Abstract
Objective:
The purpose of this in vitro study was to compare the cytotoxicity of 0.5–4.5 origanum extract solution (OES), 2% chlorhexidine gluconate (CHX) and 5.25% sodium hypochlorite (NaOCl) with WST-1 test on human periodontal ligament (hPDL) fibroblasts.
Materials and Methods:
About 0.5–4.5% OES, 2% CHX and 5.25% NaOCl solutions cytotoxicity was evaluated with cell culture test using PDL fibroblasts. Viability of hPDL cells was evaluated with WST-1 (Cell Proliferation Reagent WST-1 Roche) test at 1, 24 and 72nd h. hPDL cells were plated at 20 × 103 cells per well in 96-well plates. Absorbance values were read in optical density 480 nm by ELISA plate reader spectrophotometer. The statistical differences between various groups were evaluated using one-way ANOVA, post-hoc Duncan's Multiple Range test using SAS software. Statistically, a significant difference was considered at P < 0.001.
Results:
According to the 1-h cytotoxicity results, 0.5% OES showed the least cytotoxic effect in test groups. There were not found any statistical significance between 1% OES and 2% CHX. About 5.25% NaOCl showed more cytotoxic effect than 1% OES and 2% CHX. In 24 and 72 h, different concentrations of OES, 5.25% NaOCl, 2% CHX solutions showed similar cytotoxic effect.
Conclusions:
Based on these results, 1% OES and 2% CHX showed similar results and less cytotoxic effect than 5.25% NaOCl. It could be considered as a favorable solution concentration when OES was used as root canal irrigation solution.
Keywords: Chlorhexidine gluconate, cytotoxicity tests, origanum extract solution, sodium hypochlorite, WST-1 test
INTRODUCTION
Success of root canal treatment relies on the proper removal of pulpal remnant, bacteria and their byproducts from the root canal system.[1] Irrigation of the root canal system is one of the essential steps for the elimination of the bacteria and their byproducts effectively.[2] Therefore, ideal irrigation solution has to be a proper antibacterial and tissue dissolving effect on the necrotic pulp remnant and minimum toxic effect on the periapical tissue.[3,4]
Sodium hypochlorite (NaOCl) has been used for root canal irrigation for a long time because of its good antibacterial and tissue dissolving effects.[5] However, it is harmful when in contact with periapical tissue.[3,6,7,8] Chlorhexidine gluconate (CHX) has been suggested as an irrigation solution due to it has antimicrobial effect and binding on soft tissue and hydroxyapatite crystal.[9] However, the main disadvantage of CHX is the lack of organic tissue dissolution capabilities. Hence, different materials had been experimented alternatively to NaOCl and CHX as irrigation solutions.
Origanum minutiflorum is a plant and widespread in the eastern Mediterranean region and southwestern of Anatolia especially in Isparta, Turkey.[10,11] Its extract especially its oil and solution has an antimicrobial effect to a lot of microorganisms. Dadalioglu and Evrendilek[12] have reported that O. minutiflorum oil has an antimicrobial effect to Escherichia coli, Listeria monocytogenes, Salmonella typhimurium and Staphylococcus aureus and Simalary. Baydar et al.[13] has found 1% and 2% Origanum oil has an antimicrobial effect on Enterococcus faecalis (E. faecalis). Origanum solution was obtained from origanum oils distillation water. In a study, Ok[14] tried origanum extract solution (OES), which is mainly rich in carvacrol and thymol, and concluded that OES has an antibacterial effect against E. faecalis within the root canals alternative to NaOCl and CHX. He also reported that OES has dissolving effect on the organic compound of smear layer.[14]
The aim of this in vitro study was to evaluate and compare the cytotoxicity of 0.5–4.5% OES with 5.25% NaOCl and 2% CHX using WST-1 test on human periodontal ligament (hPDL) fibroblasts.
MATERIALS AND METHODS
Obtaining EOS
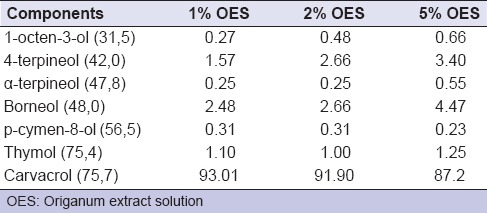
Origanum minutiflorum plant materials were dried in drying cupboard at 35°C, then they distillated in Clevenger apparatus for 3 h and origanum oil was obtained. OES, which 0.5%, 1%, 1.5%, 2%, 2.5%, 3%, 3.5%, 4%, 4.5% concentration, was obtained from mixing 100 mL distilled water and 0.5%, 1%, 1.5%, 2%, 2.5%, 3%, 3.5%, 4%, 4.5% origanum oil respectively. Gas chromatography (GC) analysis of the essential oil was performed using a Perkin Elmer Autosystem GC-type (Perkin Elmer Auto System XL, MA, USA) chromatograph. GC analysis of OES is shown in Table 1. Experimental groups in the present study were composed of different concentrations (0.5–4.5%) of OES, 2% CHX (Drogsan, Ankara, Turkiye), 5.25% NaOCl (Wizard, Ankara, Turkiye) and a control group containing only culture medium.
Table 1.
Chemical composition of the oregano solution (percentage of total peak area)
Cell culture
Cytotoxicity of solutions was evaluated on cultured hPDL fibroblast cells in Research Laboratory, Selcuk University, Faculty of Dentistry, Konya, Turkey. The cells were grown in 96-well polystyrene plates containing Dulbecco's modified Eagle's medium (DMEM) (Biological Industries, Kibbutz Beik Haemek, Israel), supplemented with 10% fetal bovine serum (FBS) (Biological Industries, Kibbutz Beik Haemek, Israel), 250 μg/ml gentamycin sulfate, (Biological Industries, Kibbutz Beik Haemek, Israel), 5 μg/ml amphotericin B (Biological Industries, Kibbutz Beik Haemek, Israel), and were incubeted in a humidified atmosphere, 95% air and 5% CO2 at 37°C for 24 h in water based incubator (NUAIRE, Fernbrook Lane N Plymouth, USA).
Cell proliferation assay
The cells were seeded in 96-well culture polystyrene plates at a fixed number of 20 × 103 cells per well. Then the cells were treated with 0.5%, 1%, 1.5%, 2%, 2.5%, 3%, 3.5%, 4%, 4.5% OES concentrations, 2% CHX and 5.25% NaOCl were placed into 96-well culture polystyrene plates. Then the cells were incubated for 24 h before applying the WST-1 cell proliferation assay reagent according to the recommendation of the manufacturer.
Cytotoxicity test
Viability of hPDL cells was evaluated with WST-1 (Roche, Basel, Switzerland) test at 1, 24 and 72th h. The test was carried out according to the protocol described by Babich et al.[15] WST-1 (4- [3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]- 1,3-benzene disulfonate) test is based on the cleavage of the tetrazolium salt WST-1 (slightly red) to formazan (dark red) by different mitochondrial dehydrogenase enzymes.[16]
This study was performed in dark medium at room temperature. In preparation stage, 18.9 ml DMEM with 5% FBS and 2.1 ml WST-1 was mixed, and totally 22 ml solution was prepared. WST-1 was diluted with the rate of 1/10 of 5% FBS DMEM. After 1 h incubation, 200 μl rate of 1:10 WST-1 including 5% FBS DMEM added to each well. The plates were wrapped with aluminum foil, placed into the incubator for 2 h, and then shaken for 1 min at room temperature. The absorbance values were read in optical density at a wavelength of 480 nm by an ELISA plate reader spectrophotometer (Quant Bio-Tek Instruments Inc., Winooski, USA). The other plates of the prepared cell culture were held in an incubator and repeated at the end of the 24th and 72nd h. The results revealed the percentage of the mean optical density of the treated negative controls, and this was set to show 100% viability.
Statistical analysis
The significance of the differences between the means was evaluated using a one-way ANOVA, post-hoc Duncan's Multiple Range test with SAS9.2 (Cary software, North Carolina SAS Institute Inc. 2004) software. A statistically significant difference was set at P < 0.001.
RESULTS
Figure 1 shows the effect on cellular viability of 1 h diluted OES concentrations, 2% CHX and 5.25% NaOCl. There was a dose-dependent increase in viability of cells that treated with OES. 0.5% OES were showed the least cytotoxic effect and OES was more dependable in lower concentrations. There wasn’t found any statistical significance between 1% OES and 2% CHX (P > 0.001). 5.25% NaOCl showed cytotoxic effect, and there weren’t found any statistical significance between 5.25% NaOCl and other (1.5–4.5%) concentrations of OES (P > 0.001). Figure 1 also shows the effect of the cellular viability of 24th and 72nd h of irrigation solutions. According to the results at 24 and 72 h, all solutions showed similar cytotoxic effect. The inverted microscope analysis showed that numerical reduction and morphological differences have been identified in periodontal ligament cells [Figures 2 and 3].
Figure 1.
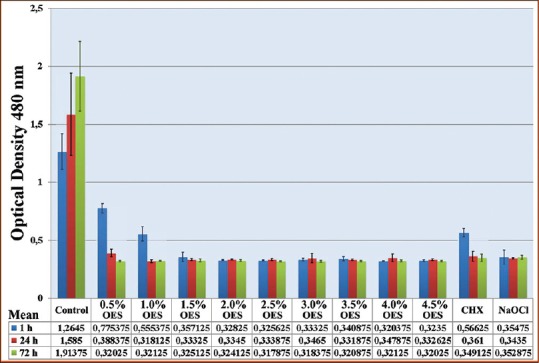
The result of WST-1 assay of the human periodontal ligament cells cultured with scaffolds for 1, 24 and 72 h
Figure 2.
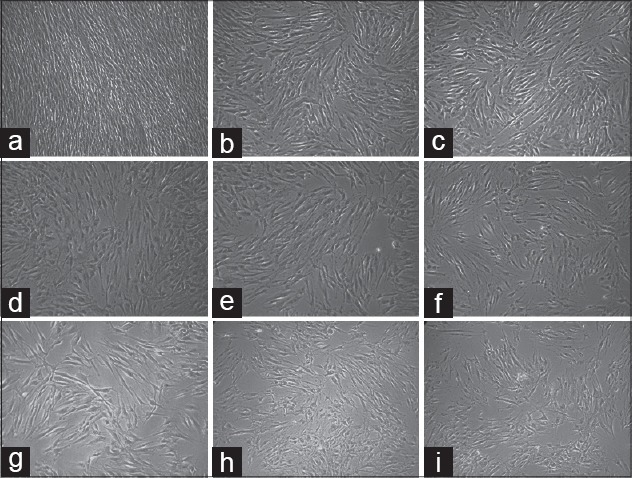
Image of periodontal ligament cells after application of 4% Origanum extract solution (OES) with an inverted microscope. After application of OES, it makes a point out that cell density was decreased. (a) Control group; human periodontal ligament (hPDL) Inverted Microscope Image (IMG ×10) that taken from unapplied OES. (b-e) hPDL image (IMG ×10) that taken 1 h interval after 4% OES application. (f-i) hPDL image (IMG ×10) that taken 1 h intervals after 24 h 4% OES application
Figure 3.
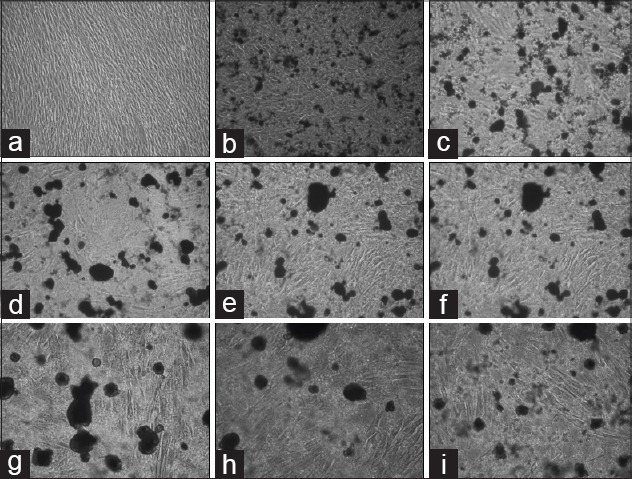
Image of periodontal ligament (PDL) cells after application of 2% Chlorhexidine gluconate (CHX) with an inverted microscope. Both numerical reduction and morphological differences have been identified in PDL cells. Fusiform morphology of PDL cells has undergone differentiation. (a) Control group; human periodontal ligament (hPDL) Inverted Microscope Image (IMG ×10) that taken from unapplied CHX (b-e) hPDL image (IMG ×10) that taken 1 h intervals after CHX application. (f-i) hPDL image (IMG ×10) that taken 1 h intervals after 24 h CHX application
DISCUSSION
A lot of in vitro and in vivo tests have been used to evaluate cytotoxicity of root canal irrigant solutions. Laboratory studies especially cell culture tests have often been used lately to evaluate cytotoxicity of materials.[17,18] Because these tests are cheap, fast and acceptable.[19] Cell culture tests are more sensitive than in vivo tests but must be evaluated within the limitations of acute toxicity tests.[20,21] In this kind of studies, permanent cell lines such as HeLa or 3T3 cells and primary diploid cells or oral fibroblasts are widely used.[22] In our study PDL fibroblasts were used. Because PDL cells are major cells that involve reaction of endodontic materials in apical tissue and are first cells that may encounter when irrigation solutions extrude to apical tissue.[23]
Cytotoxicity assays have been developed based on different parameters like metabolic activity and DNA synthesis associated with cell viability and cell proliferation.[24] Metabolic activity-based assays measure mitochondrial activity of cultured cells related with energy metabolism of cells and cell growth.[25] The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) measures mitochondrial activity of liability cells.[26] With changing metabolic activity of biomaterials, it is sensitive and easy test.[27] WST-1 (sodium5-(2,4-disulfophenyl)-2-(4-iodophenyl)-3-(4-nitrophenyl)-2H-tetrazoliuminner salt) works similarly with MTT by reacting with the mitochondrial succinate-tetrazolium reductase forming the formazan dye.[25] The WST-1 test can be quantified in 0.5–4 h without an additional solubilization step (Roche). But the MTT assay requires 52–72 h to complete. WST-1 based assays are quick and reproducible. In the present study WST-1 test was used to evaluate the effects of different irrigation solutions on the viability of PDL fibroblasts in vitro.
Irrigation plays an important role during root canal treatment. Biocompatibility with its antimicrobial and tissue solvent effect should be considered while choosing an irrigation solution. Chemical injury occurs when periapical tissue exposed to irrigation solution that is not biocompatible,[3,7] and also the tissue reaction to the root canal irrigation solution is influenced by type, volume and concentration of the irrigant.[28]
NaOCl has been widely recommended in root canal irrigation for a long time because of its necrotic and vital tissue solvent capacity and its antimicrobial activity. It has been stated that its antimicrobial effect and cytotoxic effect increases with its concentration.[3,6,29,30] Yesilsoy et al.[31] evaluated the toxic effect of 0.5%, 2.5% and 5.25% NaOCl solutions cytotoxicity at 2 h, 2 days and 2 weeks and indicated that 5.25% NaOCl cause destructive reactions when it introduced to the root apex. Onçag et al.[32] evaluated 5.25% NaOCl, cetrimide (Cetrexidine; Vebas, San Giuliano, Milan, Italy) and 2% CHX solutions cytotoxicity and found that 5.25% NaOCl had more cytotoxic effect than Cetrexidine, CHX and control groups. In their study, in the 5.25% NaOCl group, they found no statistical significant difference between the 3-time periods (2 h, 48 h, and 2 weeks). The result of their study is consistent with the present study. There was no statistical significant difference between the 3-time periods (1 h, 24 h, and 72 h) in NaOCl groups.
Alternative to NaOCl, CHX has evaluated for root canal irrigation. Boyce et al.[33] and Huth et al.[34] evaluated the cytotoxic effect of CHX and have found highly cytotoxic to fibroblast cells. Yesilsoy et al.[31] also evaluated CHX and found moderate inflammation after 2 days and reported that the formation of foreign body granuloma 2 weeks later. Tatnall et al.[35] evaluated NaOCl, H2O2, and CHX to fibroblast cells and they found all solutions showed cytotoxic effect but CHX showed the least cytotoxic effect. 1 h results of the present study revealed that CHX showed less cytotoxic effect than 5.25% NaOCl and 1.5–4.5% OES (P < 0,001), and there were not found any statistically significance between 2% CHX and 1% OES (P > 0,001). At 24 and 72nd h cytotoxicity evaluation, there were no statistical significance between irrigation solutions (P > 0,001), and all solutions showed cytotoxic effect.
CONCLUSION
The present study showed that the cytotoxicity of the solutions depends on the concentrations used. 0.5% OES showed less cytotoxic effect than other solutions. 1% OES and 2% CHX showed similar results and showed less cytotoxic effect than 5.25% NaOCl. It can be considered as a favorable solution concentration when OES was used as irrigation solution.
ACKNOWLEDGMENT
This study was supported by Suleyman Demirel University's Scientific Research Projects Unit (SDUBAP 1883-D-09).
Footnotes
Source of Support: This study was supported by Suleyman Demirel University's Scientific Research Projects Unit (SDUBAP 1883-D-09).
Conflict of Interest: None declared
REFERENCES
- 1.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–8. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 2.Bystrom A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35–40. doi: 10.1111/j.1365-2591.1985.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 3.Gatot A, Arbelle J, Leiberman A, Yanai-Inbar I. Effects of sodium hypochlorite on soft tissues after its inadvertent injection beyond the root apex. J Endod. 1991;17:573–4. doi: 10.1016/S0099-2399(06)81725-5. [DOI] [PubMed] [Google Scholar]
- 4.Spangberg L, Engström B, Langeland K. Biologic effects of dental materials 3. Toxicity and antimicrobial effect of endodontic antiseptics in vitro. Oral Surg Oral Med Oral Pathol. 1973;36:856–71. doi: 10.1016/0030-4220(73)90338-1. [DOI] [PubMed] [Google Scholar]
- 5.Yildirim C, Karaarslan ES, Ozsevik S, Zer Y, Sari T, Usumez A. Antimicrobial efficiency of photodynamic therapy with different irradiation durations. Eur J Dent. 2013;7:469–73. doi: 10.4103/1305-7456.120677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pashley EL, Birdsong NL, Bowman K, Pashley DH. Cytotoxic effects of NaOCl on vital tissue. J Endod. 1985;11:525–8. doi: 10.1016/S0099-2399(85)80197-7. [DOI] [PubMed] [Google Scholar]
- 7.Brown DC, Moore BK, Brown CE, Jr, Newton CW. An in vitro study of apical extrusion of sodium hypochlorite during endodontic canal preparation. J Endod. 1995;21:587–91. doi: 10.1016/S0099-2399(06)81108-8. [DOI] [PubMed] [Google Scholar]
- 8.Hülsmann M, Hahn W. Complications during root canal irrigation – Literature review and case reports. Int Endod J. 2000;33:186–93. doi: 10.1046/j.1365-2591.2000.00303.x. [DOI] [PubMed] [Google Scholar]
- 9.Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifácio KC, Ito IY. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25:167–71. doi: 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- 10.Baser KH. The Turkish Origanum species. In: Kintzios SE, Spiridon E, editors. London: Taylor and Francais; 2002. pp. 108–26. Oregano: The Genera Origanum and Lippia. [Google Scholar]
- 11.Vardar-Ünlü G, Ünlü M, Dönmez E, Vural N. Schwarz O, Davis PH, editors. Chemical composition and in vitro antimicrobial activity of the essential oil of Origanum minutiflorum. J Sci Food Agric. 2007;87:255–9. [Google Scholar]
- 12.Dadalioglu I, Evrendilek GA. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J Agric Food Chem. 2004;52:8255–60. doi: 10.1021/jf049033e. [DOI] [PubMed] [Google Scholar]
- 13.Baydar H, Sagdiç O, Özkan G, Karadogan T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control. 2004;15:169–72. [Google Scholar]
- 14.Ok E. Isparta: S. Demirel Üniversitesi; 2010. In vitro and ex vivo Evaluation of 1%, 2% and 5% Oregano Solution's (O. Minutiflorum) Cytotoxicity and Effect to E. faecalis and Smear Layer in Root Canal Irrigation and Disinfection. Doctoral Thesis. [Google Scholar]
- 15.Babich H, Reisbaum AG, Zuckerbraun HL. In vitro response of human gingival epithelial S-G cells to resveratrol. Toxicol Lett. 2000;114:143–53. doi: 10.1016/s0378-4274(99)00288-x. [DOI] [PubMed] [Google Scholar]
- 16.Mgbonyebi OP, Russo J, Russo IH. Antiproliferative effect of synthetic resveratrol on human breast epithelial cells. Int J Oncol. 1998;12:865–9. [PubMed] [Google Scholar]
- 17.Chang YC, Chou MY. Cytotoxicity of halothane on human gingival fibroblast cultures in vitro. J Endod. 2001;27:82–4. doi: 10.1097/00004770-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Szep S, Grumann L, Ronge K, Schriever A, Schultze M, Heidemann D. In vitro cytotoxicity of medicated and nonmedicated gutta-percha points in cultures of gingival fibroblasts. J Endod. 2003;29:36–40. doi: 10.1097/00004770-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Scotti R, Tiozzo R, Parisi C, Croce MA, Baldissara P. Biocompatibility of various root canal filling materials ex vivo. Int Endod J. 2008;41:651–7. doi: 10.1111/j.1365-2591.2008.01403.x. [DOI] [PubMed] [Google Scholar]
- 20.Granchi D, Stea S, Ciapetti G, Cavedagna D, Stea S, Pizzoferrato A. Endodontic cements induce alterations in the cell cycle of in vitro cultured osteoblasts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:359–66. doi: 10.1016/s1079-2104(05)80230-6. [DOI] [PubMed] [Google Scholar]
- 21.Korsuwannawong S, Srichan R, Vajrabhaya LO. Cytotoxicity evaluation of self-etching dentine bonding agents in a cell culture perfusion condition. Eur J Dent. 2012;6:408–14. [PMC free article] [PubMed] [Google Scholar]
- 22.Hauman CH, Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy: A review. Part 1. Intracanal drugs and substances. Int Endod J. 2003;36:75–85. doi: 10.1046/j.1365-2591.2003.00631.x. [DOI] [PubMed] [Google Scholar]
- 23.Yan P, Yuan Z, Jiang H, Peng B, Bian Z. Effect of bioaggregate on differentiation of human periodontal ligament fibroblasts. Int Endod J. 2010;43:1116–21. doi: 10.1111/j.1365-2591.2010.01786.x. [DOI] [PubMed] [Google Scholar]
- 24.Weyermann J, Lochmann D, Zimmer A. A practical note on the use of cytotoxicity assays. Int J Pharm. 2005;288:369–76. doi: 10.1016/j.ijpharm.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Ngamwongsatit P, Banada PP, Panbangred W, Bhunia AK. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J Microbiol Methods. 2008;73:211–5. doi: 10.1016/j.mimet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Lin CP, Chen YJ, Lee YL, Wang JS, Chang MC, Lan WH, et al. Effects of root-end filling materials and eugenol on mitochondrial dehydrogenase activity and cytotoxicity to human periodontal ligament fibroblasts. J Biomed Mater Res B Appl Biomater. 2004;71:429–40. doi: 10.1002/jbm.b.30107. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Ballal V. Smear layer removal agents. Aust Endod J. 2009;35:35. doi: 10.1111/j.1747-4477.2009.00167.x. author reply 36. [DOI] [PubMed] [Google Scholar]
- 29.Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CC, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:79–84. doi: 10.1016/s1079-2104(03)00360-3. [DOI] [PubMed] [Google Scholar]
- 30.Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998;24:472–6. doi: 10.1016/S0099-2399(98)80049-6. [DOI] [PubMed] [Google Scholar]
- 31.Yesilsoy C, Whitaker E, Cleveland D, Phillips E, Trope M. Antimicrobial and toxic effects of established and potential root canal irrigants. J Endod. 1995;21:513–5. doi: 10.1016/s0099-2399(06)80524-8. [DOI] [PubMed] [Google Scholar]
- 32.Onçag O, Hosgör M, Hilmioglu S, Zekioglu O, Eronat C, Burhanoglu D. Comparison of antibacterial and toxic effects of various root canal irrigants. Int Endod J. 2003;36:423–32. doi: 10.1046/j.1365-2591.2003.00673.x. [DOI] [PubMed] [Google Scholar]
- 33.Boyce ST, Warden GD, Holder IA. Cytotoxicity testing of topical antimicrobial agents on human keratinocytes and fibroblasts for cultured skin grafts. J Burn Care Rehabil. 1995;16:97–103. doi: 10.1097/00004630-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Huth KC, Jakob FM, Saugel B, Cappello C, Paschos E, Hollweck R, et al. Effect of ozone on oral cells compared with established antimicrobials. Eur J Oral Sci. 2006;114:435–40. doi: 10.1111/j.1600-0722.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 35.Tatnall FM, Leigh IM, Gibson JR. Comparative study of antiseptic toxicity on basal keratinocytes, transformed human keratinocytes and fibroblasts. Skin Pharmacol. 1990;3:157–63. doi: 10.1159/000210865. [DOI] [PubMed] [Google Scholar]


