Abstract
Objective:
The aim of this in vitro study was to evaluate the antimicrobial effect of oregano extract solution (OES) against Enterococcus faecalis within root canals and dentin tubules, and its effect on smear layer.
Materials and Methods:
A total of 180 human maxillary central incisors was selected. After removal of coronal part of the teeth, root canals were prepared using ProTaper rotary files (Dentsply, Tulsa Endodontics, OK, USA) to #F3 with the crown-down manner. The roots were randomly assigned to 15 groups (n = 12 for each). In the first seven groups, the antimicrobial effects of the test groups were evaluated. Suspensions of E. faecalis cultures were adjusted to 1.0 McFarland (1 × 108 colony-forming unit [CFU]/ml), and sterilized teeth were placed in Eppendorf tubes and kept at 37°C for 4 weeks. Samples were then taken from the root canals before irrigation using three sterile paper points. Dentin samples were taken from root canals with ProTaper #F4 and #F5 series rotary instruments after irrigation. The aliquots of samples were placed into the brain heart infusion and incubated at 37°C for 48 h and then the CFUs were counted. In the other eight groups, the efficacy of the irrigation solutions on removing the smear layer was evaluated using scanning electron microscope (Leo 440, Oxford Microscopy Ltd., Cambridge, England) analysis. Statistical evaluation of the microbiological data was performed using the Kruskall–Wallis and Mann–Witney U-test (P < 0.05).
Results:
There was a statistically difference between the groups (P < 0.05). Chlorhexidine gluconate (CHX), 5% and 2% OES wasn’t found to be statistically significant regarding their antibacterial activities against E. faecalis (P > 0.05). 1% OES and NaOCl showed similar antimicrobial effect (P > 0.05), and 1% OES and NaOCl were better than ethylenediaminetetraacetic acid (EDTA) and saline (P < 0.05) but not as successful as CHX. According to the results obtained from dentin, CHX is the most effective solution within dentinal tubules. Different concentrations of OES were not achieved smear layer removal alone but OES in conjunction with 17% EDTA was the final irrigating solution achieved the smear layer removal without dentin erosion.
Conclusions:
Within the limitations of this study, OES appears to be a possible alternative to NaOCl as a root canal irrigant on the eradication of E. faecalis and removal of smear layer.
Keywords: Antimicrobial effect, chlorhexidine gluconate, Enterococcus faecalis, irrigation solution, NaOCl, oregano extract solution, smear layer
INTRODUCTION
The success of a root canal treatment relies on the proper removal of pulpal remnants, bacteria, and their products from the root canal system.[1] Mechanical preparation alone is not able to remove the pulpal remnants and bacteria effectively from root canals due to the fact that microorganisms are present in all parts of the root canal system, especially in anastomoses, lateral canals, and dentin tubules.[2] Effectively used irrigation in the root canal system is one of the most essential steps for the elimination of microorganisms from these locations.[3]
An ideal irrigation solution has to have a proper antibacterial and tissue dissolving effect on the necrotic pulp remnant and minimum toxic effect on the periapical tissue. NaOCl is the most commonly used root canal irrigation solution up to date due to its antimicrobial effect and tissue dissolving properties,[4] but it is harmful when put in contact with periapical tissue.[5,6,7] Chlorhexidine gluconate (CHX) is an alternative irrigation solution to NaOCl that has a wide range of antimicrobial activity against both Gram-positive and Gram-negative microorganisms, especially against Enterococcus faecalis.[8] However, a main disadvantage of CHX is its lack of organic tissue dissolution capabilities. Hence, other solutions have been experimented as alternative irrigation solutions to NaOCl and CHX.
Origanum minutiflorum is a plant found throughout South-Western Anatolia, especially in Isparta, Turkey. The plant's extract, especially its oil, has an antimicrobial effect on many microorganisms. Dadalioglu and Evrendilek[9] reported that O. minutiflorum has an antimicrobial effect on Escherichia coli, Listeria monocytogenes, Salmonella typhimurium, and Staphylococcus aureus. Similarly, Baydar et al.[10] found that 1–2% origanum oil has an antimicrobial effect on E. faecalis.
Up to date, there were no in vivo or in vitro studies of oregano extract solution (OES) as a root canal irrigant in the literature. Therefore, the aim of this in vitro study was to evaluate the antimicrobial effect of OES as an intracanal irrigant against E. faecalis using bacteria culturing method, and its effect to the smear layer using a scanning electron microscope (SEM) (Leo 440, Oxford Microscopy Ltd., Cambridge, England).
MATERIALS AND METHODS
The method followed was a modification of the technique described previously by Berber et al.[11] A total of 180 extracted human mature maxillary central incisor teeth with a single root and without root resorption was selected. The crowns were removed with a water-cooled diamond saw (Buehler Ltd., Lake Bluff, IL, USA) to leave uniform 15 mm apical sections of root.
The root canal instrumentation was done using an endodontic X-Smart micro-motor (Dentsply-Maillerfer, Ballaigues, Switzerland) at a speed of 350 rpm with ProTaper NiTi rotary files (Dentsply, Tulsa Endodontics, OK, USA) to size #F3 with the crown-down manner. The 180 specimens were randomly assigned to 15 groups (n = 12 for each), antimicrobial evaluation was performed in the first seven groups. In the other eight groups, effect of the solutions on the smear layer was studied.
The smear layer was removed in the first seven groups with 3 ml 17% ethylenediaminetetraacetic acid (EDTA) (Merck KGaA, Darmstadt, Germany) for 1-min, followed by 3 ml 5.25% NaOCl (Wizard, Ankara, Turkey) for 1-min, and then 5 ml distillate water for 1-min as outlined by Teixeira et al.[12] Samples were after that autoclaved at 121°C at 1 atm for 20 min, and then placed into glass tubes containing 5 ml of brain heart infusion (BHI) broth medium (BHI, Oxoid, Basingstoke, UK).
Cultivation of the Enterococcus faecalis and contamination of samples
Pure cultures of E. faecalis (ATCC 29212) cultures were previously cultivated in a BHI broth for 24 h and then cultured in 5% defibrinated sheep blood and BHI agar plates. Suspensions of this bacterium were adjusted to 1.0 McFarland (1 × 108 colony-forming units [CFU]/ml) and sterilized teeth were placed in Eppendorf tubes (Eppendorf AG, Hamburg, Germany) and kept at 37°C for 4 weeks.
Sterile pipettes were used to remove 5 ml of sterile BHI and to replace it with 5 ml of the bacterial inoculum. The medium was changed every 7 days to avoid saturation and confirm the growth of E. faecalis.[13] Four weeks later, the purity of the cultures was confirmed by Gram staining, catalase production.
Microbial sampling of the root canals
Samples were taken from the root canals before irrigation using three sterile paper points. After that, 6 ml of each solution (Group 1: EDTA; Group 2: CHX; Groups 3-5: 1%, 2%, 5% EOS, respectively; Group 6: 5.25% NaOCl; and Group 7: Sterile saline) was used for 2 min in each canal.[13] After irrigation procures sterile paper points were placed into root canals for 1-min, and then transferred to Eppendorf tubes containing 1 ml BHI broth, and then vortexed for 1-min. After three times 10-fold serial dilutions were done for each sample, aliquots of 0.1 ml were plated onto the BHI and blood agar plates combination and incubated at 37°C for 48 h. Growing colonies were counted and recorded as CFU.
Dentin samples of root canals
Dentin samples were taken from root canals with ProTaper #F4 and #F5 series rotary instruments after irrigation. First, #F4 rotary instruments were rotated into the dentin for 15 s, and the collected dentin samples were transferred to the Eppendorf tubes containing 1 ml BHI broth. This was then repeated with the ProTaper #F5 series. All samples were vortexed for 1-min, and then the Eppendorf tubes were incubated at 37°C for 48 h. After two times 10-fold serial dilutions were done for each sample, aliquots of 0.1 ml were plated onto the BHI and blood agar plates combination and incubated at 37°C for 48 h and recorded as CFU.
Scanning electron microscope evaluation
Smear layer removal procedure was done as described by Teixeira et al.[12]
Group 8: 3 ml 5.25% NaOCl for 1-min + 3 ml 17% EDTA for 1-min + 5 ml distilled water for 1-min
Group 9: 3 ml 1% OES for 1-min + 5 ml distilled water for 1-min
Group 10: 3 ml 2% OES for 1-min + 5 ml distilled water for 1-min
Group 11: 3 ml 5% OES for 1-min + 5 ml distilled water for 1-min
Group 12: 3 ml 1% OES for 1-min + 3 ml 17% EDTA for 1-min + 5 ml distilled water for 1-min
Group 13: 3 ml 2% OES for 1-min + 3 ml 17% EDTA for 1-min + 5 ml distilled water for 1-min
Group 14: 3 ml 5% OES for 1-min + 3 ml 17% EDTA for 1-min + 5 ml distilled water for 1-min
Group 15: 3 ml sterile saline for 1-min + 3 ml 17% EDTA for 1-min + 5 ml distilled water for 1-min.
A longitudinal groove on the buccal and lingual surfaces on each specimen was prepared on each specimen using a diamond disc (SS White, Lakewood, New Jersey, USA) at low speed and split into two halves. The specimens were dehydrated, held in a 50%, 75%, 85%, and 96.5% ethyl alcohol solution, dried, mounted on metallic stubs, sputter-coated with a gold alloy (Polaron SC7620 Sputter Coater; VG Microtech Inc., Japan), and examined with a SEM (Leo 440, Oxford Microscopy Ltd., Cambridge, England) ×8,000 magnification.
The data were statistically analyzed using the SAS (SAS Institute, Raleigh, NC, USA) computer program. Each CFU count was transformed to Log10 to normalize the data before statistical evaluation. Statistical evaluation was done with the Kruskall–Wallis and Mann–Whitney U-test. The level of significance was set at P < 0.05.
RESULTS
Statistical evaluation of the groups concerning the antimicrobial effectiveness of the irrigation solutions is shown in Table 1. There was a statistically difference among the groups (P < 0.05). According to the results obtained in the root canal after irrigation, CHX, 5% and 2% OES were not found to be statistically significant regarding their antibacterial activities against E. faecalis. 1% OES and NaOCl showed a similar antimicrobial effect (P > 0.05) and were not as successful as CHX (P < 0.05).
Table 1.
Mean of the Log10 CFU and SD (±SD) of Enterococcus faecalis in experimental groups within root canals and dentin tubules
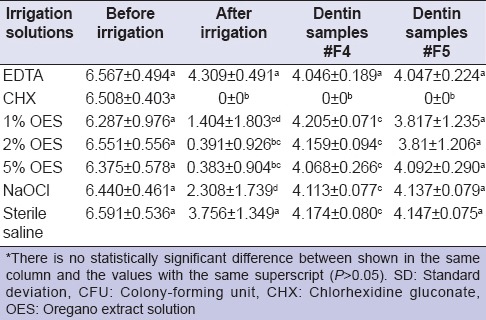
According to the results obtained from the dentin samples, CHX was the most effective solution compared to other groups (P < 0.05). CHX penetrated into the dentin tubules and eradicated all of the E. faecalis. No statistical difference was found between all OES groups and 5.25% NaOCl, which both could not penetrate into the dentin tubules.
According to the results obtained from the SEM evaluation, 1% OES, 2% OES, or 5% OES followed by 17% EDTA completely removed the smear layer without dentin erosion [Figure 1a–c]. However, % OES, 2% OES, or 5% OES without 17% EDTA failed to remove the smear layer alone [Figure 2a–c]. Irrigation with 5.25% NaOCl and 17% EDTA combination achieved the removal of the smear layer [Figure 3].
Figure 1.
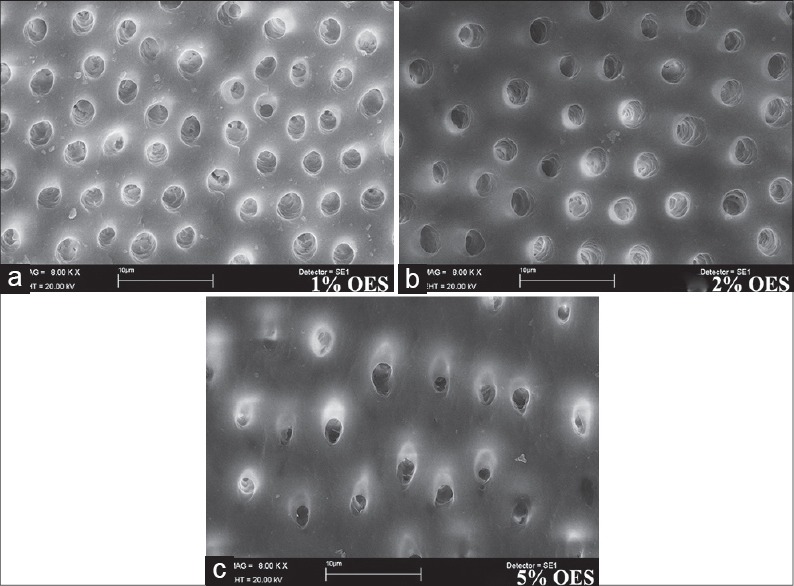
Scanning electron micrographs (a) 1% oregano extract solution (OES) + 17% ethylenediaminetetraacetic acid (EDTA) (b) 2% OES + 17% EDTA (c) 5% OES + 17% EDTA
Figure 2.
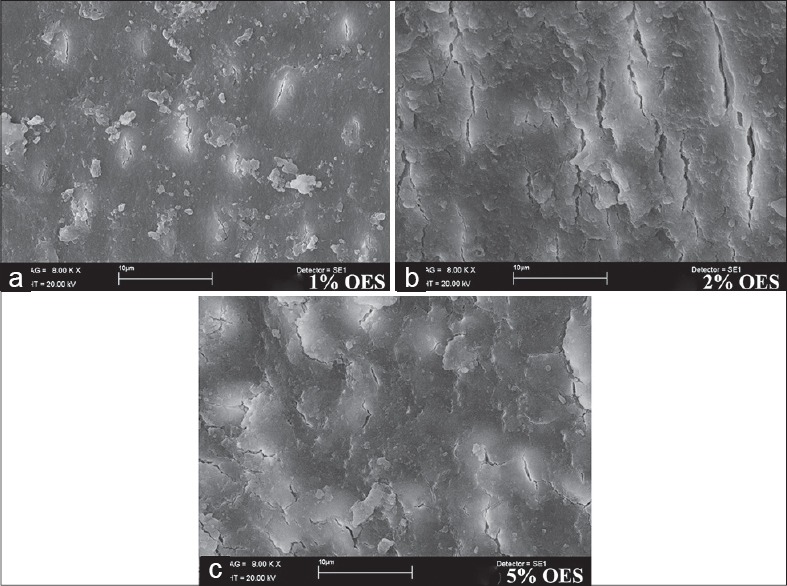
Scanning electron micrographs (a) 1% oregano extract solution (OES) (b) 2% OES (c) 5% OES
Figure 3.
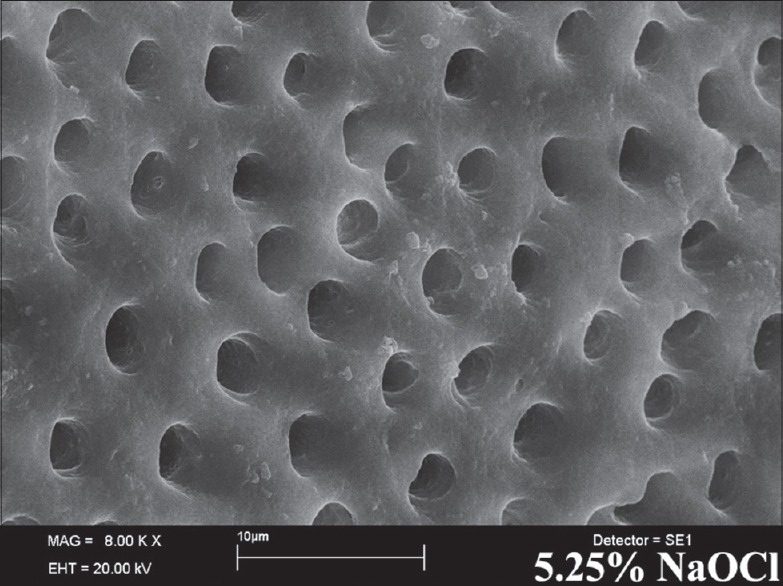
Scanning electron micrographs of 5.25 NaOCl + 17% ethylenediaminetetraacetic acid
DISCUSSION
Enterococcus faecalis, a very persistent microorganism isolated from in infected teeth that invades deep into dentin tubules, was isolated in cases of failed root canal treatment.[14,15] In the present study, E. faecalis was used to evaluate the antimicrobial efficiency of solutions. The results obtained from this study showed that 5.25% NaOCl was effective against E. faecalis within root canals. There was no statistical difference was found between 1% OES and 5.25% NaOCl (P > 0.05) in terms of their effectiveness against E. faecalis in this study. But there was a statistically significant difference between the antimicrobial effect of 2% OES and 5% OES and 5.25% NaOCl (P < 0.05). The present findings also indicate that in the eradication of E. faecalis, NaOCl was not as effective as CHX. Onçag et al.[8] found that 2% CHX was significantly more effective against E. faecalis than 5.25% NaOCl. In our study, CHX eradicated all of the E. faecalis from the root canal system and was significantly more effective than 5.25% NaOCl (P < 0.05). This result shows that our findings support the results of Onçag et al.[8]
Microorganisms may invade to the dentin tubules of both vital and necrotic pulp, but vital teeth may limit the invasion of the microorganism with a pulp defense system.[16] Microorganisms that live in dentin tubules are an important risk factor in the success of the root canal treatment.[11,17] Berber et al.[11] indicated that no statistical difference was found between 0.5% and 5.25% concentrations of NaOCl as a root canal irrigant. However, in regard to its antimicrobial effect on dentin tubules, 5.25% NaOCl was evaluated as highly effective. Vahdaty et al.[18] stated that NaOCl had an antimicrobial effect against E. faecalis in 500 μm deep dentin tubules, and Gomes et al.[19] found the same in 10 μm deep dentin tubules. Mohammadi and Shahriari[20] found that NaOCl had no antimicrobial effect when CHX had not penetrated deep into the dentin tubules, and Basrani et al.[21] and Gomes et al.[19] have stated that 2% CHX completely eradicated E. faecalis from root canals and dentin tubules. Similarly, in our findings, NaOCl and OES could not penetrate into the dentin tubules, and CHX was highly effective against E. faecalis in the dentin tubules (P < 0.05). However, 2% and 5% OES more effectively eradicate E. faecalis than 5.25% NaOCl in the root canals (P < 0.05).
In the present study, NaOCl and EDTA, combination was used to remove the smear layer. Although NaOCl was unsuccessful in removing the smear layer alone, it has been used for many years due to its capacity to act as a solvent of organic tissue. Beltz et al.[22] stated that 5.25% NaOCl dissolved over 90% of organic components, and that 17% EDTA dissolved above 70% of inorganic components. Several studies[23,24,25] have stated that an EDTA and NaOCl combination has the ability to remove the smear layer without damaging dentin walls. In the present study 5.25% NaOCl and 17% EDTA combination was used as a positive control group, it was found that the smear layer was completely removed, all dentin tubules were opened, and the root canals were clean in all parts. Contrary to the findings of Serper and Calt,[26] no intertubuler and peritubuler erosion was established. The results of our findings were similar to many articles in literature.[12,23,25,27] This difference may have arisen from the use of a 1-min 10 ml 17% EDTA solution in their study.[26] Similarly, Ballal et al.[28] reported that using 5 ml 17% EDTA did not cause erosion in peritubuler dentin.
Alternatively to NaOCl, different OES concentrations were also used alone as well as combined with EDTA to remove the smear layer, and found that OES could not remove the smear layer alone [Figure 2]. However, when the OES and EDTA combination was used, it removed the smear layer and opened dentin tubules without erosion. The combination of 1%, 2% or 5% OES and 17% EDTA removed surface smear layer, dentin tubules were opened in the coronal, middle, and apical part [Figure 1]. Torabinejad et al.[29] reported that the opening of the dentin tubules was greater in the coronal third than the apical third in connection to insufficient irrigation due to the small diameter of the apical part. Similarly, in this study, all solutions that removed the smear layer led to dentin tubules that were opened more in the coronal part than the apical part.
CONCLUSION
Within the limitations of this study, 1% OES and 5.25% NaOCl have the same antimicrobial activity against E. Feacalis and 2% or 5% OES had more effective antibacterial action than 5.25% NaOCl. OES and NaOCl have the same effect on the removing of the organic compound of the smear layer. Therefore, OES appears a possible alternative to 5.25% NaOCl as a root canal irrigant.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–8. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 2.Siqueira JF, Jr, Lima KC, Magalhães FA, Lopes HP, de Uzeda M. Mechanical reduction of the bacterial population in the root canal by three instrumentation techniques. J Endod. 1999;25:332–5. doi: 10.1016/S0099-2399(06)81166-0. [DOI] [PubMed] [Google Scholar]
- 3.Bystrom A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35–40. doi: 10.1111/j.1365-2591.1985.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 4.Siqueira JF, Jr, Machado AG, Silveira RM, Lopes HP, de Uzeda M. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal, in vitro. Int Endod J. 1997;30:279–82. doi: 10.1046/j.1365-2591.1997.00096.x. [DOI] [PubMed] [Google Scholar]
- 5.Gatot A, Arbelle J, Leiberman A, Yanai-Inbar I. Effects of sodium hypochlorite on soft tissues after its inadvertent injection beyond the root apex. J Endod. 1991;17:573–4. doi: 10.1016/S0099-2399(06)81725-5. [DOI] [PubMed] [Google Scholar]
- 6.Brown DC, Moore BK, Brown CE, Jr, Newton CW. An in vitro study of apical extrusion of sodium hypochlorite during endodontic canal preparation. J Endod. 1995;21:587–91. doi: 10.1016/S0099-2399(06)81108-8. [DOI] [PubMed] [Google Scholar]
- 7.Pashley EL, Birdsong NL, Bowman K, Pashley DH. Cytotoxic effects of NaOCl on vital tissue. J Endod. 1985;11:525–8. doi: 10.1016/S0099-2399(85)80197-7. [DOI] [PubMed] [Google Scholar]
- 8.Onçag O, Hosgör M, Hilmioglu S, Zekioglu O, Eronat C, Burhanoglu D. Comparison of antibacterial and toxic effects of various root canal irrigants. Int Endod J. 2003;36:423–32. doi: 10.1046/j.1365-2591.2003.00673.x. [DOI] [PubMed] [Google Scholar]
- 9.Dadalioglu I, Evrendilek GA. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J Agric Food Chem. 2004;52:8255–60. doi: 10.1021/jf049033e. [DOI] [PubMed] [Google Scholar]
- 10.Baydar H, Sağdic O, Özkan K, Karadoğan T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control. 2004;15:169–72. [Google Scholar]
- 11.Berber VB, Gomes BP, Sena NT, Vianna ME, Ferraz CC, Zaia AA, et al. Efficacy of various concentrations of NaOCl and instrumentation techniques in reducing Enterococcus faecalis within root canals and dentinal tubules. Int Endod J. 2006;39:10–7. doi: 10.1111/j.1365-2591.2005.01038.x. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira CS, Felippe MC, Felippe WT. The effect of application time of EDTA and NaOCl on intracanal smear layer removal: An SEM analysis. Int Endod J. 2005;38:285–90. doi: 10.1111/j.1365-2591.2005.00930.x. [DOI] [PubMed] [Google Scholar]
- 13.Johal S, Baumgartner JC, Marshall JG. Comparison of the antimicrobial efficacy of 1.3% NaOCl/BioPure MTAD to 5.25% NaOCl/15% EDTA for root canal irrigation. J Endod. 2007;33:48–51. doi: 10.1016/j.joen.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J. 2001;34:429–34. doi: 10.1046/j.1365-2591.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 15.Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 16.Nagaoka S, Miyazaki Y, Liu HJ, Iwamoto Y, Kitano M, Kawagoe M. Bacterial invasion into dentinal tubules of human vital and nonvital teeth. J Endod. 1995;21:70–3. doi: 10.1016/S0099-2399(06)81098-8. [DOI] [PubMed] [Google Scholar]
- 17.Buck R, Eleazer PD, Staat RH. In vitro disinfection of dentinal tubules by various endodontics irrigants. J Endod. 1999;25:786–8. doi: 10.1016/S0099-2399(99)80297-0. [DOI] [PubMed] [Google Scholar]
- 18.Vahdaty A, Pitt Ford TR, Wilson RF. Efficacy of chlorhexidine in disinfecting dentinal tubules in vitro. Endod Dent Traumatol. 1993;9:243–8. doi: 10.1111/j.1600-9657.1993.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 19.Gomes BP, Souza SF, Ferraz CC, Teixeira FB, Zaia AA, Valdrighi L, et al. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003;36:267–75. doi: 10.1046/j.1365-2591.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadi Z, Shahriari S. Residual antibacterial activity of chlorhexidine and MTAD in human root dentin in vitro. J Oral Sci. 2008;50:63–7. doi: 10.2334/josnusd.50.63. [DOI] [PubMed] [Google Scholar]
- 21.Basrani B, Santos JM, Tjäderhane L, Grad H, Gorduysus O, Huang J, et al. Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:240–5. doi: 10.1067/moe.2002.124002. [DOI] [PubMed] [Google Scholar]
- 22.Beltz RE, Torabinejad M, Pouresmail M. Quantitative analysis of the solubilizing action of MTAD, sodium hypochlorite, and EDTA on bovine pulp and dentin. J Endod. 2003;29:334–7. doi: 10.1097/00004770-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Khademi A, Yazdizadeh M, Feizianfard M. Determination of the minimum instrumentation size for penetration of irrigants to the apical third of root canal systems. J Endod. 2006;32:417–20. doi: 10.1016/j.joen.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Marending M, Paqué F, Fischer J, Zehnder M. Impact of irrigant sequence on mechanical properties of human root dentin. J Endod. 2007;33:1325–8. doi: 10.1016/j.joen.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Franchi M, Eppinger F, Filippini GF, Montanari G. NaOCl and EDTA irrigating solutions for endodontics: SEM findings. Bull Group Int Rech Sci Stomatol Odontol. 1992;35:93–7. [PubMed] [Google Scholar]
- 26.Serper A, Calt S. The demineralizing effects of EDTA at different concentrations and pH. J Endod. 2002;28:501–2. doi: 10.1097/00004770-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Ahmetoglu F, Keles A, Yalcin M, Simsek N. Effectiveness of different irrigation systems on smear layer removal: A scanning electron microscopic study. Eur J Dent. 2014;8:53–7. doi: 10.4103/1305-7456.126241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballal NV, Kandian S, Mala K, Bhat KS, Acharya S. Comparison of the efficacy of maleic acid and ethylenediaminetetraacetic acid in smear layer removal from instrumented human root canal: A scanning electron microscopic study. J Endod. 2009;35:1573–6. doi: 10.1016/j.joen.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Torabinejad M, Shabahang S, Aprecio RM, Kettering JD. The antimicrobial effect of MTAD: An in vitro investigation. J Endod. 2003;29:400–3. doi: 10.1097/00004770-200306000-00005. [DOI] [PubMed] [Google Scholar]


