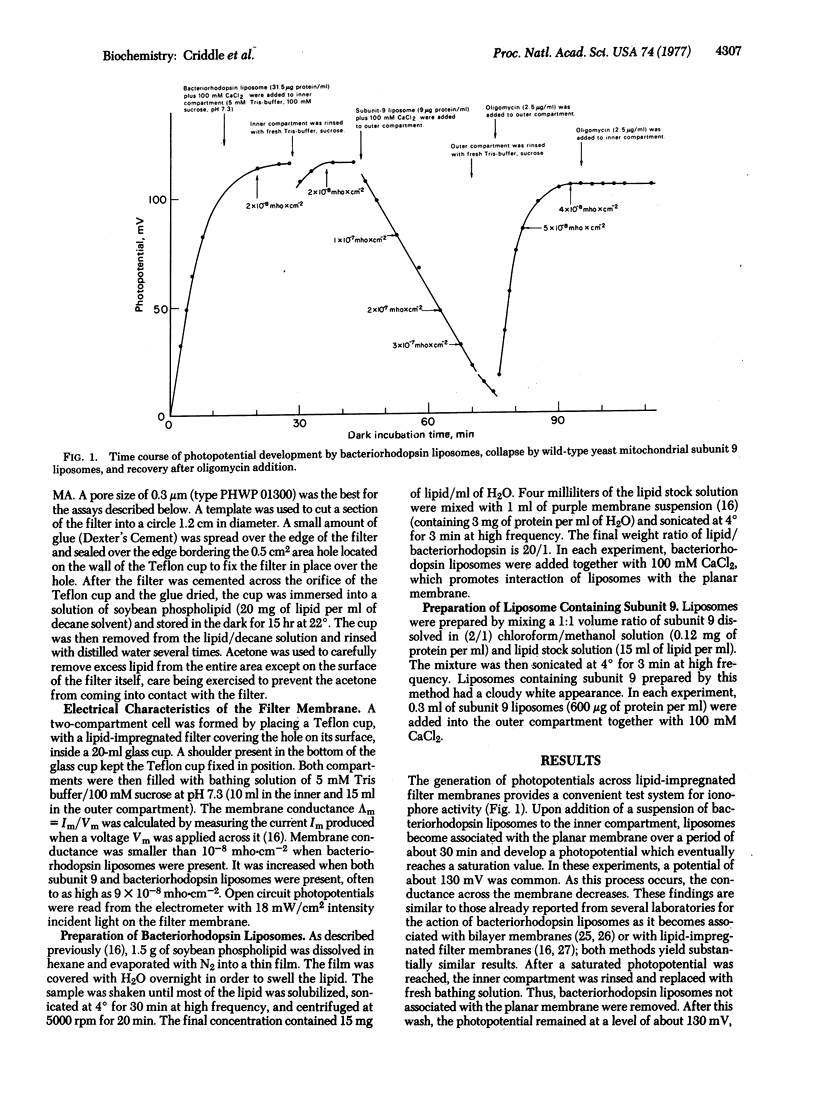
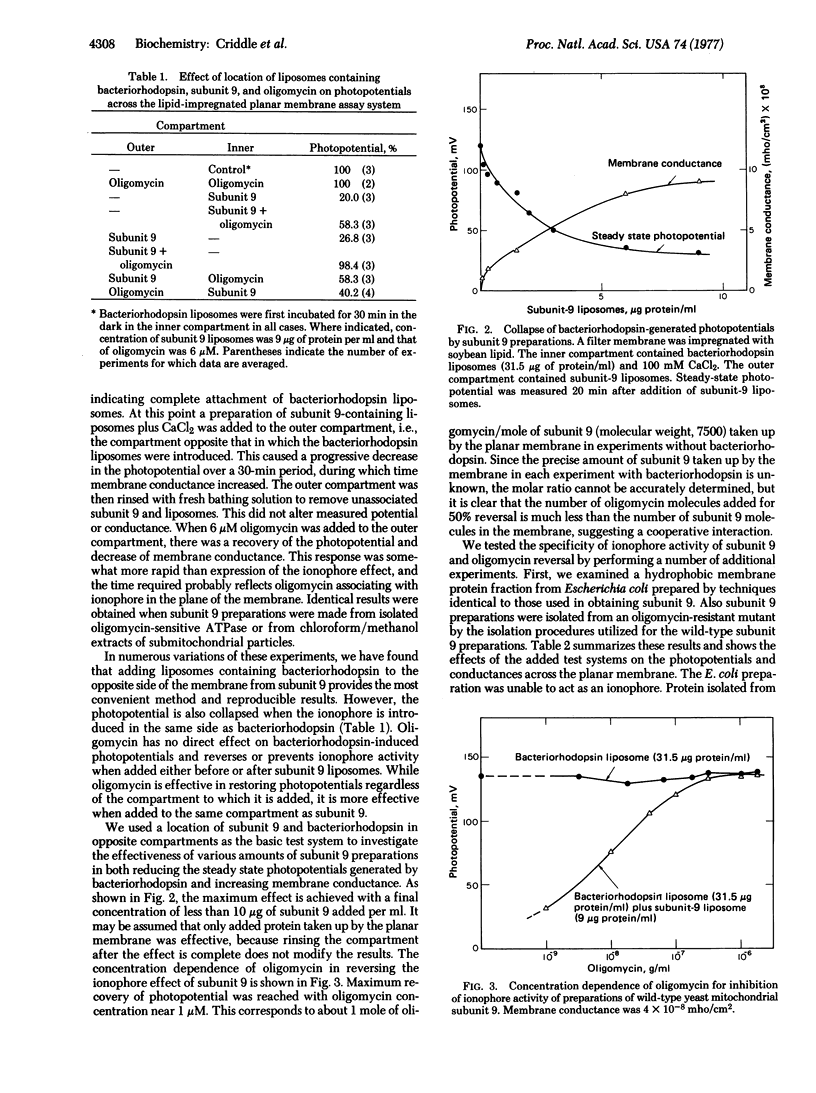
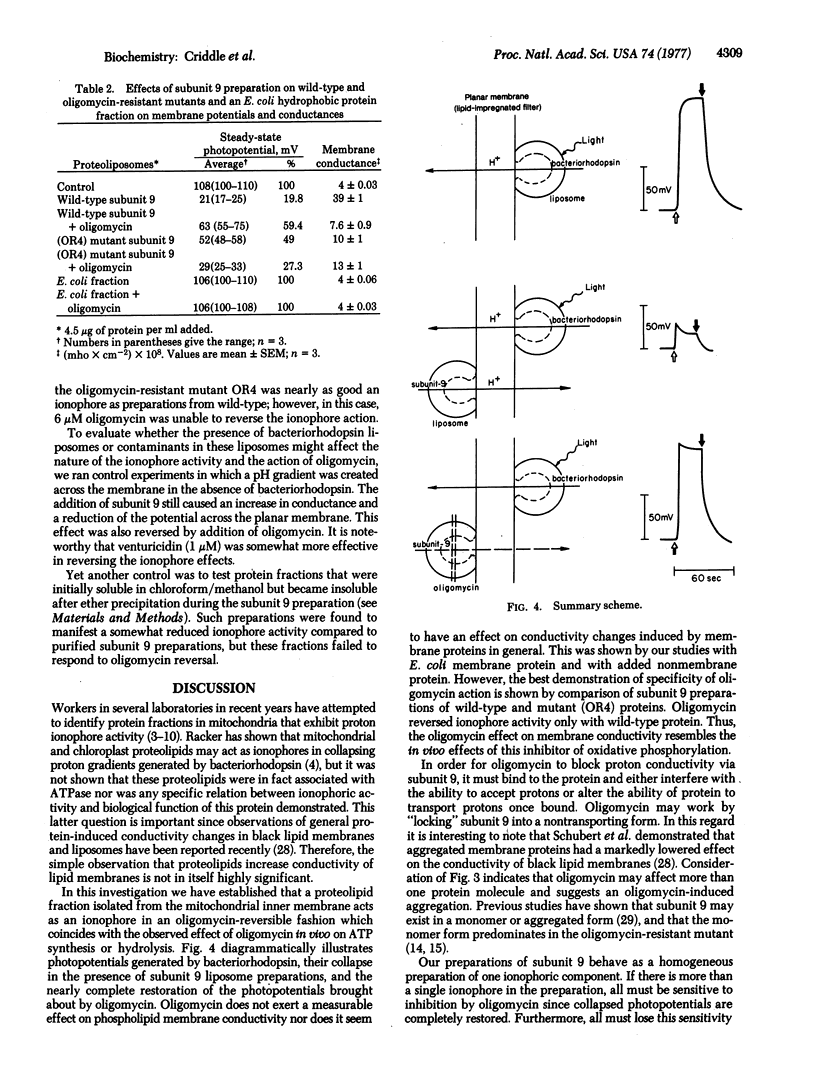
Abstract
A proteolipid isolated from yeast mitochondrial adenosinetriphosphatase (subunit 9) (ATP phosphohydrolase; EC 3.6.1.3) by chloroform/methanol extraction has been shown to discharge photo-induced potentials across a planar phospholipid membrane containing bacteriorhodopsin. Oligomycin, a specific inhibitor of oxidative phosphorylation which binds to this protein, allows the potential gradient to be reestablished. When proteolipid was isolated from an oligomycin-resistant strain, ionophoric activity was still obtained but the effect was not reversed by oligomycin. These studies suggest that the hydrophobic subunit-9 polypeptide is the ionophoric component linking ATP synthesis (hydrolysis) with proton translocation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Criddle R. S., Schatz G. Promitochondria of anaerobically grown yeast. I. Isolation and biochemical properties. Biochemistry. 1969 Jan;8(1):322–334. doi: 10.1021/bi00829a045. [DOI] [PubMed] [Google Scholar]
- Drachev L. A., Jasaitis A. A., Kaulen A. D., Kondrashin A. A., Liberman E. A., Nemecek I. B., Ostroumov S. A., Semenov AYu, Skulachev V. P. Direct measurement of electric current generation by cytochrome oxidase, H+-ATPase and bacteriorhodopsin. Nature. 1974 May 24;249(455):321–324. doi: 10.1038/249321a0. [DOI] [PubMed] [Google Scholar]
- Enns R. K., Criddle R. S. Affinity labeling of yeast mitochondrial adenosine triphosphatase by reduction with [3H]borohydride. Arch Biochem Biophys. 1977 Aug;182(2):587–600. doi: 10.1016/0003-9861(77)90540-9. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H. Identification of the dicyclohexylcarbodiimide-reactive protein component of the adenosine 5'-triphosphate energy-transducing system of Escherichia coli. J Bacteriol. 1975 Nov;124(2):870–883. doi: 10.1128/jb.124.2.870-883.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Folch-Pi J., Stoffyn P. J. Proteolipids from membrane systems. Ann N Y Acad Sci. 1972 Jun 20;195:86–107. [PubMed] [Google Scholar]
- Green D. E. The electromechanochemical model for energy coupling in mitochondria. Biochim Biophys Acta. 1974 Apr 30;346(1):27–78. doi: 10.1016/0304-4173(74)90011-1. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Ohno K., Yoshida M., Takeuchi Y., Sone N. Proton translocation by ATPase and bacteriorhodopsin. Fed Proc. 1977 May;36(6):1815–1818. [PubMed] [Google Scholar]
- Kuzela S., Kolarov J., Krempaský V. Solubility of the intramitochondrially synthesized protein and other membrane proteins of rat liver mitochondria in acidic or neutral chloroform-methanol. Biochem Biophys Res Commun. 1973 Sep 5;54(1):9–16. doi: 10.1016/0006-291x(73)90881-4. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell P. Hypothesis: cation-translocating adenosine triphosphatase models: how direct is the participation of adenosine triphosphate and its hydrolysis products in cation translocation? FEBS Lett. 1973 Jul 15;33(3):267–274. doi: 10.1016/0014-5793(73)80209-1. [DOI] [PubMed] [Google Scholar]
- Nelson N., Eytan E., Notsani B. E., Sigrist H., Sigrist-Nelson K., Gitler C. Isolation of a chloroplast N,N'-dicyclohexylcarbodiimide-binding proteolipid, active in proton translocation. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2375–2378. doi: 10.1073/pnas.74.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E., Eytan E. A coupling factor from sarcoplasmic reticulum required for the translocation of Ca2+ ions in a reconstituted Ca2+ATPase pump. J Biol Chem. 1975 Sep 25;250(18):7533–7534. [PubMed] [Google Scholar]
- Schubert D., Bleuel H., Domning B., Wiedner G. Protein-induced conductivity changes in black lipid membranes and protein aggregation. FEBS Lett. 1977 Feb 15;74(1):47–49. doi: 10.1016/0014-5793(77)80749-7. [DOI] [PubMed] [Google Scholar]
- Shamoo A. E., MacLennan D. H. A Ca++-dependent and -selective ionophore as part of the Ca++ plus Mg++-dependent adenosinetriphosphatase of sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3522–3526. doi: 10.1073/pnas.71.9.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamoo A. E., MacLennan D. H. Separate effects of mercurial compounds on the ionophoric and hydrolytic functions of the (Ca++ +Mg++)-ATPase of sarcoplasmic reticulum. J Membr Biol. 1975 Dec 4;25(1-2):65–74. doi: 10.1007/BF01868568. [DOI] [PubMed] [Google Scholar]
- Shamoo A. E., Ryan T. E., Stewart P. S., MacLennan D. H. Localization of ionophore activity in a 20,000-dalton fragment of the adenosine triphosphatase of Sarcoplasmic reticulum. J Biol Chem. 1976 Jul 10;251(13):4147–4154. [PubMed] [Google Scholar]
- Shannon C., Enns R., Wheels L., Burchiel K., Criddle R. S. Alterations in mitochondrial adenosine triphosphatase activity resulting from mutation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 May 10;248(9):3004–3011. [PubMed] [Google Scholar]
- Shieh P., Packer L. Photo-induced potentials across a polymer stabilized planar membrane, in the presence of bacteriorhodopsin. Biochem Biophys Res Commun. 1976 Jul 26;71(2):603–609. doi: 10.1016/0006-291x(76)90830-5. [DOI] [PubMed] [Google Scholar]
- Sierra M. F., Tzagoloff A. Assembly of the mitochondrial system. Purification of a mitochondrial product of the ATPase. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3155–3159. doi: 10.1073/pnas.70.11.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Akai A. Assembly of the mitochondrial membrane system. 8. Properties of the products of mitochondrial protein synthesis in yeast. J Biol Chem. 1972 Oct 25;247(20):6517–6523. [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Foury F. Assembly of the mitochondrial membrane system XVI. Modified form of the ATPase proteolipid in oligomycin-resistant mutants of Saccharomyces cerevisiae. FEBS Lett. 1976 Jun 15;65(3):391–395. doi: 10.1016/0014-5793(76)80154-8. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. I. Characterization of some enzymes of the inner membrane of yeast mitochondria. J Biol Chem. 1969 Sep 25;244(18):5020–5026. [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]



