Abstract
Objective:
Generalized aggressive periodontitis (GAgP) is a complex periodontal disease affecting the entire dentition with a rapid destruction of the periodontium and resulting in loss of teeth. We hypothesized that better clinical healing of adjunctive use of amoxicillin plus metronidazole combination may be related to the effect of this combination therapy to restore imbalance between matrix metalloproteinases (MMP) and their tissue inhibitors (TIMP) which is associated with connective tissue and alveolar bone destruction in patients with GAgP.
Materials and Methods:
Twenty-eight subjects diagnosed with GAgP were recruited. Patients were randomly assigned to test or control groups. MMP-1/TIMP-1 ratio was compared between groups receiving scaling and root planning (SRP) alone (control) or in combination with amoxicillin plus metronidazole (test). Clinical periodontal variables were measured. Gingival crevicular fluid samples were obtained and analyzed for MMP-1 and TIMP-1. Measurements were taken at baseline and repeated at 3 and 6 months after therapy.
Results:
Total MMP-1 levels were significantly decreased in both groups (P < 0.05) at 3 and 6 months. MMP-1 concentration levels showed a similar pattern to MMP-1 total levels decreasing significantly at 3 months (P < 0.05). TIMP-1 concentration levels increased in the test group throughout the study period, while the difference did not reach statistical significance (P > 0.05). TIMP-1/MMP-1 balance was restored in test group at 6 months significantly better than the control group (P < 0.05).
Conclusion:
The results of this study suggest that metronidazole and amoxicillin combination as an adjunct to SRP results in better clinical healing through restoring TIMP-1/MMP-1 balance.
Keywords: Aggressive periodontitis, amoxicilline, matrix metalloproteinases-1, metronidazole, scaling and root planing, tissue inhibitors of matrix metalloproteinases-1
INTRODUCTION
Periodontal disease is initiated as an infection where the tissue destruction is associated with host's inflammatory response.[1] Generalized aggressive periodontitis (GAgP) affects at least two permanent teeth other than incisors and first molars. Rapid destruction of the periodontium results with loss of teeth through attachment and bone loss. Specific bacteria as Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Tannerella forsythia have been strongly associated with GAgP.[2,3,4] Although immunological and microbiological etiology of AgP differ from that of chronic periodontitis (CP), treatment strategies are largely similar. Mechanical therapy using non-surgical and surgical techniques is the primary approach. However, AgP has more severe progression, and adjunctive tools may be necessary to control rapid tissue destruction through minimizing pathogenic microflora. Metronidazole plus amoxicillin combination presents a good choice with its increased bactericidal efficacy and larger spectra compared with monotherapy with each drug and other antimicrobials.[5,6]
During the pathologic process of periodontitis, microbiological factors and host-mediated inflammation trigger cells of the periodontal tissues to release enzymes responsible of tissue turnover.[7] Matrix metalloproteinases (MMPs), a family of 28 known endopeptidases with activities against extracellular matrix macromolecules such as type I collagen, which is the main structural protein in connective tissues including the periodontium are closely associated with periodontal disease activity. MMPs share a number of structural and functional features while differ in substrate specificity. MMP-1, MMP-2, MMP-8, MMP-9, MMP-12 and MMP-13 are among the most frequently studied MMPs.[7,8,9] Biological inhibitors of MMPs, known as the tissue inhibitors of MMPs (TIMPs) balance their activity and restore tissue homeostasis.[10] Thus, tissue destruction correlates with an imbalance between MMPs and TIMPs where a disturbed balance is associated with various pathological conditions.[11] This process is an ongoing physiological event, through which the healthy and “normal” tissue structure can be maintained.[12]
Several studies have explored MMPs as biomarkers for periodontal disease progression[13,14,15] especially with the aggressive forms.[16,17] MMP-9 1562 gene T allele has been associated with a decreased risk of GAgP[12] while MMP-1 gene polymorphism showed an association with GAgP.[16] MMP-2, -9 and 13 were found to be significantly elevated in diseased sites of children with AgP compared to adults with CP and healthy controls.[17] A high MMP-8 was correlated with disease activity in gingival crevicular fluid (GCF) from patients with progressive CP.[13] Several types of MMPs were associated with the enhanced severity of periodontal inflammation, indicating that these molecules could participate in the regulation of progression of periodontal diseases.[18,19,20] A recent study has further suggested that periodontal treatment has increased TIMP-1 expression and decreased the ratio of MMP to TIMP-1 in CP patients.[21]
Among various MMPs, MMP-8 is released primarily by polymorphonuclear granulocytes (neutrophils) and MMP-1 is produced by fibroblasts and keratinocytes during healing and by defense cells during inflammation to cleave type I and II collagen and regarded as key players in periodontal pathogenesis. MMP-1 may serve as an initiator of periodontal destruction and overexpression of MMP-1 may lead to accelerated matrix degradation in pathologic conditions as periodontitis.[21,22,23,24] Proteolytic activity of MMP-1 is controlled through TIMP-1 during healing and inflammatory processes.[25] The ratio of MMP-1/TIMP-1 has been proposed to be an indicator for wound healing.[26] This ratio has also been shown to decrease after non-surgical periodontal therapy.[20] Discovery of MMP's being associated with periodontal destruction has led the investigators to explore the possibility of inhibiting MMP activity using therapeutic agents such as tetracyclines and subantimicrobial doxycyclines (SDD).[27,28] We have published before clinical and microbiological data of the same patient group using metronidazole and amoxicillin in combination as adjunct to non-surgical periodontal therapy. We reported that the polypharmaceutical approach results in significant and substantial decrease in T. forsythia and prevents its recolonization for 6 months.[29] Now, in this paper we wanted to test the hypothesis that metronidazole and amoxicillin as an adjunct to non-surgical periodontal therapy may lead to a better clinical healing through restoring the balance between MMP-1 and TIMP-1 in patients with GAgP.
MATERIALS AND METHODS
Study population
A total of 28 patients consisting 19 females and 9 males aged 15–45 recruited between 2004 and 2006 to participate in the study. As GAgP has a larger age profile compared to localized type AgP, young individuals and adults were among the subjects. Subjects recruited from patients referred to the Department of Periodontology at Istanbul University Faculty of Dentistry. A detailed oral and systemic anamnesis form was fulfilled asking a series of questions to decide if the patients were meeting criteria of the study. Included patients were diagnosed with GAgP according to criteria of the American Academy of Periodontology.[30] Inclusion criteria were as follows: At least 20 teeth, at least two teeth in each quadrant with a pocket depth of at least 5 mm around, and attachment and bone loss around at least three teeth other than incisors and first molars. Exclusion criteria included having restorations on teeth to be sampled; being pregnant, lactating or allergic to the drugs used in the study; smoking more than 10 cigarettes/day; having systemic conditions that could interfere with periodontal tissues, disease progression or response to periodontal therapy; having previous periodontal treatment. The study protocol was approved by the Ethical Committee of Istanbul University. All patients were informed about the nature of the study and signed informed consent was obtained from all individuals.
Clinical measurements and treatment protocol
Study patients were randomly assigned to test and control groups by the flipping of a coin by one of the investigators (SC) who was not involved in the clinical procedures. All indices were recorded at baseline and repeated at 3 and 6 months after therapy by one of the investigators who was blinded to the randomization. Clinical parameters included plaque index (PI) (Silness-Löe),[31] gingival index (GI) (Löe-Silness),[32] periodontal probing depth (PD), and clinical attachment level (CAL). CAL was calculated by measuring the distance between cemento-enamel junction and bottom of the sulcus. Measurements were performed at six sites/tooth, excluding the third molars, using a UNC-15 periodontal probe (PCP-UNC-15, Hu-Friedy, Chicago, IL). An intraclass correlation coefficiency of 0.85 for PD measurements indicated that the examiner reliability was high. Subjects in the test group received scaling and root planning (SRP) and metronidazole (Mustafa Nevzat Pharmaceuticals, Istanbul, Turkey) (500 mg, 3 × 1)-amoxicillin (Eczacibasi Pharmaceuticals, Istanbul, Turkey) (500 mg, 3 × 1) combination while the control group received SRP alone. SRP was performed in two consecutive visits in 1 week. Systemic antibiotics were administered during the first session and continued for 7 days. All patients were treated by another investigator (EC) who was blinded to the group designations and to which subjects were receiving the medication. This investigator also provided clinical measurements and sampling. The treatment codes of the study were not available to the treating investigator until the data had been analyzed by a statistician (HI). After recruitment, clinical evaluations were carried out and sample sites determined. All patients were instructed on oral hygiene and monitored throughout the study period. All subjects were followed-up monthly for oral hygiene instructions and supragingival scaling as necessary.
Gingival crevicular fluid sampling
Gingival crevicular fluid sampling were conducted at baseline and repeated after 3 and 6 months. GCF samples were collected from the mesiobuccal aspect of the deepest single-rooted tooth of each patient with a PD of at least 5 mm. One sample was collected at each appointment. A total of three samples were collected from each patient through the study period. The same site was sampled at each time-point. Sample collections were performed 1 week after the clinical measurements. Prior to GCF sampling, the supragingival plaque was removed from the interproximal surfaces with a sterile curette. These surfaces were dried gently by an air syringe and were isolated by cotton rolls. GCF was sampled with a filter paper. Harco Electronics, Winnipeg, Canada paper strips were carefully inserted into the crevice until mild resistance was felt and left there for 30 s.[33] Care was taken to avoid mechanical injury. Strips contaminated with blood were discarded.[34] The absorbed volume of each strip was determined by a Periotron 6000 (TIMP-1 ELISA kit: Biosource International, Inc., CA, USA), after which the strips were placed in sterile Eppendorf vials and stored at − 80°C until analysis. Two parameters were analyzed from the same one strip. The readings from the Periotron 6000 were converted to an actual volume (ml) by referring to the standard curve.
Matrix metalloproteinases-1 and tissue inhibitors matrix metalloproteinases-1 analyses
Gingival crevicular fluid was retrieved from the filter strips by eluting in 250-μl phosphate-buffered saline solution (Tween buffer) for 30 min and incubating over a shaking platform overnight. The total levels of MMP-1 and TIMP-1 in the GCF samples were determined using a commercially available human-specific enzyme-linked immunosorbent assay according to the manufacturers’ instructions (TIMP-1 ELISA kit: Biosource International, Inc., CA, USA, MMP-1 kit: Calbiochem, Canada). The detection limit of the MMP-1 kit was 0.023 ng/ml, and that of the TIMP-1 kit was 1 ng/ml. The optical densities at a wavelength of 450 nm were measured.
Data analysis
Prior to initiation of this study, sample size and power were calculated. Type-1 error was assumed at 0.05, type-2 error at 0.02, and power was assumed at 80% prediction of a reduction by 30% between baseline and 3 months. The minimum sample size was 12 subjects for each group. To compensate for potential dropouts, 16 patients were initially recruited per treatment group. For the clinical measurements, average whole mouth recordings were accepted as a unit. Repeated measures of analysis of variance and paired samples t-tests were used to detect intra-group differences and Student's t-tests for inter-group differences in clinical measurements and biochemical findings. Statistical significance was accepted as a value of P < 0.05. All results were evaluated at a 95% confidence interval. The Number Cruncher Statistical System-Power Analysis and Sample Size statistical software was used for analyses (Pass 2008/NCSS 2007, NCSS, Kaysville, UT).
RESULTS
Demographic and clinical findings
The demographic information of study participants are shown in Table 1. There was no difference in terms of mean age and gender distribution in both groups (P > 0.05). Besides, there was no subject in any group who smoked more than 10 cigarettes/day. Figure 1 presents the clinical findings. There was a statistically significant improvement in plaque scores between baseline and 3 months and between baseline and 6 months in both groups (P < 0.05), with no significant differences between 3 and 6 months (P > 0.05). Similar to the PI scores, the GI decreased significantly in both groups at the end of 3 and 6 months compared to the baseline (P < 0.05), with no significant difference between the test and control groups (P > 0.05). Both treatment modalities were successful on PD measurements and CAL over 3 and 6 months compared to the baseline (P < 0.05; respectively). No significant differences were observed between the groups (P > 0.05). A detailed evaluation of the clinical findings and response to SRP alone and adjunct with antibiotics based on PD and CAL was published before (Yek et al. 2010). According to these analyses, both treatment strategies resulted in significant improvement, however the test group had a statistically significant reduction in the percentage of PD ≥ 7 mm compared to the control group at 3 and 6 months (P < 0.05).
Table 1.
Demographic features and clinical characteristics of study patients at baseline
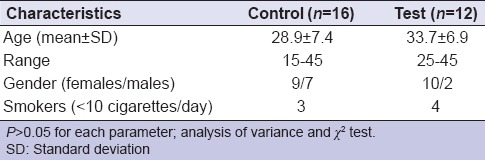
Figure 1.
Clinical findings in study groups. *P < 0.05 compared to baseline. Repeated measures of analysis of variance and generalized linear model were used to detect intragroup and intergroup differences in plaque index, gingival index, probing depth, and clinical attachment level
Matrix metalloproteinases-1 and tissue inhibitors matrix metalloproteinases-1 levels and matrix metalloproteinases-1/tissue inhibitors matrix metalloproteinases-1 ratio in gingival crevicular fluid
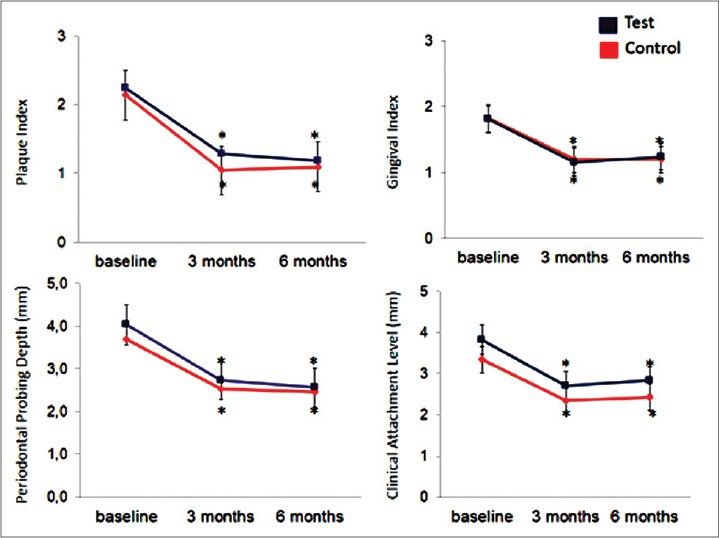
Figure 2 shows the changes in concentration and total levels of MMP-1 and TIMP-1 in the test and control groups during the course of the study. Total TIMP-1 levels did not significantly change at 3 months or after 6 months in study groups (P > 0.05). A pattern similar to that of total TIMP-1 levels was observed for the TIMP-1 concentration in the control group. Compared to the control group, the increase in test group was more stable for the concentration of TIMP-1 while the difference was not statistically significant (P > 0.05). Total MMP-1 levels were significantly decreased in both groups (P < 0.05) at 3 and 6 months. When we evaluated the MMP-1 concentration levels, a similar pattern was observed; the decrease at 3 months was significant (P < 0.05).
Figure 2.
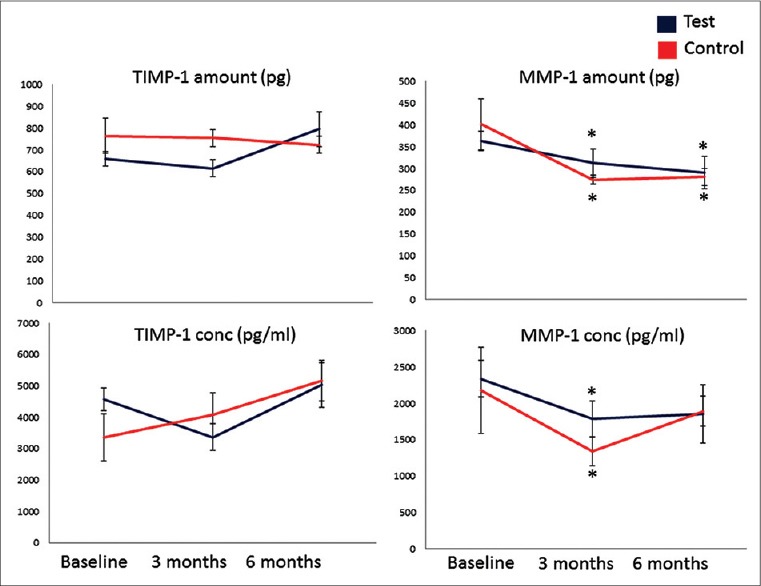
Tissue inhibitors of matrix metalloproteinases (TIMP-1) and MMP-1 levels in gingival crevicular fluid. *P < 0.05 compared to baseline. Repeated measures of analysis of variance and generalized linear model were used to detect intragroup and intergroup differences
Figure 3 demonstrates that SRP with adjunctive antibiotics changes the ratio of TIMP-1/MMP-1 at 6 months significantly better than the control group (P < 0.05). Further analysis of the TIMP-1/MMP-1 ratio based on the pocket depth demonstrated that the improvement was significantly better in the test group compared to the control group in deep pockets (P < 0.05) while there was no difference in shallow and moderately deep sites with <7 mm of PD [Figure 4].
Figure 3.
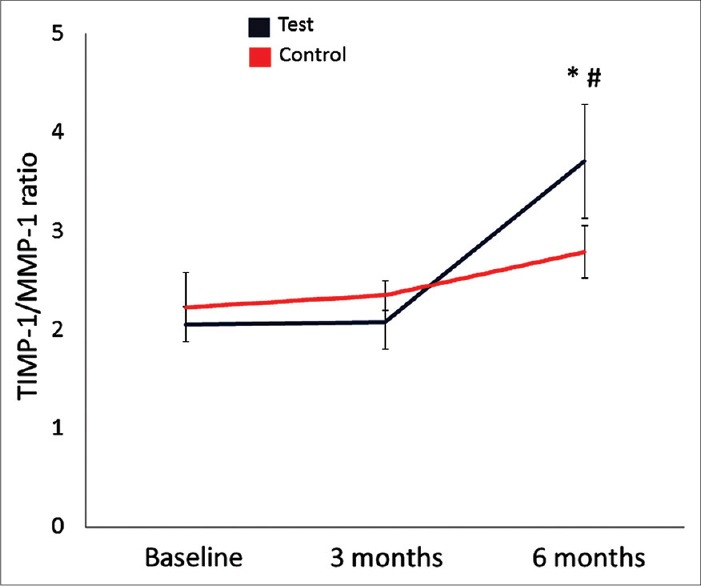
Tissue inhibitors of matrix metalloproteinases (TIMP-1)/MMP-1 ratio in gingival crevicular fluid. *P < 0.05 compared to baseline; #P < 0.05 compared to the control group
Figure 4.
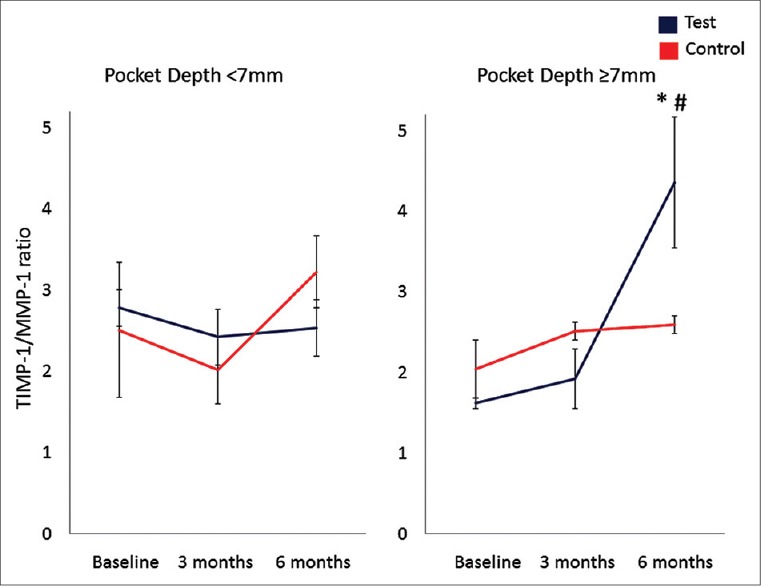
Tissue inhibitors of matrix metalloproteinases (TIMP-1)/MMP-1 ratio in gingival crevicular fluid based on the initial pocket depth. *P < 0.05 compared to baseline; #P < 0.05 compared to the control group
DISCUSSION
The aim of this randomized clinical trial was to evaluate the impact of systemic metronidazole–amoxicillin therapy as an adjunct to non-surgical therapy in patients with GAgP on the MMP-1 and TIMP-1 content in the GCF. The outcome of treatment was evaluated over a period of 6 months. The findings indicate that both treatment modalities resulted in significant and stable improvements in clinical parameters with reduced clinical inflammation, pocket depth and a gain in CAL. Deeper pockets benefited from the adjunctive use of the antibiotic combination and with a more stable healing compared to the shallow and moderately deep pockets. The clinical data were supported by the restoration of a more favorable balance between TIMP-1 and MMP-1 collectively suggesting that adjunctive use of amoxicillin and metronidazole facilitates the healing in areas where SRP alone may have a limited access and efficacy.
Treatment of AgP is a challenge for clinicians. As the disease progression is severe and rapid, adjunctive treatment is usually necessary to improve the outcomes of the therapy. In view of the specific bacterial etiology, systemic antibiotics can be a valuable choice of adjunctive therapy of AgP. As there is a specific bacterial biofilm in the etiology, this pathological structure should first be disrupted mechanically. Hence, mechanical therapy must precede systemic antibiotic therapy as biofilm can protect bacteria from the effects of antibiotic.[35] Among the antibiotics that have been studied for the treatment of AgP,[6,36,37] metronidazole plus amoxicilline presents comparatively a better choice for the improvement of clinical parameters as reported by Sgolastra et al.[37] Recently, Emingil et al. used azithromycin for the management AgP, however they found no additional benefit over non-surgical periodontal treatment on either clinical and microbiological parameters or in gingival crevicular fluid biochemical markers investigated in patients with GAgP.[6]
In periodontitis, the degradation of extracellular matrix is thought to be induced by an imbalancement between MMPs and their specific inhibitors TIMPs.[38,39] To the best of our knowledge this is the only study evaluating the effects of the amoxicilline and metronidazole combination therapy as an adjunct to SRP on MMP-1 and TIMP-1 levels in GCF of GAgP patients. Up to date there is a very limited number of studies reporting the effects of different treatment choices as adjunct to SRP for AgP or chronic periodontitis on MMP levels. In these limited number of studies authors have reported a decrease in MMP-1 levels after SRP with or without any other kind of adjunctive therapy (SDD, ozonotherapy, laser, combined antibiotics).[40,41,42,43] Recently, Emingil et al. demonstrated that MMP-8 levels in the GCF significantly decreased over a 6-month period after periodontal treatment, along with clinical and microbiological improvements.[6] However, the authors utilized either SRP or adjunctive azithromycin therapy in a population of generalized GAgP patients. Another study by Skurska et al. evaluated salivary MMP levels after SRP alone or with ozonotherapy in patients with AgP and CP.[40] Adjunctive treatment in this study in AgP group did not result in better healing either in a clinical situation or MMP levels contrary to our study that used combined antibiotic therapy as adjunctive treatment.
Very recently, the same combination therapy was reported by Gonçalves et al.[41] to substantially decrease MMP levels after SRP at 3 and 6 months parallel to our findings. However, in that study the subject population consisted of Localized AgP patients. All these studies reported different amount of decrease or no decrease at different time points in MMP levels. In another recent study by Saglam et al., diode laser was applied as adjunct to SRP and GCF levels of MMP-1, MMP-8, TIMP-1.[43] The total amounts of MMP-1, MMP-8, and TIMP-1 decreased after treatment in SRP alone and SRP plus laser groups (P < 0.05). This study was carried out in a group with CP. In our study, TIMP-1 levels were not affected by SRP or adjunctive therapy contrary to the findings of this study. The differences in the amount of reductions in MMP levels may be related to different treatment approaches, different methods of detection and disease severity.
Matrix metalloproteinases play a crucial role in tissue homeostasis, for which the balance between MMPs and TIMPs is important. Various periodontal pathogens such as P. gingivalis, T. forsythia, and F. nucleate are known to induce secretion of MMPs by host cells.[44,45,46] P. gingivalis is also known to secrete MMPs, albeit in quantities much less than that produced by host cells; promotes the activity of the host-derived MMP-2 and MMP-1, suggesting a pathological role in the progression of periodontitis.[47] In our previous study on the same patient group, we have reported that T. forsythia, T. denticola, P. intermedia and P. gingivalis were the most prevalent bacteria isolated from all sites at baseline.[29] Reductions in these bacterial species taken together with the current findings, suggest that host-mediated resolution of the inflammation and restoration of the homeostasis is critical for tissue healing. These observations are in parallel with the literature on the SDD and chemically modified tetracyclines.
In summary, SRP procedure has an impact on good healing of the periodontal situation in clinical and biochemical level in patients with AgP. Combination of the selected antibiotics caused further improvement in clinical periodontal parameters in deeper pockets and the levels of MMP-1 and TIMP-1/MMP-1 ratio. Our data also suggest that the combination of amoxicillin and metronidazole is a valuable approach to both eliminations of the bacteria and stabilizing the MMP-1/TIMP-1 ratio during treatment of the GAgP.
Weakness and strength of the study
We were able to measure the volume of the strips; therefore, we could calculate the concentrations as well as the total amounts. In the present study, only a single combination of TIMP/MMP was assessed, and this could indicate the tissue response only in part. Other related members and their specific indicators should be investigated to be able to evaluate the exact role of systemic antibiotics on soft tissue response to mechanical therapy. GCF was used as a tool to assess MMP and TIMP as the content of this unique fluid is accepted as an accurate representation of tissue response. The difference observed in the clinical parameters of the deeper sites related to adjunctive antibiotics as confirmed by the change in MMP-1/TIMP-1 ratio as the main finding of the present study can in part be attributed to the hawthorn effect, as the control group did not use placebo pills as a limitation of the present study. Sample size of the study is comparatively limited and could be expanded to increase power of the study.
Footnotes
Source of Support: This study was supported by the Research Fund of Istanbul University (project no. T657/170305).
Conflict of Interest: None declared
REFERENCES
- 1.Kornman KS. Mapping the pathogenesis of periodontitis: A new look. J Periodontol. 2008;79(8 Suppl):1560–8. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- 2.Tolo K, Schenck K. Activity of serum immunoglobulins G, A, and M to six anaerobic, oral bacteria in diagnosis of periodontitis. J Periodontal Res. 1985;20:113–21. doi: 10.1111/j.1600-0765.1985.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho RP, Mesquita JS, Bonomo A, Elsas PX, Colombo AP. Relationship of neutrophil phagocytosis and oxidative burst with the subgingival microbiota of generalized aggressive periodontitis. Oral Microbiol Immunol. 2009;24:124–32. doi: 10.1111/j.1399-302X.2008.00484.x. [DOI] [PubMed] [Google Scholar]
- 4.Faveri M, Mayer MP, Feres M, de Figueiredo LC, Dewhirst FE, Paster BJ. Microbiological diversity of generalized aggressive periodontitis by 16S rRNA clonal analysis. Oral Microbiol Immunol. 2008;23:112–8. doi: 10.1111/j.1399-302X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 5.Pavicic MJ, van Winkelhoff AJ, Douqué NH, Steures RW, de Graaff J. Microbiological and clinical effects of metronidazole and amoxicillin in Actinobacillus actinomycetemcomitans-associated periodontitis. A 2-year evaluation. J Clin Periodontol. 1994;21:107–12. doi: 10.1111/j.1600-051x.1994.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 6.Emingil G, Han B, Ozdemir G, Tervahartiala T, Vural C, Atilla G, et al. Effect of azithromycin, as an adjunct to nonsurgical periodontal treatment, on microbiological parameters and gingival crevicular fluid biomarkers in generalized aggressive periodontitis. J Periodontal Res. 2012;47:729–39. doi: 10.1111/j.1600-0765.2012.01488.x. [DOI] [PubMed] [Google Scholar]
- 7.Pussinen PJ, Paju S, Mäntylä P, Sorsa T. Serum microbial- and host-derived markers of periodontal diseases: A review. Curr Med Chem. 2007;14:2402–12. doi: 10.2174/092986707781745604. [DOI] [PubMed] [Google Scholar]
- 8.Sexton WM, Lin Y, Kryscio RJ, Dawson DR, 3rd, Ebersole JL, Miller CS. Salivary biomarkers of periodontal disease in response to treatment. J Clin Periodontol. 2011;38:434–41. doi: 10.1111/j.1600-051X.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–84. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 10.Verstappen J, Von den Hoff JW. Tissue inhibitors of metalloproteinases (TIMPs): Their biological functions and involvement in oral disease. J Dent Res. 2006;85:1074–84. doi: 10.1177/154405910608501202. [DOI] [PubMed] [Google Scholar]
- 11.López-Otín C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–8. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 12.Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: Destruction or repair? Nat Clin Pract Rheumatol. 2008;4:128–35. doi: 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- 13.Hernández M, Gamonal J, Tervahartiala T, Mäntylä P, Rivera O, Dezerega A, et al. Associations between matrix metalloproteinase-8 and -14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: A longitudinal study. J Periodontol. 2010;81:1644–52. doi: 10.1902/jop.2010.100196. [DOI] [PubMed] [Google Scholar]
- 14.Reinhardt RA, Stoner JA, Golub LM, Lee HM, Nummikoski PV, Sorsa T, et al. Association of gingival crevicular fluid biomarkers during periodontal maintenance with subsequent progressive periodontitis. J Periodontol. 2010;81:251–9. doi: 10.1902/jop.2009.090374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota T, Itagaki M, Hoshino C, Nagata M, Morozumi T, Kobayashi T, et al. Altered gene expression levels of matrix metalloproteinases and their inhibitors in periodontitis-affected gingival tissue. J Periodontol. 2008;79:166–73. doi: 10.1902/jop.2008.070159. [DOI] [PubMed] [Google Scholar]
- 16.Cao Z, Li C, Jin L, Corbet EF. Association of matrix metalloproteinase-1 promoter polymorphism with generalized aggressive periodontitis in a Chinese population. J Periodontal Res. 2005;40:427–31. doi: 10.1111/j.1600-0765.2005.00806.x. [DOI] [PubMed] [Google Scholar]
- 17.Alfant B, Shaddox LM, Tobler J, Magnusson I, Aukhil I, Walker C. Matrix metalloproteinase levels in children with aggressive periodontitis. J Periodontol. 2008;79:819–26. doi: 10.1902/jop.2008.070513. [DOI] [PubMed] [Google Scholar]
- 18.Emingil G, Tervahartiala T, Mãntylã P, Määttä M, Sorsa T, Atilla G. Gingival crevicular fluid matrix metalloproteinase (MMP)-7, extracellular MMP inducer, and tissue inhibitor of MMP-1 levels in periodontal disease. J Periodontol. 2006;77:2040–50. doi: 10.1902/jop.2006.060144. [DOI] [PubMed] [Google Scholar]
- 19.Emingil G, Kuula H, Sorsa T, Atilla G. Gingival crevicular fluid matrix metalloproteinase-25 and -26 levels in periodontal disease. J Periodontol. 2006;77:664–71. doi: 10.1902/jop.2006.050288. [DOI] [PubMed] [Google Scholar]
- 20.Tüter G, Kurtis B, Serdar M, Yücel A, Ayhan E, Karaduman B, et al. Effects of phase I periodontal treatment on gingival crevicular fluid levels of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1. J Clin Periodontol. 2005;32:1011–5. doi: 10.1111/j.1600-051X.2005.00816.x. [DOI] [PubMed] [Google Scholar]
- 21.Mouzakiti E, Pepelassi E, Fanourakis G, Markopoulou C, Tseleni-Balafouta S, Vrotsos I. Expression of MMPs and TIMP-1 in smoker and nonsmoker chronic periodontitis patients before and after periodontal treatment. J Periodontal Res. 2012;47:532–42. doi: 10.1111/j.1600-0765.2011.01465.x. [DOI] [PubMed] [Google Scholar]
- 22.Sorsa T, Tjäderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10:311–8. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 23.Peake NJ, Khawaja K, Myers A, Jones D, Cawston TE, Rowan AD, et al. Levels of matrix metalloproteinase (MMP)-1 in paired sera and synovial fluids of juvenile idiopathic arthritis patients: Relationship to inflammatory activity, MMP-3 and tissue inhibitor of metalloproteinases-1 in a longitudinal study. Rheumatology. 2005;44:1383–9. doi: 10.1093/rheumatology/kei025. [DOI] [PubMed] [Google Scholar]
- 24.Myers A, Lakey R, Cawston TE, Kay LJ, Walker DJ. Serum MMP-1 and TIMP-1 levels are increased in patients with psoriatic arthritis and their siblings. Rheumatology (Oxford) 2004;43:272–6. doi: 10.1093/rheumatology/keh032. [DOI] [PubMed] [Google Scholar]
- 25.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 26.Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: The ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med. 2008;25:419–26. doi: 10.1111/j.1464-5491.2008.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golub LM, McNamara TF, Ryan ME, Kohut B, Blieden T, Payonk G, et al. Adjunctive treatment with subantimicrobial doses of doxycycline: Effects on gingival fluid collagenase activity and attachment loss in adult periodontitis. J Clin Periodontol. 2001;28:146–56. doi: 10.1034/j.1600-051x.2001.028002146.x. [DOI] [PubMed] [Google Scholar]
- 28.Emingil G, Gürkan A, Atilla G, Kantarci A. Subantimicrobial-dose doxycycline and cytokine-chemokine levels in gingival crevicular fluid. J Periodontol. 2011;82:452–61. doi: 10.1902/jop.2010.100036. [DOI] [PubMed] [Google Scholar]
- 29.Yek EC, Cintan S, Topcuoglu N, Kulekci G, Issever H, Kantarci A. Efficacy of amoxicillin and metronidazole combination for the management of generalized aggressive periodontitis. J Periodontol. 2010;81:964–74. doi: 10.1902/jop.2010.090522. [DOI] [PubMed] [Google Scholar]
- 30.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 32.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 33.Lamster IB, Mandella RD, Gordon JM. Lactate dehydrogenase activity in gingival crevicular fluid collected with filter paper strips: Analysis in subjects with non-inflamed and mildly inflamed gingiva. J Clin Periodontol. 1985;12:153–61. doi: 10.1111/j.1600-051x.1985.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 34.Cimasoni G. Crevicular fluid updated. Monogr Oral Sci. 1983;12:III–VII. 1. [PubMed] [Google Scholar]
- 35.Mombelli A. Heresy? Treatment of chronic periodontitis with systemic antibiotics only. J Clin Periodontol. 2006;33:661–2. doi: 10.1111/j.1600-051X.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- 36.Haas AN, de Castro GD, Moreno T, Susin C, Albandar JM, Oppermann RV, et al. Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial. J Clin Periodontol. 2008;35:696–704. doi: 10.1111/j.1600-051X.2008.01254.x. [DOI] [PubMed] [Google Scholar]
- 37.Sgolastra F, Petrucci A, Gatto R, Monaco A. Effectiveness of systemic amoxicillin/metronidazole as an adjunctive therapy to full-mouth scaling and root planing in the treatment of aggressive periodontitis: A systematic review and meta-analysis. J Periodontol. 2012;83:731–43. doi: 10.1902/jop.2011.110432. [DOI] [PubMed] [Google Scholar]
- 38.Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26:230–42. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 39.Tüter G, Kurtis B, Serdar M. Effects of phase I periodontal treatment on gingival crevicular fluid levels of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1. J Periodontol. 2002;73:487–93. doi: 10.1902/jop.2002.73.5.487. [DOI] [PubMed] [Google Scholar]
- 40.Skurska A, Pietruska MD, Paniczko-Drezek A, Dolinska E, Zelazowska-Rutkowska B, Zak J, et al. Evaluation of the influence of ozonotherapy on the clinical parameters and MMP levels in patients with chronic and aggressive periodontitis. Adv Med Sci. 2010;55:297–307. doi: 10.2478/v10039-010-0048-x. [DOI] [PubMed] [Google Scholar]
- 41.Gonçalves PF, Huang H, McAninley S, Alfant B, Harrison P, Aukhil I, et al. Periodontal treatment reduces matrix metalloproteinase levels in localized aggressive periodontitis. J Periodontol. 2013;84:1801–8. doi: 10.1902/jop.2013.130002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tüter G, Kurtis B, Serdar M, Aykan T, Okyay K, Yücel A, et al. Effects of scaling and root planing and sub-antimicrobial dose doxycycline on oral and systemic biomarkers of disease in patients with both chronic periodontitis and coronary artery disease. J Clin Periodontol. 2007;34:673–81. doi: 10.1111/j.1600-051X.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 43.Saglam M, Kantarci A, Dundar N, Hakki SS. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: A randomized, controlled clinical trial. Lasers Med Sci. 2014;29:37–46. doi: 10.1007/s10103-012-1230-0. [DOI] [PubMed] [Google Scholar]
- 44.Söder B, Airila Månsson S, Söder PO, Kari K, Meurman J. Levels of matrix metalloproteinases-8 and -9 with simultaneous presence of periodontal pathogens in gingival crevicular fluid as well as matrix metalloproteinase-9 and cholesterol in blood. J Periodontal Res. 2006;41:411–7. doi: 10.1111/j.1600-0765.2006.00888.x. [DOI] [PubMed] [Google Scholar]
- 45.Ding Y, Haapasalo M, Kerosuo E, Lounatmaa K, Kotiranta A, Sorsa T. Release and activation of human neutrophil matrix metallo- and serine proteinases during phagocytosis of Fusobacterium nucleatum, Porphyromonas gingivalis and Treponema denticola. J Clin Periodontol. 1997;24:237–48. doi: 10.1111/j.1600-051x.1997.tb01837.x. [DOI] [PubMed] [Google Scholar]
- 46.Kumagai Y, Yagishita H, Yajima A, Okamoto T, Konishi K. Molecular mechanism for connective tissue destruction by dipeptidyl aminopeptidase IV produced by the periodontal pathogen Porphyromonas gingivalis. Infect Immun. 2005;73:2655–64. doi: 10.1128/IAI.73.5.2655-2664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Windsor LJ. Porphyromonas gingivalis affects host collagen degradation by affecting expression, activation, and inhibition of matrix metalloproteinases. J Periodontal Res. 2006;41:47–54. doi: 10.1111/j.1600-0765.2005.00835.x. [DOI] [PubMed] [Google Scholar]


