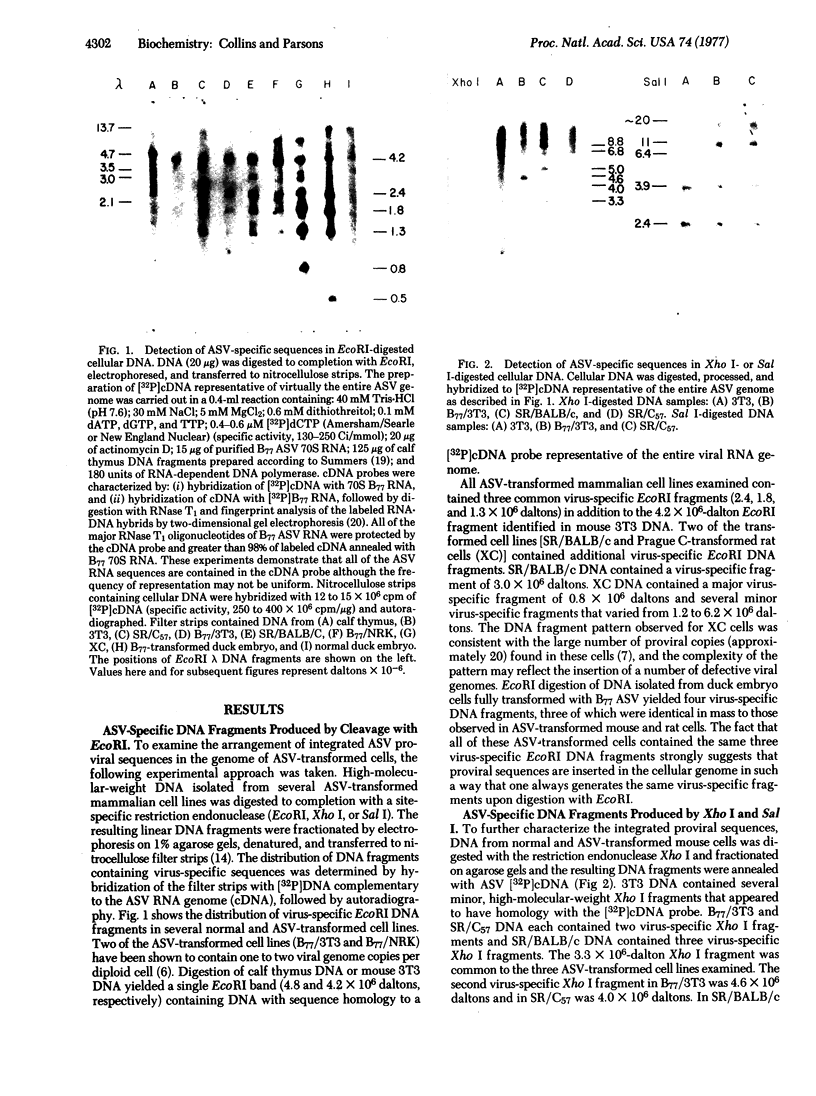
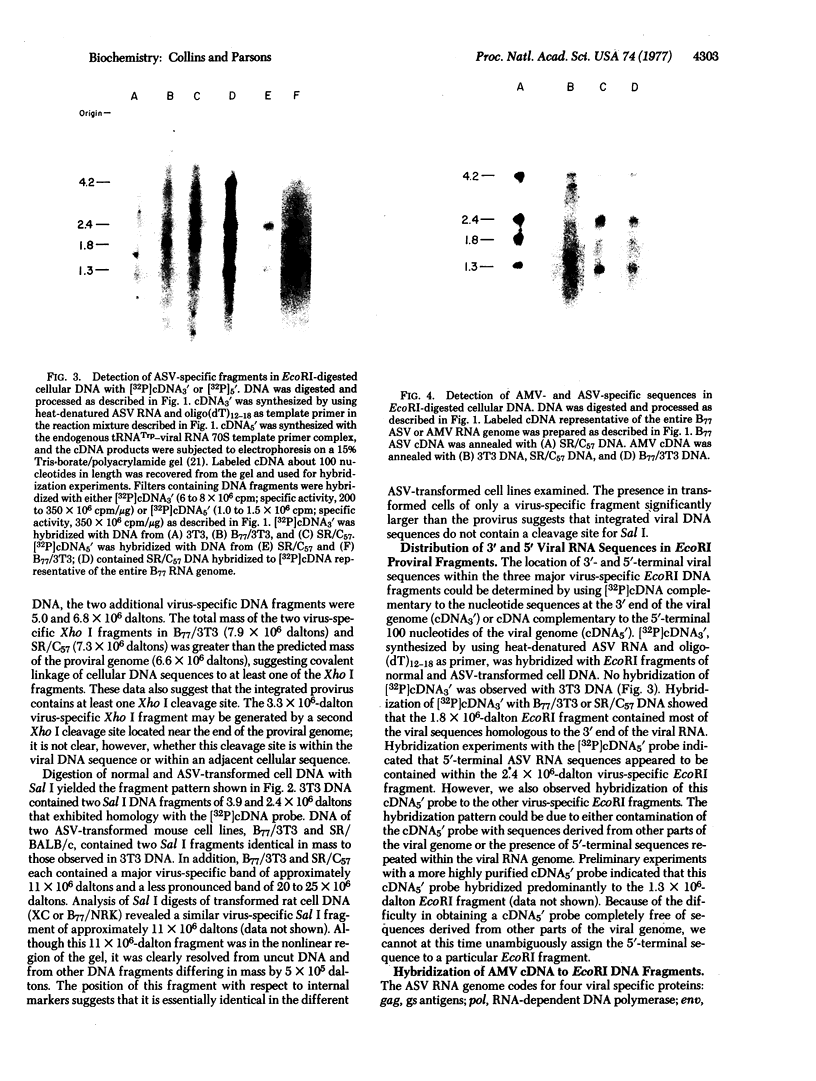
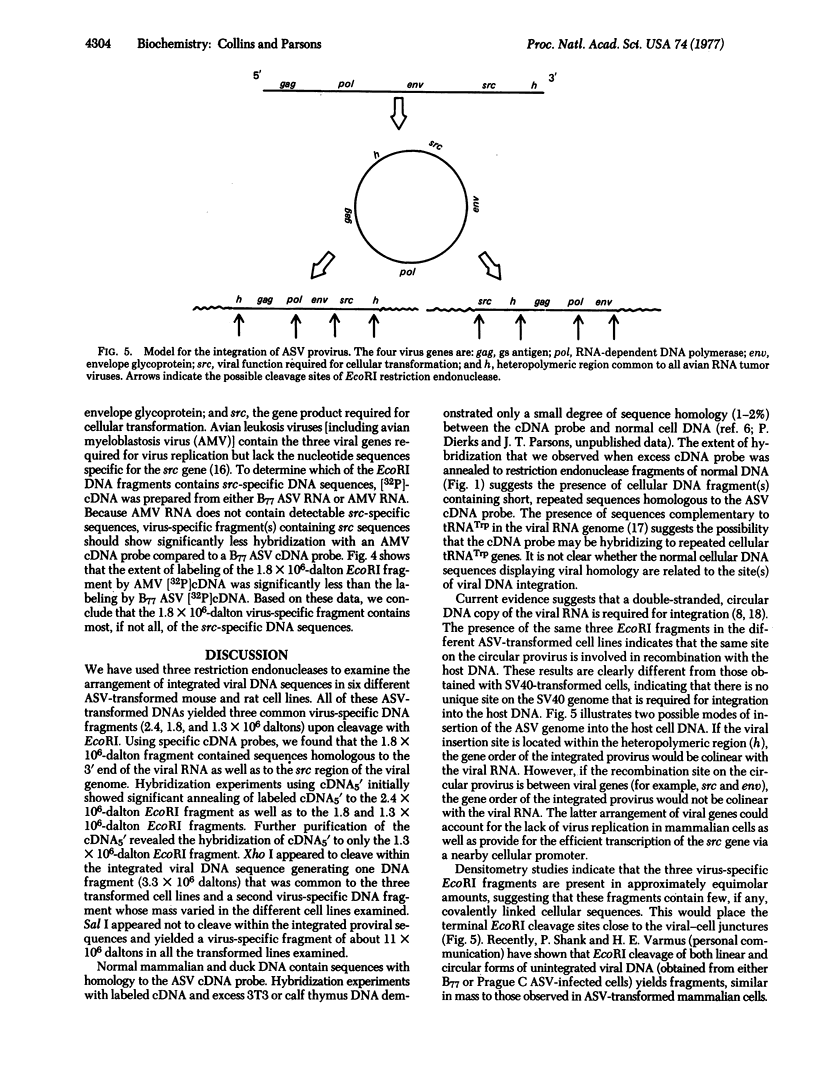
DNA from six avian sarcoma virus (ASV)-transformed mammalian cell lines was digested with the restriction endonucleases EcoRI, Xho I, or Sal I, fractionated by agarose gel electrophoresis, transferred to nitrocellulose filter strips, and hybridized with specific ASV [32P]cDNA probes. DNA from all of the ASV-transformed cell lines yielded three common virus-specific DNA fragments (2.4, 1.8, and 1.3 X 10(6) daltons) upon cleavage with EcoRI. Xho I appeared to cleave at least once within the integrated provirus and yielded a common fragment of 3.3 X 10(6) daltons as well as a second virus-specific DNA fragment whose size varied from 4.0 to 5.0 X 10(6) daltons in the different transformed cell lines. Sal I did not cleave within the provirus and yielded a single major virus-specific fragment of about 11 X 10(6) daltons in all transformed lines examined. Using specific cDNA probes, we show that the 1.8 X 10(6)-dalton EcoRI fragment contains sequences homologous to the 3' end of the viral RNA as well as to the src region of the viral genome. These studies clearly demonstrate that the same region on the ASV genome is utilized for provirus integration in different ASV-transformed cell lines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Parsons J. T., Rymo L., Haroz R. K., Weissmann C. A new approach to the isolation of RNA-DNA hybrids and its application to the quantitative determination of labeled tumor virus RNA. J Mol Biol. 1974 Jun 25;86(2):373–396. doi: 10.1016/0022-2836(74)90026-6. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Dierks P., Cahill J. F., Faras A. J., Parsons J. T. Terminally repeated sequences in the avian sarcoma virus RNA genome. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2389–2393. doi: 10.1073/pnas.74.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. J., Jackson D. A., DeVries A. J. Biochemical construction of specific chimeric plasmids from ColE1 DNA and unfractionated Escherichia coli DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3838–3842. doi: 10.1073/pnas.73.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Stavnezer E., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence that binds primer for DNA synthesis to the avian sarcoma virus genome. J Virol. 1976 Aug;19(2):548–558. doi: 10.1128/jvi.19.2.548-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Thomas C. A., Jr Molecular cloning of DNA fragments produced by restriction endonucleases Sa1I and BamI. Proc Natl Acad Sci U S A. 1976 May;73(5):1537–1541. doi: 10.1073/pnas.73.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Coffin J. M., Haroz R. K., Bromley P. A., Weissmann C. Quantitative determination and location of newly synthesized virus-specific ribonucleic acid in chicken cells infected with Rous sarcoma virus. J Virol. 1973 May;11(5):761–774. doi: 10.1128/jvi.11.5.761-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summers J. Physical map of polyoma viral DNA fragments produced by cleavage with a restriction enzyme from Haemophilus aegyptius, endonuclease R-HaeIII. J Virol. 1975 Apr;15(4):946–953. doi: 10.1128/jvi.15.4.946-953.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. The cellular and molecular biology of RNA tumor viruses, especially avian leukosis-sarcoma viruses, and their relatives. Adv Cancer Res. 1974;19(0):47–104. doi: 10.1016/s0065-230x(08)60052-4. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Deng C. T., Bishop J. M. Synthesis, structure and function of avian sarcoma virus-specific DNA in permissive and nonpermissive cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):987–996. doi: 10.1101/sqb.1974.039.01.113. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. Structure of the intermediates leading to the integrated provirus. Biochim Biophys Acta. 1977 Mar 21;473(1):39–55. doi: 10.1016/0304-419x(77)90006-3. [DOI] [PubMed] [Google Scholar]