Abstract
Objective
To examine our institutional experience in patients treated with partial nephrectomy (PN) for renal cortical tumours (RCTs) of ≥7 cm, as PN is an accepted surgical approach for appropriate RCTs of <7 cm but there are limited data on the use of PN for larger tumours.
Patients and Methods
After Institutional Review Board approval, we examined our prospectively collected surgical database for patients treated with PN for RCTs of ≥7 cm between 1989 and 2008. Pertinent demographic, clinical, surgical and pathological data were reviewed.
Results
In all, 34 patients (37 renal units) were identified for analysis with a median (interquartile range, IQR) age of 63 (52-71) years, median (IQR) tumour size of 7.5 (7.2-9.0) cm with the largest tumour being 19 cm. In 31 renal units (28 patients, 84%) carcinoma was evident, with 16 renal units (43%) having conventional clear cell carcinoma, followed by papillary in eight renal units (21%). Currently, 20 of these 28 patients (71%) are disease free, three are alive with metastatic disease (two had known preoperative metastatic disease), three died from disease and two died from other causes. The median (IQR) preoperative estimated glomerular filtration rate was 65 (55-73) mL/min/1.73 m2, compared with 55 (47-74) mL/min/1.73 m2 after PN (P = 0.003, paired Student's t-test).
Conclusions
Our findings suggest that PN for RCTs of ≥7 cm can be safely performed and provide effective tumour control for selected patients. PN should be considered for patients with appropriate tumours, solitary kidneys or pre-existing renal insufficiency.
Keywords: renal cell carcinoma, partial nephrectomy, large renal tumours
Introduction
According to the latest estimates, ≈54 000 people were diagnosed with a renal cortical tumour (RCT) in 2008, with just over 13 000 patients dieing from their tumour [1]. Since Robson's work delineating radical nephrectomy (RN) as the standard of care for any RCT almost 50 years ago, the surgical treatment of RCTs has progressed with partial nephrectomy (PN) considered the standard of care for most patients with appropriately selected RCTs of <7 cm [2–4]. Current studies have indicated that up to 26% of patients with RCTs have pre-existing chronic kidney disease (CKD) with an estimated GFR (eGFR) of <60 mL/min/1.73 m2 with RN being a significant risk factor for developing CKD [5]. Moreover, CKD has been associated with increased risk of death, cardiovascular events and hospitalization [6]. These contemporary findings underscore the importance of minimizing the impact of surgical intervention for the treatment of RCTs. The purpose of the present study was to examine our institutional experience in a cohort of patients treated with PN for RCTs of ≥7 cm.
Patients and Methods
After Institutional Review Board approval, we reviewed our prospectively collected nephrectomy database for patients treated with PN for RCTs of ≥7 cm (Fig. 1). Of the 3238 patients in the database, 1339 were treated with a PN during this study, of which we identified 34 patients (2.5%) accounting for 37 renal units. Patients from our cohort had operations between 1 July 1989 and 30 June 2008 performed by eight different surgeons, with 32 open PN and five laparoscopic procedures. In most of the cases, the decision to perform an elective PN was based on surgeon discretion after complete evaluation with preoperative imaging showing a large tumour that was amenable to resection, combined with a significant portion of the kidney not being involved by tumour. Five patients in the present study had a solitary kidney from a previous RN, therefore PN was performed as imperative indication with all of these patients undergoing an open procedure. Pertinent demographic, clinical, surgical and pathological variables were recorded. The eGFR was determined before and after PN using the modified Modification of Diet in Renal Disease equation [7].
Fig. 1.
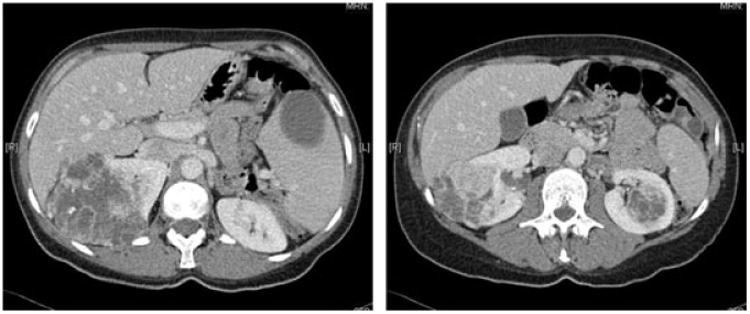
Patient with bilateral RCTs of ≥7 cm treated with staged bilateral PN.
In all statistical analyses P < 0.05 was considered to indicate statistical significance.
Results
During the study period, we identified 34 patients (37 renal units) who had a PN for RCTs of ≥7 cm. The median (interquartile range, IQR) age of our cohort was 63 (52–71) years, with the median (range) tumour size being 7.5 (7–19) cm and median (IQR) follow-up of 17 (6–40) months (Table 1). In all, 31 of 37 (84%) renal units (28 patients) had carcinoma evident on final pathology, with conventional clear cell carcinoma being the most common pathology (16 renal units, 43%), followed by papillary renal cell carcinoma in eight renal units (21%), and four renal units (11%) with chromophobe. Other malignant pathologies consisted of translocation associated RCC (two renal units, 5%), and one renal unit with a dedifferentiated liposarcoma. Benign pathologies included three renal units (8%) with multilocular cystic kidney, two kidneys with angiomyolipoma and one kidney with oncocytoma (Table 1). All patients had negative surgical margins and Table 1 describes the complete cohort and operative characteristics. There were three patients with known metastatic disease (lung nodules) at the time of PN with one of these patients having baseline chronic renal impairment (eGFR of 43 mL/min/1.73 m2).
Table 1. The cohort and tumour characteristics.
| Variable | Value |
|---|---|
| Median (IQR) | |
| Age, years | 63 (52–71) |
| Tumour size, cm | 7.5 (7.2–9.0) |
| N | |
| Indication | |
| Elective | 32 |
| Solitary kidney | 5 |
| Approach: | |
| Open | 32 |
| Laparoscopic | 5 |
| Median (IQR) | |
| Cold ischaemia time*, min | 45 (36–55) |
| Operative duration, min | 170 (150–240) |
| EBL, mL | 500 (200–750) |
| Hospital stay, days | 4 (3–5) |
| eGFR, mL/min/1.73 m2 | |
| Preoperative | 65 (55–73) |
| Postoperative | 55 (47–74) |
| Distribution of tumour size (cm)†, n (%) | |
| 7–7.9 | 21 (57) |
| 8.0–8.9 | 6 (16) |
| 9.0–9.9 | 6 (16) |
| >10 | 4 (11) |
| Pathological characteristics†, n (%): | |
| Conventional clear cell | 16 (43) |
| Papillary | 8 (22) |
| Chromophobe | 4 (11) |
| Multilocular cystic benign | 3 (8) |
| Angiomyolipoma | 2 (5) |
| RCC: translocation associated | 2 (5) |
| Dedifferentiated liposarcoma | 1 (3) |
| Oncocytoma | 1 (3) |
EBL, estimated blood loss;
n = 29;
n = 37.
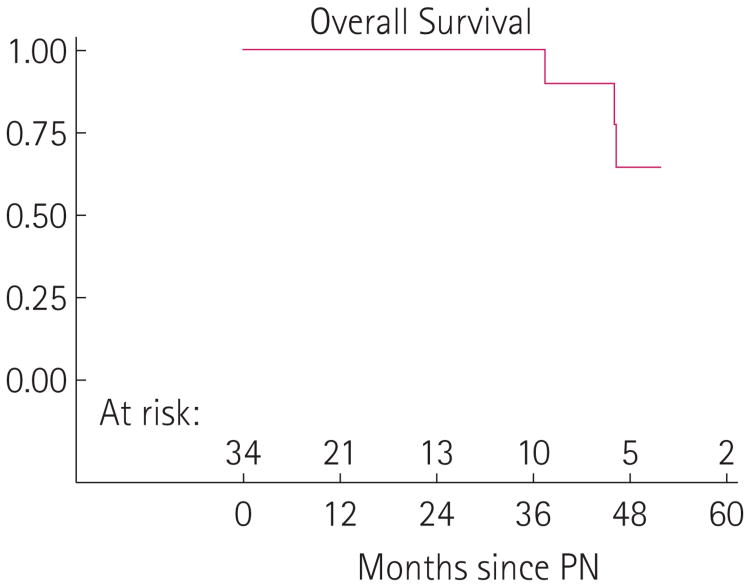
At the time of our review, 20 of the 28 patients (71%) with carcinoma present in their specimen were alive with no evidence of disease, three (11%) were alive with disease, three (11%) died from clear cell RCC, and two (7%) were deceased from unknown causes (Fig. 2). Two of the three patients with known preoperative metastatic disease (lung nodules, clear cell primary) are currently alive with stable disease while one patient has died from disease. The three deaths from disease consisted of one patient with known lung nodules preoperatively who had a 7.5-cm mass removed at surgery and lived for 47 months after PN. The second patient had a 7-cm tumour, developed lung nodules 24 months after PN, and died from his disease 47 months later, while the last patient had a 9-cm tumour, developed lung metastasis at 15 months and died from his disease 39 months later. Of the three patients currently alive with stable disease, two had known preoperative lung nodules with a primary lesion of 7 cm and 9 cm, respectively. The third patient alive with stable disease had a 7.5-cm primary tumour and developed lung metastasis 30 months after PN. All patients who dead from disease or who are alive with metastatic disease had clear cell as their primary pathology.
Fig. 2.
Kaplan–Meier overall survival curve.
The median (IQR) preoperative eGFR for our cohort was 65 (55–73) mL/min/1.73 m2 compared with an eGFR of 55 (47–74) mL/ min/1.73 m2 after PN (P = 0.003, paired Student's t-test). One patient required permanent haemo-dialysis after PN (preoperative eGFR of 46 mL/min/1.73 m2, had a solitary kidney with a 7.5-cm primary tumour, and cold ischaemia time 65 min). In all, 29 of the 37 (78%) operations were performed with renal hypothermia with a median (IQR) renal artery clamp time of 45 (36–55) min.
Four patients (11%) had postoperative urinary fistulae with a median (IQR) duration of leakage of 144 (39-174) days. Three of the four patients with urinary leaks had tumour sizes of 7 cm, 7.3 cm and 7.3 cm, respectively, while one patient had a 19-cm tumour. Three of four were managed conservatively by leaving the surgical drain in place until the leakage resolved, while the remaining patient required drain replacement 1 week after PN. There were no major complications such as pulmonary embolism, cardiac events, haemorrhage, or death.
Discussion
The use of PN as an accepted standard of care for the surgical treatment of small RCTs (T1) has gained acceptance over the past decade. Many authors and institutions have shown equivalent oncological outcomes for patients treated with PN for RCTs of up to 7 cm showing effective cancer control with preservation of renal function [3,4,8]. Despite these data, PN is under-utilized for patients with small RCTs. Hollenbeck et al. [9] reported that according to SEER data from 1988 to 2001 only 9.6% of patients with RCTs of <7 cm were treated with PN. Over the latter years of their study, the rate of PN for T1 tumours increased to 17.6%, but for patients with the smallest tumours (<2 cm), only 42% were treated with PN. In contrast to the national data, the use of PN at our institution for T1a tumours is now ≈90%, while almost 60% of patients with T1b lesions are treated with PN [10].
There are several reasons supporting the use of PN for the appropriately selected patient with a RCT. With the increased use of cross-sectional imaging (CT, MRI) as well as ultrasound to assist in the diagnostic evaluation of non-urological conditions, the detection rate of incidental RCTs has increased. In the present series, 25 of 34 patients (74%) (28 of 37 renal units) with RCTs of ≥7 cm had their tumour detected incidentally, with ≈20% of these RCTs being benign [11]. Conventional clear cell RCC is the most common (43% in the present study) and aggressive histological subtype, and conveys the highest risk for metastatic disease. Moreover, ≈25% of patients with a RCT will have a more indolent tumour such as papillary or chromophobe RCC [12]. However, current radiographic imaging methods cannot reliably determine the histological composition of the RCT, therefore a proportion of patients with large RCTs could be subjected to over treatment with RN.
Up to 26% of patients with RCTs have preexisting CKD defined as a GFR of <60 mL/min/1.73 m2, with RN being a significant risk factor for developing CKD [5]. Additionally, the recent observation showing significantly higher risks of hospitalization, cardiovascular morbidity, and death in patients with CKD (GFR <60 mL/min/m2) [6], underscores the importance of minimizing the impact of surgical treatment for RCTs.
Does size really matter? According to several studies, including a recent study from our institution, tumour size directly correlates with increasing malignant potential for patients with RCTs. The authors concluded that for every 1 cm increase in tumour size, the risk of increased malignancy increases by 16% [13]. Data from that study indicate that for patients with RCTs of >7 cm, 74% of their patients had clear cell RCC, which compares favourably to the present overall carcinoma rate (28 of 34 patients, 84%); however, only 16 patients (47%) in the present study had clear cell pathology. Additionally, six patients (17%) in the present study had benign tumours compared with 7.1% in their study. One patient in the present study had a 19-cm upper pole mass that on two different imaging studies was reported as indeterminate and was considered either adrenal or renal in origin. The mass involved ≈10% of the upper pole of the left kidney with the remaining portion of the kidney uninvolved and ultimately was an angiomyolipoma on final diagnosis. For this patient in whom imaging was indeterminate, would have lost his entire kidney and associated renal function for a benign process treated by a RN. Although current imaging methods cannot adequately distinguish histology, investigational ‘immuno-positron emission tomography’ imaging studies hold promise to differentiate clear cell pathologies from non-clear cell RCTs. In a pilot study of 25 patients, an iodine-labelled antibody to cG250 (carbonic anhydrase IX) present in conventional clear cell carcinomas detected 13 of 14 cases [14]. If these preliminary results are validated by ongoing study, this type of imaging study could assist in differentiating conventional clear cell RCTs from the more indolent non-clear cell types or benign RCTs. The three patients in the present study who had preoperative metastatic disease and the three patients who died from disease all had tumour sizes that were 7, 7.5 and 9 cm, respectively. While these patients had tumours that were larger than the standard 7 cm size criteria often used as a limit for PN, their tumours were adequately resected with negative surgical margins, therefore it is unlikely that a RN would have altered their disease course.
The preservation of renal function is an advantage of PN. In the present cohort the median preoperative eGFR was 65 mL/min/ 1.73 m2 compared with 55 mL/min/1.73 m2 after PN. While this change was statistically significant (P = 0.003), only one patient required haemodialysis after his PN. Moreover, if these patients had been treated with RN for their large renal mass, the change in eGFR would have been more significant, and at least the five patients with a solitary kidney would have been faced with dialysis.
Urinary fistula was the most common complication in the present series, occurring in four of 34 patients (11%), with a median (IQR) time of leakage of 144 (39–174) days. Three of four fistulae occurred in patients whose RCTs were ≈7 cm and were managed conservatively by leaving the postoperative surgical drain in place, while the other patient had a drain replaced. Our urinary leakage rate compares favourably with a recent series described by Meeks et al. [15] where 21 patients (13.3%) had a urine leak after PN. They reported that a larger tumour size was significantly associated with leakage, with their large tumour size being 3.2 cm, compared with our median tumour size of 7.5 cm. Prevention of urinary fistulae with meticulous intraoperative repair of the collecting system, as well a proper nutrition postoperatively remains the best strategy to avoid this complication. However, the risk of immediate, delayed, or prolonged urinary fistula should be discussed with all patients during preoperative counselling.
There are several limitations to the present study, including a small cohort of patients, relatively short duration of follow-up, and a single institution experience. Most of the RCTs in the present series (21 of 37 tumours, 57%) were 7–7.9 cm, thus not markedly larger than the traditional 7 cm size criteria for PN. However, based on published practice patterns, most if not all of these RCTs would have been treated by RN at other institutions [9]. Our small group of patients maybe indicative of a selection bias, as this cohort reflects a highly selected group of patients who were referred to a tertiary care centre for treatment of their large renal mass. Longer follow-up of these patients for the development of metastatic disease or morbidities related to CKD would be preferred. Perhaps a larger cohort of patients with larger RCTs could be obtained through a multicentre review.
In conclusion, the present study suggests that PN for RCTs of ≥7 cm can be performed without major surgical complications and with effective tumour control for carefully selected patients. We feel that strict size criteria should not be an absolute contraindication to this approach and PN should be considered for patients with appropriate tumours, solitary kidneys or preexisting renal insufficiency.
Acknowledgments
Supported by: The Sidney Kimmel Center for Prostate and Urologic Cancers.
Source of Funding: Division of Urology of MSKCC.
Abbreviations
- RCT
renal cortical tumour
- (R)(P)N
(radical) (partial) nephrectomy
- CKD
chronic kidney disease
- eGFR
estimated GFR
- IQR
interquartile range
Footnotes
Conflict of Interest: None declared.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. Ca Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Robson CJ. Radical nephrectomy for renal cell carcinoma. J Urol. 1963;89:37–42. doi: 10.1016/S0022-5347(17)64494-X. [DOI] [PubMed] [Google Scholar]
- 3.Dash A, Vickers AJ, Schachter LR, Bach AM, Snyder ME, Russo P. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4–7 cm. Bju Int. 2006;97:939–45. doi: 10.1111/j.1464-410X.2006.06060.x. [DOI] [PubMed] [Google Scholar]
- 4.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zinke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066–70. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 5.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–40. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Patard JJ, Shvarts O, Lam JS, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171:2181–5. doi: 10.1097/01.ju.0000124846.37299.5e. [DOI] [PubMed] [Google Scholar]
- 9.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. Use of nephrectomy at select medical centers – a case of follow the crowd? J Urol. 2006;175:670–4. doi: 10.1016/S0022-5347(05)00146-1. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RH, Kaag M, Vickers A, et al. Contemporary use of partial nephrectomy at a tertiary care center in the United States. J Urol. 2009;181:993–7. doi: 10.1016/j.juro.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder ME, Bach A, Kattan MW, Raj GV, Reuter VE, Russo P. Incidence of benign lesions for clinically localized renal masses smaller than 7 cm in radiological diameter: influence of sex. J Urol. 2006;176:2391–6. doi: 10.1016/j.juro.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170:2163–72. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 13.Thompson RH, Kurta JM, Kaag M, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol. 2009;181:2033–6. doi: 10.1016/j.juro.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo P. Contemporary understanding and management of renal cortical tumors. Urol Clin North Am. 2008;35:xiii–xvii. doi: 10.1016/j.ucl.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Meeks JJ, Zhao LC, Navai N, Perry KT, Jr, Nadler RB, Smith ND. Risk factors and management of urine leaks after partial nephrectomy. J Urol. 2008;180:2375–8. doi: 10.1016/j.juro.2008.08.018. [DOI] [PubMed] [Google Scholar]