Abstract
Objective:
This study aimed to evaluate in vitro the effect of formulations containing Biosilicate to treat enamel and dentin bovine samples exposed to dental bleaching agents.
Materials and Methods:
On enamel and dentin bleached with commercial gels containing 16% carbamide peroxide (CP) (14 days/4 h) or 35% hydrogen peroxide (single session/45 min), desensitizing dentifrices (Sensodyne®; experimental dentifrice of Biosilicate®; Odontis RX®; Sorriso®) were applied along 14 days and desensitizing pastes (Biosilicate®/water 1:1; Dessensebilize NanoP®; Bioglass type 45S5/water 1:1) were applied on days 1, 3, 7, 10 and 14. Distilled water was the control. Microhardness (MH) and roughness measurements were the variables measured on the samples before and after the treatments. Student's t-test analyzed differences before and after the treatments. Two-way analysis of variance and post-hoc Tukey test analyzed differences among the factors desensitizing, bleaching agents and substrate.
Results:
Tukey test showed no differences in roughness for both bleaching treatments and among the desensitizing agents (P > 0.05). Differences in MH appeared on enamel treated with in-home bleaching when control group (lower values) was compared with Sensodyne, Biosilicate dentifrice, Biosilicate paste, and Bioglass paste (higher values). Comparisons between desensitizing agents on dentin treated with both bleaching gels showed no statistical differences.
Conclusions:
The effect of formulations containing Biosilicate (Biosilicate dentifrice and paste) was significant in the MH of enamel bleached with 16% CP.
Keywords: Biomaterial, carbamide peroxide, dental bleaching, hydrogen peroxide
INTRODUCTION
Bleaching treatment is in high demand by patients, and it is considered as part of most esthetic dental treatments. Despite the tooth whitening esthetic benefits, some side-effects have been reported, including: Tooth sensitivity[1,2,3,4,5,6,7] and structural changes such as microhardness (MH) reduction[8,9,10,11] and increased roughness.[12,13,14,15]
For minimizing the side-effects of the bleaching treatment, the application of desensitizing and remineralizing agents before, during or after the bleaching procedure has been used, clinically. These agents include fluoride, calcium,[9,10,16] potassium nitrate,[17,18] and recently bioactive materials have been used as well.[19,20]
Despite the wide information available, there is still controversy about the use of desensitizing agents during and after bleaching treatment can significantly protect bleached enamel and dentin or not. Desensitizing products currently available in the market are not always capable of eliminating tooth sensitivity caused by tooth bleaching. Clinical trials have shown tooth sensitivity incidence even when these products are applied,[21,22] this condition can be associated to structural changes on enamel and dentin.
Bioactive glasses when in contact with biological fluids start a reaction that quickly culminates in the formation of hydroxyapatite on its surface. Bioactive glasses can obliterate exposed and opened dentinal tubules with hydroxyapatite, which is the main component of enamel and dentin.[23,24,25,26,27] Due to this property, bioglass materials are considered as a new strategy in the treatment for tooth sensitivity.
Bioactive glass materials have been incorporated into toothpastes and even into bleaching gels, as remineralizing agent, as well as desensitizing agent in tooth sensitivity treatment. Previous in vitro studies investigated the influence of these remineralizing agents on bleached dental tissues. Gjorgievska and Nicholson[20] showed that bioactive materials are able to increase the calcium and phosphate content on the enamel damaged by bleaching agents. The investigation done by Cunha et al.[28] concluded that the application of casein phosphopeptide-amorphous calcium phosphate (ACP) before and after high concentration of hydrogen peroxide (HP) and carbamide peroxide (CP) exposure was able to prevent negative structural changes. However, De Abreu et al.[29] showed no beneficial effects of adding ACP to bleaching formulas on enamel MH.
In this context, two experimental formulations with a new bioactive material were being proposed in this study to treat dental tissues submitted to the bleaching gel. The experimental proposal consists in to insert a bioactive nanoparticles powder named Biosilicate[19,27,30,31,32] in a dentifrice and a paste. One in vitro study by Tirapelli et al.[27] showed that micron-sized particles of Biosilicate® were able to induce hydroxyl carbonate apatite deposition in open dentinal tubules. In a clinical study, Tirapelli et al.[32] evaluated experimental formulations containing Biosilicate® and different commercial desensitizing agents in dentin hypersensitivity treatment, showing Biosilicate® mixed with distilled water as the best and the fastest method to reduce dentin hypersensitivity, suggesting this biomaterial could be used as a desensitizing agent. In another laboratorial study,[19] authors indicated that when Biosilicate is used immediately after bleaching treatment it could reduce or even avoid the demineralization effect of bleaching products and prevent exposing dentinal tubules.
Nevertheless, there is little information in the literature evaluating this novel bioglass-ceramic associated to bleaching agents. Therefore, this in vitro study considers the null hypothesis that there is no difference between experimental formulations containing Biosilicate® and other commercial desensitizing agents regarding roughness and MH of enamel and dentin bleached with 16% CP or 35% HP.
MATERIALS AND METHODS
Experimental design
Variables MH and surface roughness (Ra) (RG) were studied under four levels: (1) Tooth tissue; (2) bleaching treatment type; (3) desensitizing agent; (4) time (before and after). Materials used are listed in Table 1 and the experimental design is illustrated in Figure 1.
Table 1.
Products utilized in the study
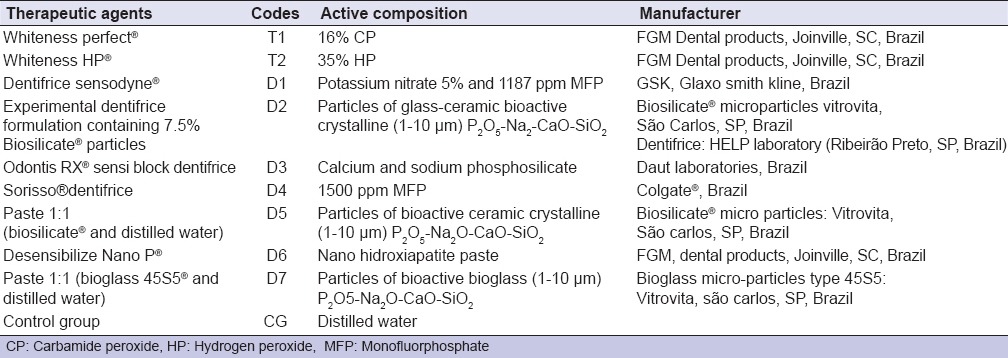
Figure 1.
Schematic arrangement of the experimental design
Sample preparation
This in vitro study utilized 320 samples (160 of enamel and 160 of dentin). Extracted permanent bovine incisors (intact and noncarious) were used. The teeth were cleaned with periodontal curette, and the crowns were separated from the root using carborundum disks in a low-speed dental handpiece (Dabi Atlante®, Ribeirao Preto, Sao Paulo, Brazil) under refrigeration. Square enamel sections (4 mm × 4 mm × 3 mm) were obtained from the middle third of the crown and the dentin sections (4 mm × 4 mm × 3 mm) from the cervical region of the root. The samples were polished under water-cooling on a polishing device (Struers®/Denmark). Grit abrasive papers (200, 500, 600 and 800) were used to obtain parallel planar surfaces required for the MH and roughness tests and 1200 grit abrasive paper to obtain standard smoothness. The thickness of the samples was measured with micrometer (Mitutoyo®-Pocket Gage, Japan). Enamel samples with thickness lower than 3 mm were discarded. Selected samples were stored in distilled water.
Treatments
Samples were removed from the individual containers for applying the bleaching product and the desensitizing agent. Treatments were carried out at room temperature (30–36°C). The same protocol was established for enamel and dentin, as follows:
For the in-home bleaching treatment, the specimens were exposed to 16% CP (T1) for 4 h a day (according the manufacturer instructions) for 14 consecutive days. After 4 h of bleaching gel exposition, samples were gently washed using tap water for 30 s. Then, they were treated with the desensitizing agents according to the experimental groups:
T1/D1, T1/D2, T1/D3 and T1/D4 Groups: Samples were immersed in desensitizing solution of D1, D2, D3 and D4 respectively and tap water (20% wt/vol) for 15 min; during the 14 days of the bleaching procedure
T1/D5 Group: A paste obtained from micron-sized particles of Biosilicate® and distilled water (50% wt/vol) was applied using a micro-applicator (Microbrush®) on the sample surface and stayed active for 15 min on days 1, 3, 7 and 10 of the bleaching procedure
T1/D6 Group: Desensibilize NanoP® was applied on the sample surface using a micro-applicator (Microbrush®), the product was rubbed for 10 s, and then it stayed active for 5 min (according to the manufactures instruction). This procedure was carried out on days 1, 3, 7 and 10 of the bleaching procedure
T1/D7 Group: The same as T1/D5, replacing D5 with D7
T1/CG Group: Control for T1, samples immersed in distilled water for 14 days.
For in-office bleaching treatment, samples were treated with 35% HP (T2) for 45 min (with three 15 min – applications each) according to manufacturer instructions. T2 had a single application. When the time of exposure to T2 was finalized, samples were also washed with tap water for 30 s and they were followed by desensitizing products application:
T2/D1, T2/D2, T2/D3 and T2/D4 Groups: Samples were immersed in desensitizing solution of D1, D2, D3 and D4 respectively, and tap water (20% wt/vol) for 15 min during the 14 days of the bleaching procedure
T2/D5 Group: A paste obtained from micron-sized particles of Biosilicate® and distilled water (50% wt/vol) was applied using a micro-applicator (Microbrush®) on the sample surface and stayed active for 15 min on days 1, 3, 7 and 10 of the bleaching procedure
T2/D6 Group: Desensibilize NanoP® was applied on the sample surface using a micro-applicator (Microbrush®), the product was rubbed for 10 s, and then it stayed active for 5 min (according to the manufactures instruction). This procedure was carried out on days 1, 3, 7 and 10 of the bleaching procedure
T2/D7 Group: The same of T2/D5 replacing D5 with D7
T2/CG Group: Control for T2, samples immersed in distilled water for 14 days.
After each treatment, samples were gently washed using tap water for 30 s and stored in distilled water, which was changed every day during the experiment period (14 days). Immediately, after the treatment period, samples were evaluated regarding MH and surface roughness.
Measurements
Microhardness and surface roughness were assessed for all samples before (t0) and after the study time (t1).
Roughness measurement
To measure surface roughness (Ra), a contact profilometer device (Mitutoyo, SJ-201P, Japão) was used. The cut-off was 0.8 mm calibrated and determined previously, and three measurements were performed on surface of each sample in different directions with a distance of 0.5 mm between them, for baseline values and for the measurements after the application of the bleaching and the desensitizing agents.
Microhardness measurement
Knoop MH measurement was made using a MH tester (Shimadzu HMV 2000, Kyoto, Japan) at a load of 1N for 30 s. Measurements were made before and after experimental period of treatment. For baseline measurement, three indentations were performed in each sample, with 0.5 mm of distance between them, following the mark 1. For the final measurement, we set a mark 2 on the opposite side (considering the first one), and the three indentation was performed following it. Then, the values were averaged.
Scanning electronic microscope
Representative samples of each experimental group of enamel and dentin were dehydrated in a desiccator at 37°C for 24 h and coated with a conductive layer of gold by evaporation under vacuum and analyzed by scanning electron microscope (SEM) (Zeiss, EVO® 50, Cambridge Instruments Co., UK). Each sample was screened using a magnification of ×200, ×2000 and × 5000. SEM was performed at the baseline (t0) and at the end of treatment (t1).
Statistical analysis
The units of the study were the dentin or enamel samples. Each sample was measured 3 times for roughness and MH before and after the treatments proposed - a total of 1920 measurements were obtained for each variable. The RG or MH value for each sample was the average obtained from the three measurements. The authors analyzed the data statistically with the software Prism version 7.0 (GraphPad software, California, USA). The factor “before-after” treatments were analyzed using Student's t-test to compare the means in each group. To analyze the factors desensitizers/bleaching agents, and the tooth tissues (dentin and enamel), two-way analysis of variance (ANOVA) was used comparing the mean of the differences between the measurements moments’ (after minus before) for each variable. The Tukey multiple comparison test post-hoc (α = 0.05) was performed. The mean's groups could assume positive values if MH and RG had increased or negative values if had decreased.
RESULTS
Table 2 summarizes the results obtained for both variables.
Table 2.
Mean values and standard deviation of the difference between before and after appliance of desensitizing agents in bleached enamel and dentin samples
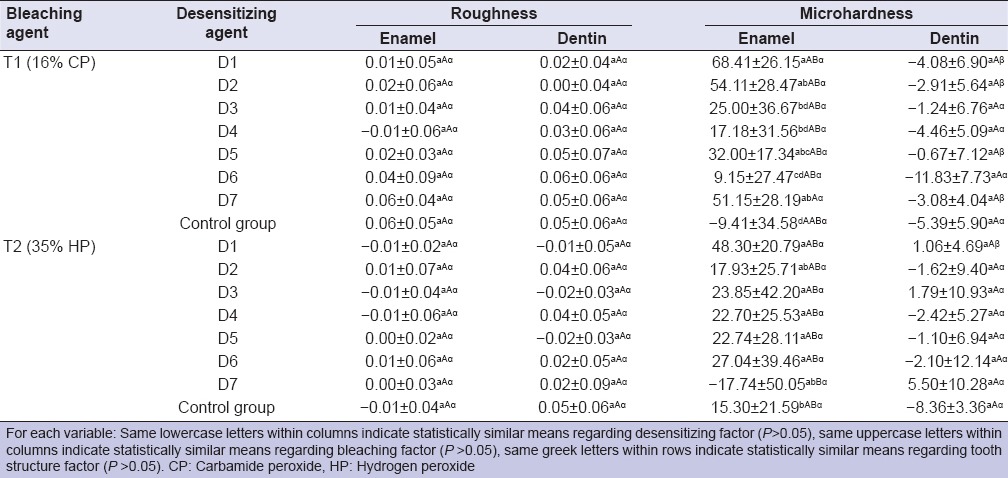
Roughness data
Student's t-test showed to the factor “before-after” no statistical difference between the baseline and posttreatment means for roughness (P > 0.05) at any group.
Descriptive statistics presents the mean of the differences between the measurements moments (after-before) as positive (~70%) or negative values (~30%) indicating an increased or decreased surface roughness. However, two-way ANOVA showed that the interaction among the factors (desensitizing, bleaching agents and tooth structure) was no significant (P = 0.6267).
Data analysis revealed that there was no statistical difference in surface roughness of enamel and dentin in the control group and experimental groups.
Microhardness data
Student's t-test showed to the factor “before-after” statistical difference in enamel only in the group D1 when treated with 16% CP where MH increased and in dentin samples for the group D6 where MH decreased significantly. The multiple comparison among the factors showed statistical difference (P < 0.0001). Regarding the desensitizing factors, in enamel, the MH of the control group after in-home bleaching treatment decreased (showing a negative difference), and it was statistical different to D1, D2, D5 and D7 experimental groups, in which were observed the higher increase of MH. After the in-office bleaching treatment, the control group showed an increase in MH that was no statically different to the other groups, but the MH of D7 group decreased, showing a statistical difference with D1, D3, D4, D5 and D6. About dentin, statistical analysis revealed no differences among the groups for both bleaching agents. However, descriptive statistic showed that the MH decreased for all groups after in-home bleaching and in D2, D4, D5 and D6 after in-office bleaching. Regarding the bleaching factor, Tukey test revealed a significant difference between bleaching agents to D7 (P < 0.0001). Finally, considering the factor tooth structure, the desensitizing D1, D2, D5 and D7 after in-home bleaching treatment showed different action on substrates enamel and dentin. After in-office bleaching treatment, only D1 showed different effect in enamel and dentin.
Scanning electron micrographs
The SEM micrographs [Figure 2a and b] show both untreated tooth structure enamel and dentin as regular images from these tissues.
Figure 2.
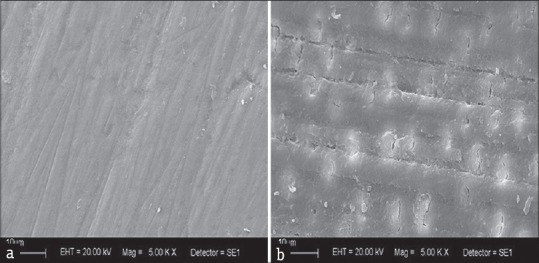
Representative scanning electron microscope photomicrographs of the untreated samples: (a) Enamel; (b) dentin
Additionally, Figure 3 shows a compilation of SEM images from specimens of enamel and dentin submitted to the bleaching treatments and the subsequent application of the desensitizing agents. In dentin control group, compared with untreated tooth tissue, it was observed a different pattern on sample surfaces treated with 16% CP, with darkened regions suggesting the attacked part of the tissues by the bleaching gel.
Figure 3.
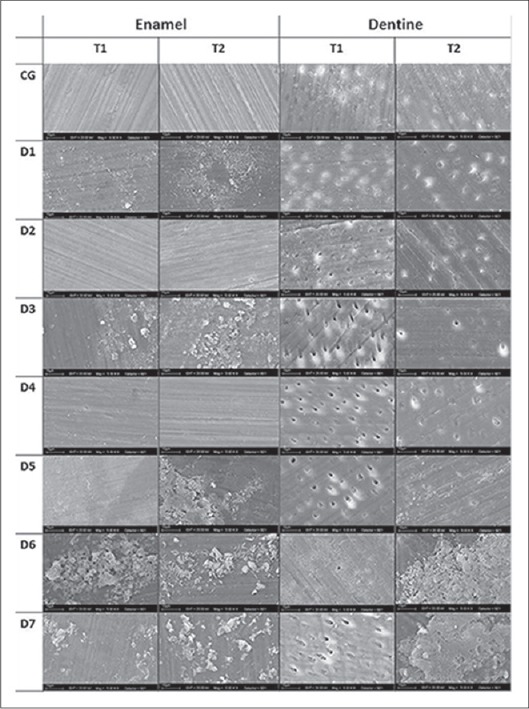
Scanning electron microscope micrographs of enamel and dentin bovine samples. The images on the top correspond to control group where the bleaching products (T1 or T2) were applied. All other images are allocated in accordance with the subsequent desensitizing treatment
In the groups treated with 45S5 (D7) and Biosilicate® (D5), on samples bleached with T1 suggested the deposition of these materials is present on both dentin surfaces. The dentinal tubules appeared to be completely obliterated when treated with Biosilicate® for samples bleached with T2; however, this pattern (dentinal tubule occlusion) is also observed in the control group.
DISCUSSION
Adverse effects of tooth bleaching have been described in previous research[1,4,18] indicating that this procedure can cause defects on enamel and dentin surfaces. These effects include roughness increase, MH decrease and potential alteration of the chemical composition of these hard tissues.[33] Enamel matrix is altered allowing the diffusion of peroxides from enamel to dentin, and thus could cause increase dentin permeability[4] which is reported in clinical trials as tooth sensitivity.[34,35]
Thus, in an attempt to reduce the loss of mineral of the dental tissues[4,13,20,33] different desensitizing and remineralizing agents were used before, during or after the bleaching phase and the investigations revealed that their use could be a contributing factor in preventing this damage.[9,10,19,28,36,37]
This study analyzed the MH and surface roughness because it is possible to quantitatively determine changes on the dental structure that indicate loss or gain of mineral of the dental structure.[38] In addition, SEM has been used for qualitative analyze of the surface morphology of enamel and dentin samples following bleaching therapy in several studies.[8,19,20,29,33,36,39]
The findings of this study rejected the null hypothesis that there is no difference between experimental formulations containing Biosilicate® and other commercial desensitizing agents regarding MH of enamel bleached with 16% CP. This is because data supported comparatively that the experimental dentifrice containing micro particles of Biosilicate (D2) and paste containing micro particles of Biosilicate (D5) increased enamel MH treated after in-home bleaching.
On the other hand, considering the roughness results, we observed no significant difference among the desensitizing agents at any factor (before-after, bleaching/desensitizing agents and tooth structure). Apparently, bleaching agents did not cause significant alteration on both tooth enamel and dentin surface roughness.
Similarly, in vitro studies have been related no alteration in roughness on enamel and dentin bleached.[13,15,29,40,39,37] Abouassi et al.[39] found no significant changes on enamel roughness after CP or HP use; Pedreira De Freitas et al.,[40] showed no statistically significant changes on enamel roughness after HP 38% exposure. De Abreu et al.[29] demonstrated that the surface roughness only is altered when HP is applied, but after immersion in artificial saliva the values get similar to the baseline. Sa et al. (2012)[15] found no changes on enamel exposure even using a high concentration of HP.
In contrast, Azrak et al.[14] indicated roughness alteration on an enamel surface after 10 h of 35% HP bleaching; the authors attributed that fact to the high concentration of peroxide or low pH on eroded enamel. In our study, both of the bleaching agents tested had neutral pH, and relatively lower CP concentration. Despite we used a high concentration of HP (35%); the total exposure time was 45 min with 15 min-interleaved (according the manufacturer instructions).
Regarding MH evaluation, the results of this study showed that when comparing before (t0) and after (t1) enamel values for in-home bleaching, MH increased when desensitizing products were applied and reduced only in the control group (no application of desensitizing agents). The comparison among the desensitizing agents revealed that the control group was statistically different from D1, D2, D5 and D7 products (MH higher values). Concerning MH reduction observed in the control group, one study has pointed that this condition is associated with the loss of mineral content because demineralization.[41] Previous studies have reported that different concentrations of CP may influence the chemical composition of enamel and dentin substrates.[20,33] Thus, the good performance shown specifically by D2, D5 (experimental desensitizing agents containing Biosilicate) and D7 (Bioglass 45S5) may have been because of they are capable of bonding chemically to hard dental tissues and their components may impart bioactivity,[24] inducing hydroxycarbonate apatite deposition on dental surface.[27]
Also for the enamel MH, treated by in-office bleaching agent, no differences were verified among experimental and control groups. It may be explained due to 35% HP (T2) did not cause alteration on enamel in our study. Similarly, Abouassi et al.[39] reported no difference on enamel MH after bleaching with different HP concentrations. Also, Sa et al. (2012)[15] indicated that in vitro conditions, enamel MH is not affected by bleaching agents containing high concentrations. Conversely, other studies[10,37] have related that 35% HP is able to reduce enamel MH. Enamel MH reduction is attributed to contact time between HP gel and substrate[42] or to acid pH of bleaching gel.[10] The difference in our results could be due to a shorter HP exposure time (45 min) recommended by the manufacturer or to pH, which not was acid.
On the other hand, another situation was observed in dentin tissue regarding MH. Considering time factor, we observed a MH reduction in all groups treated by in-home bleaching although it was no significant, exceptionally to D6. This pattern of reduction could explain why multiple comparison did not find difference among the desensitizing products. They were not able to recover the baseline values of MH, showing a similar effect on dentin.
At this regard, it is important to consider that radicular dentin is a soluble tissue and thus is more susceptible to demineralization.[43] An in vitro study, Faraoni-Romano et al.[13] related that bleaching does not alter enamel MH and surface roughness, but in the root dentin is capable of reducing its MH.
Studies[8,33] that investigated the surface morphology using SEM found no significant changes following bleaching and Abouassi et al.[39] reported that changes occurred in the surface morphology of the bleached enamel, but with no major changes to the enamel composition. In contrast, other studies[19,20,28] revealed alterations on the morphological surface of hard dental tissues in different degrees of severity, characterized by an increased surface porosity, depressions, and superficial irregularities.
In our study, it is possible to observe that in SEM image from dentin control group [Figure 3] there was not significant change, the dentinal tubules were obliterated, grinding grooves were evident and part of smear layer was still persisted. In addition, particles can be observed on dentin surface for the group treated with Dessensiblize NanoP® paste; however, images from samples bleached with 16% CP and treated with Bioglass and Biosilicate suggested incorporation of the particles on dentin surfaces. Also, the dentinal tubules seemed to be completely obliterated when treated with Biosilicate® for samples bleached with 35% HP.
Finally, it is important to consider that our study had some limitations. Although some studies[8,10,13,15,29,36] have used artificial saliva as storing solution in order to closely simulate intra-oral conditions, in our study samples were stored in distilled water to avoid the remineralizing effect of artificial saliva,[29] and evaluate if the products tested are able to re-harden the surface softened enamel and dentin samples. Moreover, it is important to consider other evaluation methods to identifying morphological and chemical changes and further clinical studies will be needed to clarify these findings.
CONCLUSION
According to the results of this in vitro study, it was concluded that:
Roughness was not affected by dental bleaching agents on enamel and dentin or when associated to desensitizing agents
Microhardness was increased by both experimental formulations containing Biosilicate®, Sensodyne dentifrice and paste of Bioglass on enamel bleached with 16% CP
Dentin MH was affected differently than enamel when treated with in-home bleaching agents together with both experimental formulations containing Biosilicate®, Sensodyne dentifrice and paste of Bioglass.
ACKNOWLEDGMENTS
The authors are grateful to FAPESP (the State of Sao Paulo Research Foundation) for the financial support of this study.
Footnotes
Source of Support: The State of Sao Paulo Research Foundation.
Conflict of Interest: None declared.
REFERENCES
- 1.Dahl JE, Pallesen U. Tooth bleaching – A critical review of the biological aspects. Crit Rev Oral Biol Med. 2003;14:292–304. doi: 10.1177/154411130301400406. [DOI] [PubMed] [Google Scholar]
- 2.Tredwin CJ, Naik S, Lewis NJ, Scully C. Hydrogen peroxide tooth-whitening (bleaching) products: Review of adverse effects and safety issues. Br Dent J. 2006;200:371–6. doi: 10.1038/sj.bdj.4813423. [DOI] [PubMed] [Google Scholar]
- 3.Kihn PW. Vital tooth whitening. Dent Clin North Am. 2007;51:319–31. doi: 10.1016/j.cden.2006.12.001. viii. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg M, Grootveld M, Lynch E. Undesirable and adverse effects of tooth-whitening products: A review. Clin Oral Investig. 2010;14:1–10. doi: 10.1007/s00784-009-0302-4. [DOI] [PubMed] [Google Scholar]
- 5.FDI World Dental Federation. Int Dent J 2013. Vol. 63. Mexico City, Mexico: 2011. Sep 17, FDI policy statement on dental bleaching materials: Adopted by the FDI General Assembly; pp. 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basting RT, Amaral FL, França FM, Flório FM. Clinical comparative study of the effectiveness of and tooth sensitivity to 10% and 20% carbamide peroxide home-use and 35% and 38% hydrogen peroxide in-office bleaching materials containing desensitizing agents. Oper Dent. 2012;37:464–73. doi: 10.2341/11-337-C. [DOI] [PubMed] [Google Scholar]
- 7.Özcan M, Abdin S, Sipahi C. Bleaching induced tooth sensitivity: Do the existing enamel craze lines increase sensitivity? A clinical study. Odontology. 2014;102:197–202. doi: 10.1007/s10266-013-0104-7. [DOI] [PubMed] [Google Scholar]
- 8.Zantner C, Beheim-Schwarzbach N, Neumann K, Kielbassa AM. Surface microhardness of enamel after different home bleaching procedures. Dent Mater. 2007;23:243–50. doi: 10.1016/j.dental.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Borges AB, Samezima LY, Fonseca LP, Yui KC, Borges AL, Torres CR. Influence of potentially remineralizing agents on bleached enamel microhardness. Oper Dent. 2009;34:593–7. doi: 10.2341/08-081-L. [DOI] [PubMed] [Google Scholar]
- 10.Borges AB, Yui KC, D’Avila TC, Takahashi CL, Torres CR, Borges AL. Influence of remineralizing gels on bleached enamel microhardness in different time intervals. Oper Dent. 2010;35:180–6. doi: 10.2341/09-117-L. [DOI] [PubMed] [Google Scholar]
- 11.Zanet CG, Fava M, Alves LA. In vitro evaluation of the microhardness of bovine enamel exposed to acid solutions after bleaching. Braz Oral Res. 2011;25:562–7. doi: 10.1590/s1806-83242011000600015. [DOI] [PubMed] [Google Scholar]
- 12.Markovic L, Jordan RA, Lakota N, Gaengler P. Micromorphology of enamel surface after vital tooth bleaching. J Endod. 2007;33:607–10. doi: 10.1016/j.joen.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Faraoni-Romano JJ, Da Silveira AG, Turssi CP, Serra MC. Bleaching agents with varying concentrations of carbamide and/or hydrogen peroxides: Effect on dental microhardness and roughness. J Esthet Restor Dent. 2008;20:395–402. doi: 10.1111/j.1708-8240.2008.00216.x. [DOI] [PubMed] [Google Scholar]
- 14.Azrak B, Callaway A, Kurth P, Willershausen B. Influence of bleaching agents on surface roughness of sound or eroded dental enamel specimens. J Esthet Restor Dent. 2010;22:391–9. doi: 10.1111/j.1708-8240.2010.00372.x. [DOI] [PubMed] [Google Scholar]
- 15.Sa Y, Sun L, Wang Z, Ma X, Liang S, Xing W, et al. Effects of two in-office bleaching agents with different pH on the structure of human enamel: An in situ and in vitro study. Oper Dent. 2012;38:100–10. doi: 10.2341/11-173-L. [DOI] [PubMed] [Google Scholar]
- 16.Gladwell J, Simmons D, Wright JT. Remineralization potential of a fluoridated carbamide peroxide whitening gel. J Esthet Restor Dent. 2006;18:206–12. doi: 10.1111/j.1708-8240.2006.00021_1.x. [DOI] [PubMed] [Google Scholar]
- 17.Tay LY, Kose C, Loguercio AD, Reis A. Assessing the effect of a desensitizing agent used before in-office tooth bleaching. J Am Dent Assoc. 2009;140:1245–51. doi: 10.14219/jada.archive.2009.0047. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz K. Pretty painful: Why does tooth bleaching hurt? Med Hypotheses. 2010;74:835–40. doi: 10.1016/j.mehy.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 19.Pinheiro H, Lopes B, Klautau EB, Cardoso J, Silva BR, Cardoso PE. Influence of bioactive materials used on the dentin surface whitened with carbamide peroxide 16% Mat Res. 2010;13:273–8. [Google Scholar]
- 20.Gjorgievska E, Nicholson JW. Prevention of enamel demineralization after tooth bleaching by bioactive glass incorporated into toothpaste. Aust Dent J. 2011;56:193–200. doi: 10.1111/j.1834-7819.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- 21.Reis A, Tay LY, Herrera DR, Kossatz S, Loguercio AD. Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent. 2011;36:590–6. doi: 10.2341/10-173-C. [DOI] [PubMed] [Google Scholar]
- 22.Bonafé E, Loguercio AD, Reis A, Kossatz S. Effectiveness of a desensitizing agent before in-office tooth bleaching in restored teeth. Clin Oral Investig. 2014;18:839–45. doi: 10.1007/s00784-013-1055-7. [DOI] [PubMed] [Google Scholar]
- 23.Siqueira RL, Zanotto ED. Biosilicate® : historical of a highly bioactive Brazilian glass-ceramic. Quim Nova. 2011;34:1231–41. [Google Scholar]
- 24.Renno AC, Bossini PS, Crovace MC, Rodrigues AC, Zanotto ED, Parizotto NA. Characterization and in vivo biological performance of biosilicate. Biomed Res Int 2013. 2013:141427. doi: 10.1155/2013/141427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17:967–78. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 26.Kokubo T, Kim HM, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24:2161–75. doi: 10.1016/s0142-9612(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 27.Tirapelli C, Panzeri H, Soares RG, Peitl O, Zanotto ED. A novel bioactive glass-ceramic for treating dentin hypersensitivity. Braz Oral Res. 2010;24:381–7. doi: 10.1590/s1806-83242010000400002. [DOI] [PubMed] [Google Scholar]
- 28.Cunha AG, De Vasconcelos AA, Borges BC, Vitoriano Jde O, Alves-Junior C, Machado CT, et al. Efficacy of in-office bleaching techniques combined with the application of a casein phosphopeptide-amorphous calcium phosphate paste at different moments and its influence on enamel surface properties. Microsc Res Tech. 2012;75:1019–25. doi: 10.1002/jemt.22026. [DOI] [PubMed] [Google Scholar]
- 29.De Abreu DR, Sasaki RT, Amaral FL, Flório FM, Basting RT. Effect of home-use and in-office bleaching agents containing hydrogen peroxide associated with amorphus calcium phosphate on enamel microhardness and surface roughness. J Esthet Restor Dent. 2011;23:158–68. doi: 10.1111/j.1708-8240.2010.00394.x. [DOI] [PubMed] [Google Scholar]
- 30.Moura J, Teixeira LN, Ravagnani C, Peitl O, Zanotto ED, Beloti MM, et al. In vitro osteogenesis on a highly bioactive glass-ceramic (Biosilicate) J Biomed Mater Res A. 2007;82:545–57. doi: 10.1002/jbm.a.31165. [DOI] [PubMed] [Google Scholar]
- 31.Roriz VM, Rosa AL, Peitl O, Zanotto ED, Panzeri H, de Oliveira PT. Efficacy of a bioactive glass-ceramic (Biosilicate) in the maintenance of alveolar ridges and in osseointegration of titanium implants. Clin Oral Implants Res. 2010;21:148–55. doi: 10.1111/j.1600-0501.2009.01812.x. [DOI] [PubMed] [Google Scholar]
- 32.Tirapelli C, Panzeri H, Lara EH, Soares RG, Peitl O, Zanotto ED. The effect of a novel crystallised bioactive glass-ceramic powder on dentine hypersensitivity: A long-term clinical study. J Oral Rehabil. 2011;38:253–62. doi: 10.1111/j.1365-2842.2010.02157.x. [DOI] [PubMed] [Google Scholar]
- 33.Cakir FY, Korkmaz Y, Firat E, Oztas SS, Gurgan S. Chemical analysis of enamel and dentin following the application of three different at-home bleaching systems. Oper Dent. 2011;36:529–36. doi: 10.2341/11-050-L. [DOI] [PubMed] [Google Scholar]
- 34.Browning WD, Cho SD, Deschepper EJ. Effect of a nano-hydroxyapatite paste on bleaching-related tooth sensitivity. J Esthet Restor Dent. 2012;24:268–76. doi: 10.1111/j.1708-8240.2011.00437.x. [DOI] [PubMed] [Google Scholar]
- 35.Reis A, Kossatz S, Martins GC, Loguercio AD. Efficacy of and effect on tooth sensitivity of in-office bleaching gel concentrations: A randomized clinical trial. Oper Dent. 2013;38:386–93. doi: 10.2341/12-140-C. [DOI] [PubMed] [Google Scholar]
- 36.Dominguez JA, Bittencourt B, Michel M, Sabino N, Gomes JC, Gomes OM. Ultrastructural evaluation of enamel after dental bleaching associated with fluoride. Microsc Res Tech. 2012;75:1093–8. doi: 10.1002/jemt.22035. [DOI] [PubMed] [Google Scholar]
- 37.Klaric E, Marcius M, Ristic M, Sever I, Prskalo K, Tarle Z. Surface changes of enamel and dentin after two different bleaching procedures. Acta Clin Croat. 2013;52:419–29. [PubMed] [Google Scholar]
- 38.Joiner A. Review of the effects of peroxide on enamel and dentine properties. J Dent. 2007;35:889–96. doi: 10.1016/j.jdent.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Abouassi T, Wolkewitz M, Hahn P. Effect of carbamide peroxide and hydrogen peroxide on enamel surface: An in vitro study. Clin Oral Investig. 2011;15:673–80. doi: 10.1007/s00784-010-0439-1. [DOI] [PubMed] [Google Scholar]
- 40.Pedreira De Freitas AC, Botta SB, Teixeira Fde S, Salvadori MC, Garone-Netto N. Effects of fluoride or nanohydroxiapatite on roughness and gloss of bleached teeth. Microsc Res Tech. 2011;74:1069–75. doi: 10.1002/jemt.20996. [DOI] [PubMed] [Google Scholar]
- 41.Featherstone JD, ten Cate JM, Shariati M, Arends J. Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res. 1983;17:385–91. doi: 10.1159/000260692. [DOI] [PubMed] [Google Scholar]
- 42.Rodrígues JA, Basting RT, Serra MC, Rodrígues Júnior AL. Effects of 10% carbamide peroxide bleaching materials on enamel microhardness. Am J Dent. 2001;14:67–71. [PubMed] [Google Scholar]
- 43.Wefel JS. Root caries histopathology and chemistry. Am J Dent. 1994;7:261–5. [PubMed] [Google Scholar]


