Abstract
Objective:
Tooth bleaching tends to increase enamel roughness and porosity, in addition to reducing surface microhardness. The aim of this in vitro study was to evaluate the effects of bleaching treatments using different hydrogen peroxide (HP) concentrations, with and without light activation on bovine enamel microhardness.
Materials and Methods:
The buccal surfaces of sixty bovine incisors were flattened and polished and the enamel specimens were divided into six groups: G1 : c0 ontrol, exposed to artificial saliva; G2: 35% HP applied in two sessions (45’ each); G3: 35% HP applied in two sessions (3 × 15’ each); G4: 35% HP applied in one session (3 × 7’30”) plus hybrid light (HL); G5: 25% HP applied in one session (3 × 7’30”) plus HL; and G6: 15% HP applied in one session (3 × 7’30”) plus HL. After the treatment, the enamel specimens were stored in artificial saliva. The surface microhardness (Knoop) was measured at the baseline, 24 h and 7 days after bleaching. The data was analyzed using the ANOVA test, followed by the Tukey–Krummer test (P < 0.05).
Results:
All bleaching procedures lead to a decrease in surface microhardness when compared with the control group after 24 h. The lowest change in surface microhardness was found in the specimens treated with 15% HP plus HL. However, 35% HP plus HL induced the highest decrease in surface microhardness. After 7 days of remineralization, the surface microhardness returned to normal levels for all bleached specimens.
Conclusion:
Therefore, it can be concluded that the bleaching protocols caused a slight enamel surface alteration. However, the remineralization process minimized these effects.
Keywords: Hardness, tooth bleaching agents, tooth remineralization
INTRODUCTION
Today, people are continually looking for aesthetic perfection, and this includes a beautiful smile with white teeth. The use of peroxides to promote tooth bleaching is not a recent phenomenon since there is some evidence from earlier in the middle of the XIX century that showed hydrogen peroxide (HP) as one of the substances capable of changing tooth color (value and chrome).[1]
Usually, the technique for in-office bleaching uses 35% HP. This can be used alone or in association with heat or light, which can accelerate the color changing process.[2] When bleaching is performed using high concentrations of HP at 25-38%, in conjunction with light activation, it is possible to achieve success in just one appointment.[3]
Although the efficiency of bleaching agents to vital and nonvital teeth is well-documented, the widespread use of bleaching techniques generates some concern about the effects promoted by these agents onto the bleached substrates.[4] Risks to soft tissues, such as burning, a possible co-carcinogenic effect and lesions, are correlated to the use of hydrogen and/or carbamide peroxide in high concentrations.[5] Some alterations in the enamel and dentin, such as an increase of roughness, porosity and diminished microhardness have also been observed.[4,6,7,8,9,10,11] When observed with a scanning electron microscope (SEM), the enamel presented an increase in porosity, erosion and superficial demineralization.[12] Contradictory results have also been observed in spite of the chemical composition, physical and mechanical properties of bleached human enamel with minimal alterations.[11,12] Regarding the microhardness properties, there are many controversial results, possibly due to the variations in methodologies.[6,7,8,9,13]
The importance of understanding the effects of bleaching agents on the dental enamel, especially microhardness, can help determine which bleaching treatment will be safest to obtain the best bleaching effects with maximum preservation of the dental tissues. Therefore, the objective of this study was to evaluate the effects of an in-office bleaching treatment, employing different concentrations and application times of HP gel, with and without LED/Laser hybrid light (HL) activation, in relation to the superficial microhardness of bovine dental enamel.
MATERIALS AND METHODS
Specimen preparation
Freshly extracted bovine incisors were stored in 0.9% NaCl plus 0.1% thymol solution (pH 7.0) at 4°C, until the specimen preparation. The crown and root were separated using an Isomet Low Speed Saw (Buehler Ltd., Lake Bluff, IL, USA) and a diamond disk (Extec Corp., Enfield, CT, USA). Sixty bovine incisor crowns were embedded in acrylic resin cylinders (JET, São Paulo, SP, Brazil), and the buccal surfaces were ground flat with water-cooled carborundum paper (1200, 2400 and 4000 grits, Buehler, Lake Bluff, IL, USA), and polished with wet felt paper using a diamond spray (1 μm; Buehler, Lake Bluff, IL, USA). After the preparation, the specimens were stored in distilled water until used for the experiment in order to avoid dehydration.
Microhardness testing
Initially, the enamel surface microhardness (SH) was measured using a microhardness tester (HMV-2000; Shimadzu Corporation, Tokyo, Japan) and a Knoop diamond with a 25 g load applied for 5s. Five indentations, 100 μm apart, were made in the center of the enamel specimens. After 24 h and 7 days of bleaching treatments, the microhardness measurement (SH24h and SH7d) was repeated close to the initial measurements points.
Bleaching protocols
After the initial microhardness measurement, the specimens were randomly divided in to six groups (n = 10) to receive the bleaching procedures.
The bleaching agents were manipulated and applied according to the manufacturer's instructions. A 1.0 mm thick coat of bleaching gel was applied over the experimental enamel area. For the new bleaching gel applications, the specimens were cleaned with deionized water until the complete removal of the gel and dried with absorbent paper.
For the groups activated with HL, the LED/Laser device, Ultrablue IV (DMC Equipamentos Ltda., São Carlos, SP, Brazil), was used. This device is equipped with a one-point diode laser with 830 nm of wavelength and 200 mW/cm2 and 19 LEDs with 460-480 nm and 73 mW/cm2 each.
At the end of each session, the specimens were polished with a felt disc impregnated with abrasive polishing paste, cleaned and followed by an application of a 1.0 mm coat of desensitizer gel with 2% neutral sodium fluoride and 5% potassium nitrate (Lase Sensy, DMC Equipaments, Ltda., São Carlos, SP, Brazil), for 5 min. During all periods in which the specimens were not being submitted to the bleaching procedures, the specimens were kept in artificial saliva at 37 ± 1°C, specifically formulated for the re-mineralization of the dental hard tissues. The composition of the artificial saliva was: 1.5 mM Ca(NO3)2.4H2O, 0.9 mM Na2HPO4. 2H2O, 0.15 M KCl, 0.02 M TRIS and 0.05 ppm F (pH 7.0).[13]
For the control group, all the clinical steps were followed, but the bleaching gel was substituted by a glycerin based gel that did not require any manipulation and was applied over the specimen's buccal face with a microbrush (Cavibrush, FGM produtos Odontológicos, Joenvile, SC, Brazil).
The protocols employed and substances used are listed in Table 1.
Table 1.
Products and application protocol of different treatment groups
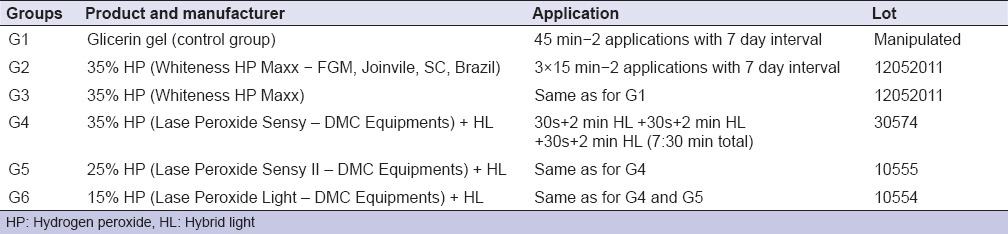
After the bleaching procedures, the specimens remained in unstirred artificial saliva, at 37°C, for 24 h and 7 days after bleaching. The artificial saliva was changed daily.
Statistical analysis
Means and standard deviations were calculated from the surface microhardness values (Knoop hardness number). Equality of variances and normal distribution of the data were tested for all the variables using the Bartlett and Kolmogorov–Smirnov tests, respectively (GraphPad InStat for Windows, version 4.0, San Diego, CA, USA). All data showed equal variances and normal distributions. Therefore, a one-way ANOVA test, followed by a post-hoc Tukey's test were used (P < 0.05).
RESULTS
In the comparison between groups, there were no significant statistical differences in the initial enamel microhardness (ANOVA, P = 0.99) [Table 2]. Twenty-four hours after bleaching, the enamel microhardness diminished significantly in all groups compared with the control group. The greatest microhardness alteration was presented by group G4 (ANOVA, P < 0.0001), whereas groups G2, G3, G5 and G6 did not differ significantly. However, 7 days after bleaching, the experimental groups did not present significant differences in comparison to the control group (P > 0.05).
Table 2.
Means of enamel microhardness (KHN±SD) before bleaching, 24 h after and 7 days after bleaching
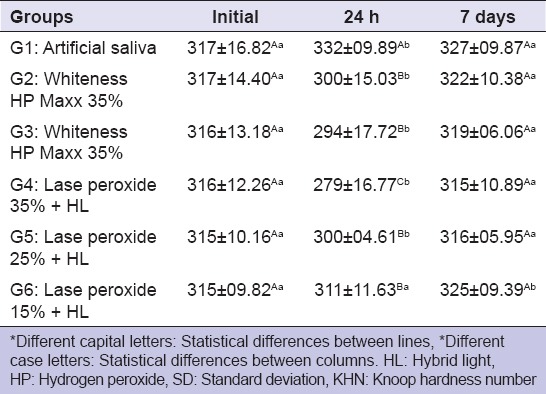
There was an increase in the microhardness values in the control group (G1) after 24 h inside the artificial saliva, however, the microhardness returned to prebleaching levels after 7 days in saliva (ANOVA, P = 0.0096). Group G6 was the only group that did not present statistical differences 24 h after bleaching, however, it was also the only group that had a significant increase in microhardness after 7 days (ANOVA, P = 0.003).
DISCUSSION
The possible effects that peroxides can have on dental tissues have generated numerous studies. Many authors have shown that bleaching gels can alter dental tissue, such as enamel microhardness,[6,10,14] superficial roughness[15,16] and chemical composition,[10] as well as the bond strenght of the adhesive system to the enamel after bleaching.[17] The alterations in microhardness can be related to the loss or gain of minerals (demineralization and remineralization) of the dental structure. Some studies showed that microhardness tests are adequate to determine small differences in the superficial enamel, which can be caused by the effects of acids, colas, and also bleaching gels.[7]
Oxygen ions have a short lifespan, are unstable and react with other free substances or substrates presenting weak reactions. This is possible because of its high electro-negativity, which promotes a powerful reaction characterized by ions seeking molecular stability. This process probably occurs due to the redox mechanism or a simple reduction promoted by the oxygen ion that reacts with the molecules that stain the teeth, becoming more simple, whiter or eliminated.[6,7,8,11,18] Although the exact mechanism of dental bleaching isn’t totally apparent, it's believed that the permeability of the dental structure to the bleaching agents can reduce the size of the chromogenous molecules.[8]
In the present study, all the experimental groups, except the G6 group (15% HP), showed a significant reduction in the microhardness values in relation to the control group (G1). Twenty-four hours after treatment, the G4 group (35% HP + HL) presented the greatest changes in microhardness for this period. Other studies also observed a decrease in enamel and dentin microhardness after bleaching.[6,9,13,19,20] The effect on the microhardness properties is probably dependent on the concentration and light activation, combined with a high concentrated HP that could exacerbate this alteration.[21,22]
Light activation with bleaching procedures have also been studied, with positive results.[23] In the present study, the light activation did not present a significant influence on the microhardness values. The results are in agreement with other studies which analyzed the effect of different light sources (LED, Diode Laser and neodymium-doped yttrium aluminium garnet Laser) on the microhardness of enamel submitted to bleaching with 35% HP and observed a decrease in the enamel microhardness in all groups, independent of light source.[2] Parreiras et al.,[24] in 2014, also reported nonsignificant enamel changes comparing light activated and nonlight activated bleaching protocols right after the bleaching process, and also reported that, after 1 week storage in artificial saliva, all the specimens´ microhardness were similar to their initial values.
Several studies also showed that, despite enamel microhardness changes by the effects of HP, this demineralization can be reversed by the remineralization potential of the saliva that replaces the lost calcium and phosphate ions.[20] Araujo et al.,[14] in 2010, in an in situ study, concluded that changes in microhardness are not significant and can be recovered 14 days after bleaching, due to the absorption and precipitation of the calcium and phosphate present in the saliva. In 2013, Araujo et al.,[25] reported that after 15 days, all the specimens bleached with a HP based gel, with neutral pH, were able to re-establish their baseline microhardness. In the present results, after 7 days of remineralization, there were no significant differences between all experimental groups and the control group. Lewinstein et al.,[9] in 2004, concluded that exposure to low concentrations of fluoride can also restore the superficial microhardness after bleaching.
These aspects show that all the tested protocols are completely safe when considering the microhardness, but it is important to say that the option for a specific concentration of HP and light activation must be made according to the patient characteristics and preferences. For example, an elderly patient with a narrow pulpal chamber can be submitted to a protocol with a higher HP concentration and HL, with a slight chance of sensibility; on the other hand, a patient with a wide pulpal chamber should be treated with a smaller concentrated bleaching agent and HL, but a greater number of appointments may be necessary.
In contrast with these results, some studies showed no changes in the enamel surface after bleaching.[11,26]
In 2000, Potocnik et al.,[27] also found no significant changes in the enamel microhardness after treatment with 10% carbamide peroxide. It was also corroborated by Götz et al.,[18] in 2007, that analyzed the effects of HP in low concentrations (13 and 16%) over the enamel surface and subsurface, and found no changes in the microhardness.
Sulieman et al.,[10] in 2004, evaluated the effects of high concentrations of HP used in office on the enamel and dentin. The results showed no change in abrasion, hardness and topography in the enamel and dentin. The authors concluded that, even with the use of high concentrations of HP, no deleterious effects were observed. Finally, they suggested that the deleterious effects may not be caused by the concentration of the peroxide bleaching agent, but by the pH level of the gel used.
These contradictory studies regarding the microhardness alterations can be explained by the fact that surveys have different methodologies, such as using different bleaching agents (with different concentrations, application times and methods of application), different forms of hardness evaluation (Knoop, Vickers, weight and length indentation), pH level and storage method of the specimens.[8] Thus, it becomes difficult to find concrete data to compare the results.
Considering the pH level effect, Alexandrino et al.,[28] in 2014, reported that the use of an HP based gel capable of a 7.0 pH maintenance didn’t affect the enamel microhardness, but under SEM evaluation, none of the tested gels caused morphological changes over the specimens´ surface.
It's important to consider that the demineralization after bleaching covered a relatively normal range when compared with acidic drinks and daily drinking,[29] as well as the importance of patient post-bleaching orientation about their diet in order to avoid acid and/or high coloured foods and beverages, because the demineralized enamel is more susceptible to staining by pigments, which could cause undesirable colour changes.[25]
Therefore, the results obtained in the present study and the information found in the literature, indicate an important role of saliva in the recovery of the enamel microhardness, and its related effects on the remineralizing action. Furthermore, one should consider that the changes of enamel microhardness may be insignificant from a clinical standpoint,[13] but it's important to follow the manufactures´ instructions and to apply fluoride and/or desensitizing agents prior, during or after the bleaching protocol in order to minimize the demineralizing effects.[30] However, in the clinical situation, besides the bleaching procedure, other challenges might be presented in the oral cavity, such as erosion, abrasion and cariogenesis, which may develop an important role together with the bleaching procedure, leading to enamel surface alterations.[31] Therefore, the association between bleaching and acid exposure and abrasive challenges should be done in further studies. In situ studies should be done to better understand the role of human saliva in this process.
CONCLUSION
Considering the limitations of this study, it can be concluded that:
All bleaching treatments, except for group 6 (15% HP), promoted a significant reduction in the microhardness values compared to the control group.
After 7 days of bleaching and storage in artificial saliva, all experimental groups showed a microhardness recovery when compared to the control group.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Borges A, Zanatta R, Barros A, Silva L, Pucci C, Torres C. Effect of Hydrogen Peroxide Concentration on Enamel Color and Microhardness. Oper Dent. 2014 doi: 10.2341/13-371-L. Ahead of print. Doi: 10.2341/13-371-L. [DOI] [PubMed] [Google Scholar]
- 2.Cervantes A, Bolanho A, Valera MC, Araújo MA. Microhardness study of bovine enamel to bleaching treatment activated for different sources of light. Brazilian Dental Science. 2006;9:78–86. [Google Scholar]
- 3.Mondelli RF, Azevedo JF, Francisconi AC, Almeida CM, Ishikiriama SK. Comparative clinical study of the effectiveness of different dental bleaching methods - Two year follow-up. J Appl Oral Sci. 2012;20:435–43. doi: 10.1590/S1678-77572012000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giannini M, Silva AP, Cavalli V, Paes Leme AF. Effect of carbamide peroxide-based bleaching agents containing fluoride or calcium on tensile strength of human enamel. J Appl Oral Sci. 2006;14:82–7. doi: 10.1590/S1678-77572006000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam L. Vital tooth bleaching: Review and current status. J Can Dent Assoc. 1992;58:654–5. 659. [PubMed] [Google Scholar]
- 6.Attin T, Schmidlin PR, Wegehaupt F, Wiegand A. Influence of study design on the impact of bleaching agents on dental enamel microhardness : A review. Dent Mater. 2009;25:143–57. doi: 10.1016/j.dental.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Joiner A. Review of the effects of peroxide on enamel and dentine properties. J Dent. 2007;35:889–96. doi: 10.1016/j.jdent.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Joiner A. The bleaching of teeth : A review of the literature. J Dent. 2006;34:412–9. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Lewinstein I, Fuhrer N, Churaru N, Cardash H. Effect of different peroxide bleaching regimens and subsequent fluoridation on the hardness of human enamel and dentin. J Prosthet Dent. 2004;92:337–42. doi: 10.1016/j.prosdent.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Sulieman M, Addy M, Macdonald E, Rees JS. A safety study in vitro for the effects of an in-office bleaching system on the integrity of enamel and dentine. J Dent. 2004;32:581–90. doi: 10.1016/j.jdent.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Soldani P, Amaral CM, Rodrigues JA. Microhardness evaluation of in situ vital bleaching and thickening agents on human dental enamel. Int J Periodontics Restorative Dent. 2010;30:203–11. [PubMed] [Google Scholar]
- 12.Akal N, Over H, Olmez A, Bodur H. Effects of carbamide peroxide containing bleaching agents on the morphology and subsurface hardness of enamel. J Clin Pediatr Dent. 2001;25:293–6. [PubMed] [Google Scholar]
- 13.Pinto CF, Paes Leme AF, Cavalli V, Giannini M. Effect of 10% carbamide peroxide bleaching on sound and artificial enamel carious lesions. Braz Dent J. 2009;20:48–53. doi: 10.1590/s0103-64402009000100008. [DOI] [PubMed] [Google Scholar]
- 14.Araujo Fde O, Baratieri LN, Araújo E. In situ study of in-office bleaching procedures using light sources on human enamel microhardness. Oper Dent. 2010;35:139–46. doi: 10.2341/08-033-C. [DOI] [PubMed] [Google Scholar]
- 15.Mondelli RF, Azevedo JF, Francisconi PA, Ishikiriama SK, Mondelli J. Wear and surface roughness of bovine enamel submitted to bleaching. Eur J Esthet Dent. 2009;4:396–403. [PubMed] [Google Scholar]
- 16.Tezel H, Ertas OS, Ozata F, Dalgar H, Korkut ZO. Effect of bleaching agents on calcium loss from the enamel surface. Quintessence Int. 2007;38:339–47. [PubMed] [Google Scholar]
- 17.Josey AL, Meyers IA, Romaniuk K, Symons AL. The effect of a vital bleaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil. 1996;23:244–50. doi: 10.1111/j.1365-2842.1996.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 18.Götz H, Duschner H, White DJ, Klukowska MA. Effects of elevated hydrogen peroxide ‘strip’ bleaching on surface and subsurface enamel including subsurface histomorphology, micro-chemical composition and fluorescence changes. J Dent. 2007;35:457–66. doi: 10.1016/j.jdent.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Al-Salehi SK, Wood DJ, Hatton PV. The effect of 24h non-stop hydrogen peroxide concentration on bovine enamel and dentine mineral content and microhardness. J Dent. 2007;35:845–50. doi: 10.1016/j.jdent.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Basting RT, Rodrigues AL, Jr, Serra MC. The effects of seven carbamide peroxide bleaching agents on enamel microhardness over time. J Am Dent Assoc. 2003;134:1335–42. doi: 10.14219/jada.archive.2003.0047. [DOI] [PubMed] [Google Scholar]
- 21.Elfallah HM, Swain MV. A review of the effect of vital teeth bleaching on the mechanical properties of tooth enamel. N Z Dent J. 2013;109:87–96. [PubMed] [Google Scholar]
- 22.Jiang T, Ma X, Wang Y, Tong H, Shen X, Hu Y, et al. Investigation of the effects of 30% hydrogen peroxide on human tooth enamel by Raman scattering and laser-induced fluorescence. J Biomed Opt. 2008;13:014019. doi: 10.1117/1.2870114. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Zhu Y, Li J, Liao S, Ai H. Efficacy of cold light bleaching using different bleaching times and their effects on human enamel. Dent Mater J. 2013;32:761–6. doi: 10.4012/dmj.2013-109. [DOI] [PubMed] [Google Scholar]
- 24.Parreiras SO, Vianna P, Kossatz S, Loguercio AD, Reis A. Effects of light activated in-office bleaching on permeability, microhardness, and mineral content of enamel. Oper Dent. 2014;39:E225–30. doi: 10.2341/13-031-L. [DOI] [PubMed] [Google Scholar]
- 25.Araujo NC, da Costa Soares MU, Nery MM, Sales WS, Gerbi ME. Effect of pH values of two bleaching gels on enamel microhardness. Gen Dent. 2013;61:55–8. [PubMed] [Google Scholar]
- 26.Mellberg JR. Hard-tissue substrates for evaluation of cariogenic and anti-cariogenic activity in situ. J Dent Res. 1992;71(Spec No):913–9. doi: 10.1177/002203459207100S25. [DOI] [PubMed] [Google Scholar]
- 27.Potocnik I, Kosec L, Gaspersic D. Effect of 10% carbamide peroxide bleaching gel on enamel microhardness, microstructure, and mineral content. J Endod. 2000;26:203–6. doi: 10.1097/00004770-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Alexandrino L, Gomes Y, Alves E, Costi H, Rogez H, Silva C. Effects of a bleaching agent with calcium on bovine enamel. Eur J Dent. 2014;8:320–5. doi: 10.4103/1305-7456.137634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Chen Z, Lin Y, Shao J, Yin L. Effects of tooth whitening agents and acidic drinks on the surface properties of dental enamel. Hua Xi Kou Qiang Yi Xue Za Zhi. 2013;31:530–2. [PubMed] [Google Scholar]
- 30.Yesilyurt C, Sezer U, Ayar MK, Alp CK, Tasdemir T. The effect of a new calcium-based agent, Pro-Argin, on the microhardness of bleached enamel surface. Aust Dent J. 2013;58:207–12. doi: 10.1111/adj.12063. [DOI] [PubMed] [Google Scholar]
- 31.Salomão D, Santos D, Nogueira R, Palma-Dibb R, Geraldo-Martins V. Acid demineralization susceptibility of dental enamel submitted to different bleaching techniques and fluoridation regimens. Oper Dent. 2014;39:E178–85. doi: 10.2341/13-140. [DOI] [PubMed] [Google Scholar]


