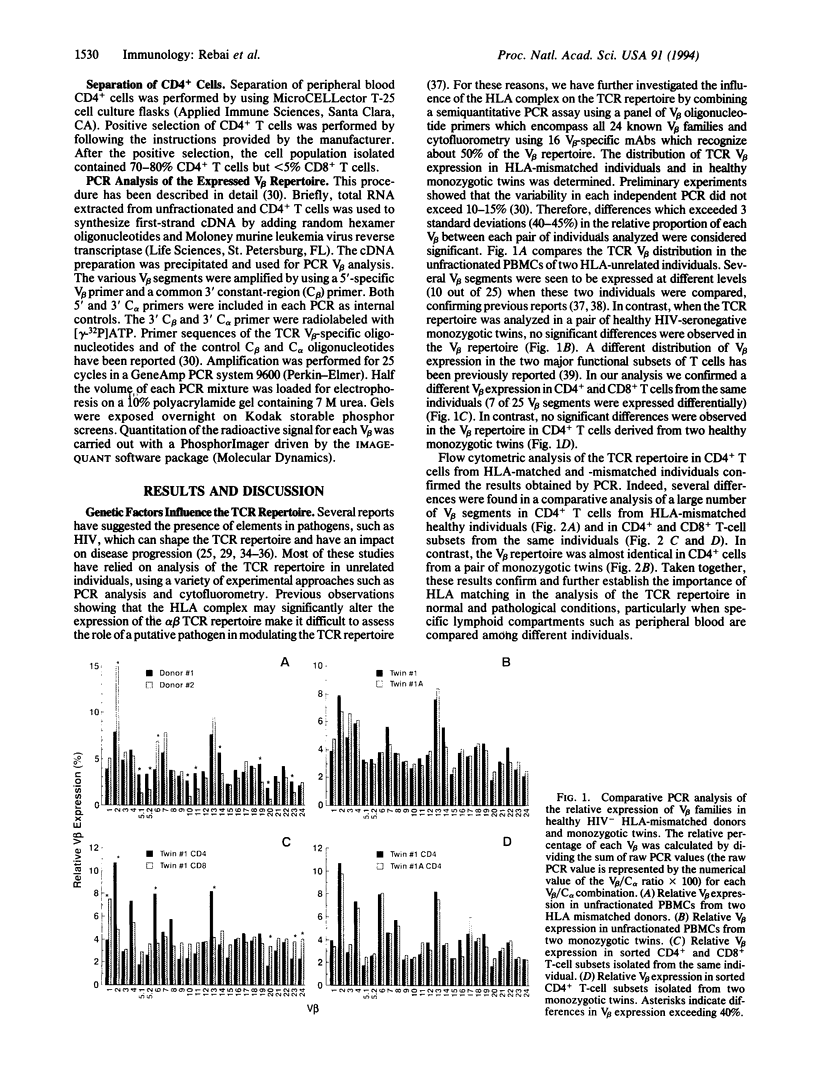
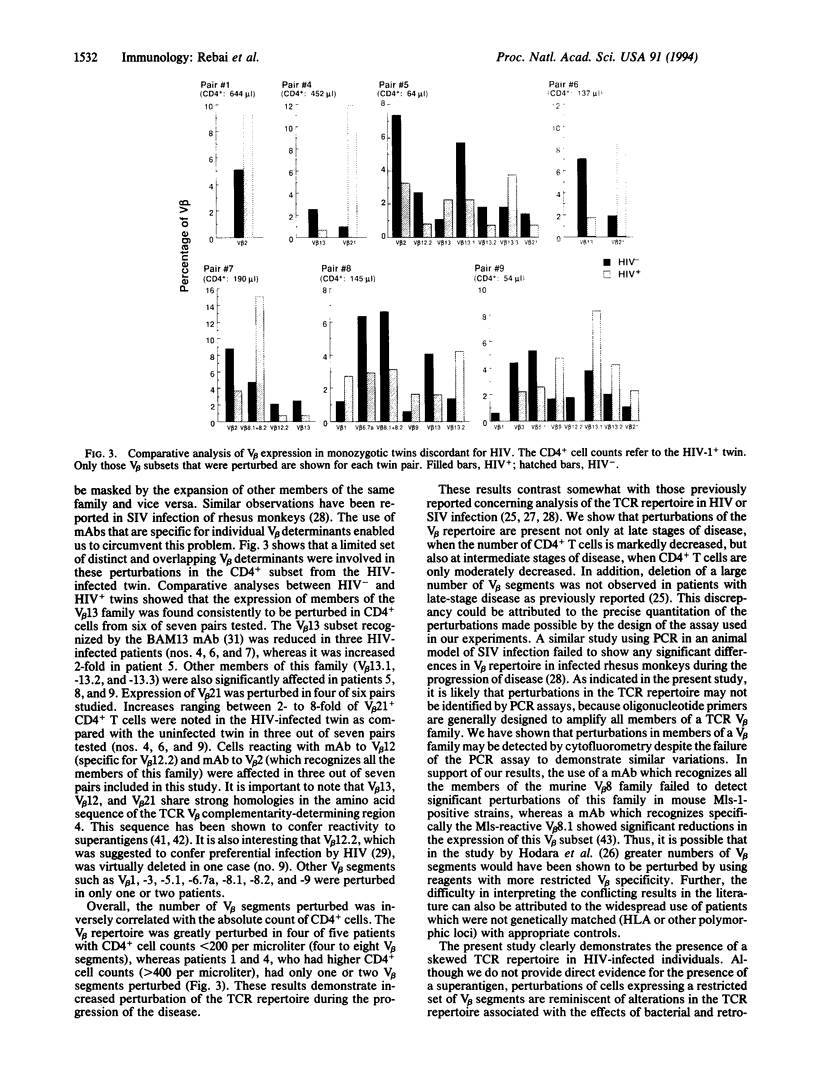
Abstract
We analyzed the T-cell receptor (TCR) V beta repertoire in human immunodeficiency virus type 1 (HIV-1)-infected individuals at different stages of disease. To circumvent the effect of HLA and other loci on the expressed TCR repertoire, we compared the TCR repertoire in nine pairs of monozygotic twins who were discordant for HIV infection. A semiquantitative polymerase chain reaction (PCR) assay and flow cytometry enabled us to show distinct differences in the V beta repertoire in the HIV-positive twin compared with the HIV-negative twin. By combining PCR and cytofluorometry, these differences were restricted to a specific set of TCR V beta segments, with members of the V beta 13 family perturbed in six out of seven cases and those of the V beta 21 family perturbed in four out of seven cases studied. Most of the other V beta families remained unchanged. Our results provide direct evidence for a skewed TCR repertoire in HIV infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe R., Foo-Phillips M., Hodes R. J. Analysis of Mlsc genetics. A novel instance of genetic redundancy. J Exp Med. 1989 Oct 1;170(4):1059–1073. doi: 10.1084/jem.170.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acha-Orbea H., Shakhov A. N., Scarpellino L., Kolb E., Müller V., Vessaz-Shaw A., Fuchs R., Blöchlinger K., Rollini P., Billotte J. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991 Mar 21;350(6315):207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- Bekoff M. C., Cole B. C., Grey H. M. Studies on the mechanism of stimulation of T cells by the Mycoplasma arthritidis-derived mitogen. Role of class II IE molecules. J Immunol. 1987 Nov 15;139(10):3189–3194. [PubMed] [Google Scholar]
- Bill J., Kanagawa O., Woodland D. L., Palmer E. The MHC molecule I-E is necessary but not sufficient for the clonal deletion of V beta 11-bearing T cells. J Exp Med. 1989 Apr 1;169(4):1405–1419. doi: 10.1084/jem.169.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave P. A., Marche P. N., Jouvin-Marche E., Voegtlé D., Bonhomme F., Bandeira A., Coutinho A. V beta 17 gene polymorphism in wild-derived mouse strains: two amino acid substitutions in the V beta 17 region greatly alter T cell receptor specificity. Cell. 1990 Nov 16;63(4):717–728. doi: 10.1016/0092-8674(90)90138-5. [DOI] [PubMed] [Google Scholar]
- Chen Z. W., Kou Z. C., Shen L., Reimann K. A., Letvin N. L. Conserved T-cell receptor repertoire in simian immunodeficiency virus-infected rhesus monkeys. J Immunol. 1993 Aug 15;151(4):2177–2187. [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Lafferty J., White J., Pigeon M., Kubo R., Kappler J., Marrack P. A method for production of antibodies to human T-cell receptor beta-chain variable regions. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8357–8361. doi: 10.1073/pnas.88.19.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Choi Y., Lafferty J. A., Clements J. R., Todd J. K., Gelfand E. W., Kappler J., Marrack P., Kotzin B. L. Selective expansion of T cells expressing V beta 2 in toxic shock syndrome. J Exp Med. 1990 Sep 1;172(3):981–984. doi: 10.1084/jem.172.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux B., Bjorkman P. J., Stevenson C., Greif W., Elliott J. F., Sagerström C., Clayberger C., Krensky A. M., Davis M. M. Generation of monoclonal antibodies against soluble human T cell receptor polypeptides. Eur J Immunol. 1991 Sep;21(9):2111–2119. doi: 10.1002/eji.1830210920. [DOI] [PubMed] [Google Scholar]
- Dyson P. J., Knight A. M., Fairchild S., Simpson E., Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991 Feb 7;349(6309):531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Rudy C., Coffin J. M., Huber B. T. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature. 1991 Feb 7;349(6309):526–528. doi: 10.1038/349526a0. [DOI] [PubMed] [Google Scholar]
- Golding H., Shearer G. M., Hillman K., Lucas P., Manischewitz J., Zajac R. A., Clerici M., Gress R. E., Boswell R. N., Golding B. Common epitope in human immunodeficiency virus (HIV) I-GP41 and HLA class II elicits immunosuppressive autoantibodies capable of contributing to immune dysfunction in HIV I-infected individuals. J Clin Invest. 1989 Apr;83(4):1430–1435. doi: 10.1172/JCI114034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkina T. V., Chervonsky A., Dudley J. P., Ross S. R. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell. 1992 May 15;69(4):637–645. doi: 10.1016/0092-8674(92)90227-4. [DOI] [PubMed] [Google Scholar]
- Groux H., Torpier G., Monté D., Mouton Y., Capron A., Ameisen J. C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992 Feb 1;175(2):331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulwani-Akolkar B., Posnett D. N., Janson C. H., Grunewald J., Wigzell H., Akolkar P., Gregersen P. K., Silver J. T cell receptor V-segment frequencies in peripheral blood T cells correlate with human leukocyte antigen type. J Exp Med. 1991 Nov 1;174(5):1139–1146. doi: 10.1084/jem.174.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Hawes G. E., Struyk L., van den Elsen P. J. Differential usage of T cell receptor V gene segments in CD4+ and CD8+ subsets of T lymphocytes in monozygotic twins. J Immunol. 1993 Mar 1;150(5):2033–2045. [PubMed] [Google Scholar]
- Held W., Waanders G. A., Shakhov A. N., Scarpellino L., Acha-Orbea H., MacDonald H. R. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell. 1993 Aug 13;74(3):529–540. doi: 10.1016/0092-8674(93)80054-i. [DOI] [PubMed] [Google Scholar]
- Hodara V. L., Jeddi-Tehrani M., Grunewald J., Andersson R., Scarlatti G., Esin S., Holmberg V., Libonatti O., Wigzell H. HIV infection leads to differential expression of T-cell receptor V beta genes in CD4+ and CD8+ T cells. AIDS. 1993 May;7(5):633–638. doi: 10.1097/00002030-199305000-00004. [DOI] [PubMed] [Google Scholar]
- Imberti L., Sottini A., Bettinardi A., Puoti M., Primi D. Selective depletion in HIV infection of T cells that bear specific T cell receptor V beta sequences. Science. 1991 Nov 8;254(5033):860–862. doi: 10.1126/science.1948066. [DOI] [PubMed] [Google Scholar]
- Janeway C. Immune recognition. Mls: makes a little sense. Nature. 1991 Feb 7;349(6309):459–461. doi: 10.1038/349459a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kappler J., Kotzin B., Herron L., Gelfand E. W., Bigler R. D., Boylston A., Carrel S., Posnett D. N., Choi Y., Marrack P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989 May 19;244(4906):811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Leung D. Y., Kappler J., Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- Labrecque N., McGrath H., Subramanyam M., Huber B. T., Sékaly R. P. Human T cells respond to mouse mammary tumor virus-encoded superantigen: V beta restriction and conserved evolutionary features. J Exp Med. 1993 Jun 1;177(6):1735–1743. doi: 10.1084/jem.177.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A., Roosnek E., Gregory T., Berman P., Abrignani S. T cells can present antigens such as HIV gp120 targeted to their own surface molecules. Nature. 1988 Aug 11;334(6182):530–532. doi: 10.1038/334530a0. [DOI] [PubMed] [Google Scholar]
- Laurence J., Hodtsev A. S., Posnett D. N. Superantigen implicated in dependence of HIV-1 replication in T cells on TCR V beta expression. Nature. 1992 Jul 16;358(6383):255–259. doi: 10.1038/358255a0. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature. 1991 Feb 7;349(6309):524–526. doi: 10.1038/349524a0. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Fauci A. S. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993 Feb 4;328(5):327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Koenig S., Baseler M., Lane H. C., Fauci A. S. Defective clonogenic potential of CD8+ T lymphocytes in patients with AIDS. Expansion in vivo of a nonclonogenic CD3+CD8+DR+CD25- T cell population. J Immunol. 1990 Mar 1;144(5):1696–1704. [PubMed] [Google Scholar]
- Posnett D. N., Kabak S., Hodtsev A. S., Goldberg E. A., Asch A. T-cell antigen receptor V beta subsets are not preferentially deleted in AIDS. AIDS. 1993 May;7(5):625–631. doi: 10.1097/00002030-199305000-00003. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Marrack P., Kappler J. W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988 Oct 27;335(6193):796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Wade T., Marrack P., Kappler J. W. Identification of the region of T cell receptor beta chain that interacts with the self-superantigen MIs-1a. Cell. 1990 Jun 29;61(7):1365–1374. doi: 10.1016/0092-8674(90)90700-o. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Soudeyns H., Rebai N., Pantaleo G. P., Ciurli C., Boghossian T., Sékaly R. P., Fauci A. S. The T cell receptor V beta repertoire in HIV-1 infection and disease. Semin Immunol. 1993 Jun;5(3):175–185. doi: 10.1006/smim.1993.1021. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Woodland D. L., Happ M. P., Gollob K. J., Palmer E. An endogenous retrovirus mediating deletion of alpha beta T cells? Nature. 1991 Feb 7;349(6309):529–530. doi: 10.1038/349529a0. [DOI] [PubMed] [Google Scholar]
- Woodland D., Happ M. P., Bill J., Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science. 1990 Feb 23;247(4945):964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]






