Abstract
Objective
HIV infection is associated with cancer risk. This relationship has resulted in a growing cancer burden, especially in resource-limited countries where HIV is highly prevalent. Little is known, however, about how HIV affects cancer survival in these settings. We therefore investigated the role of HIV in cancer survival in Uganda.
Design
Retrospective cohort (N = 802).
Methods
Eligible cancer patients were residents of Kyadondo County, at least 18 years of age at cancer diagnosis, and diagnosed between 2003 and 2010 with one of the following: breast cancer, cervical cancer, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, or esophageal cancer. Patients were classified as HIV-infected at cancer diagnosis based on a documented positive HIV antibody test, medical history indicating HIV infection, or an HIV clinic referral letter. The primary outcome, vital status at 1 year following cancer diagnosis, was abstracted from the medical record or determined through linkage to the national hospice database. The risk of death during the year after cancer diagnosis was compared between cancer patients with and without evidence of HIV infection using Cox proportional hazards regression.
Results
HIV-infected cancer patients in Uganda experienced a more than two-fold increased risk of death during the year following cancer diagnosis compared to HIV-uninfected cancer patients [hazard ratio 2.28; 95% confidence interval (CI) 1.61–3.23]. This association between HIV and 1-year cancer survival was observed for both cancers with (hazard ratio 1.56; 95% CI 1.04–2.34) and without (hazard ratio 2.68; 95% CI 1.20–5.99) an infectious cause.
Conclusion
This study demonstrates the role of HIV in cancer survival for both cancers with and without an infectious cause in a resource-limited, HIV-endemic setting.
Keywords: cancer in Africa, cancer survival, HIV, immunosuppression, Uganda
Introduction
Introduction of HAART in 1996 led to marked improvements in immune status and life expectancy for HIV-infected individuals in the US and Europe [1]. Although HIV treatment rollout has been delayed in resource-limited settings such as East Africa, a 2010 report demonstrated greater than 65% reductions in overall mortality for HIV-infected Ugandan patients after the introduction of antiretroviral therapy (ART) [2]. Recent evidence demonstrates that patients who initiate ART can in fact achieve life expectancy comparable to the Ugandan general population [3].
This increased life expectancy exposes HIV-infected patients to the risk of developing chronic diseases, including cancer. HIV infection and cancer risk have been linked in the US since the beginning of the HIV epidemic [4–11]. Importantly, cancer is a complication of HIV infection that is increasingly recognized to impact not just resource-rich but also resource-limited regions [12–14]. One Ugandan study utilized data from the Kampala Cancer Registry (KCR) to compared cancer rates in patients from a large HIV clinic to those in the general population, and observed significantly elevated cancer risk associated with an AIDS diagnosis [15]. Data from Uganda and Kenya have also recently demonstrated declines in individual Kaposi sarcoma (KS) risk in HIV-infected patients after ART treatment [16]. However, the association between successful HIV treatment and declining KS incidence on a population level in Uganda is not as clear as the dramatic declines observed after the introduction of ART in the US [13,17–22]. KCR trend data report that KS incidence has only begun declining [annual percentage change: −4.5%; 95% confidence interval (CI) −5.6%, −3.4%] for men under the age of 50, with no significant declines observed for either men aged greater than 50 or any subset of women [13,23].
As expected, increased HIV treatment uptake in resource-limited settings has increased HIV-infected patient life expectancy. Against this backdrop, the consistently elevated risk for cancer in this ageing population will result in a growing number of patients diagnosed with both diseases over time. Therefore, the question of whether and how HIV plays a role in cancer outcomes is of public health importance.
Little is known, however, about the role HIV plays in cancer survival in either resource-rich or resource-limited settings. Since the introduction of ART in Uganda in the early 2000s, results from only one cancer survival study have been reported. In that study, 154 non-Hodgkin lymphoma (NHL) patients were ascertained from the Uganda Cancer Institute (UCI), and the study found that HIV-infected NHL patients not receiving HIV treatment experienced significantly poorer 1-year cancer survival than both HIV-uninfected NHL patients and HIV-infected NHL patients receiving appropriate HIV treatment [24]. The growing importance of the role of immunosuppression in cancer patient outcomes and the lack of data specific to resource-limited settings motivated us to investigate the role of HIV in survival after a cancer diagnosis in Uganda.
Methods
Eligibility
Eligible cancer patients were residents of Kyadondo County, Uganda, at least 18 years of age at the time of cancer diagnosis, and diagnosed from 2003 to 2010 with one of the following: breast cancer, cervical cancer, NHL, Hodgkin lymphoma (HL) or esophageal cancer. Patient residence, age at diagnosis, and year of diagnosis were abstracted from the medical record. For men, we selected two cancers with (NHL, HL) and one without (esophageal) an established infectious cause. For women, we selected the two most common cancers: one with (cervical) and one without (breast) an infectious cause. Patients with a prior malignancy noted in the medical record were excluded.
Case ascertainment
Eligibility criteria were used to generate lists of cancer patients using the KCR database, a population-based cancer registry that has performed cancer registration in Kyadondo County since the 1950s, with the exception of selected years in the 1970–1980s due to political turmoil [14,25]. Cases are ascertained for the KCR primarily from the Pathology Department at Mulago Hospital, the largest tertiary referral hospital in Uganda, as well as the four main hospitals in Kampala and the national hospice system. Patient lists were then transferred to the UCI, the only comprehensive cancer treatment facility for Uganda, where record departments facilitated medical record retrieval. Lists were also provided to appropriate clinics at Mulago Hospital in order to survey for records for cancer patients who may not have presented to the UCI. To achieve more complete case ascertainment, eligibility criteria were also given directly to the record officers at the UCI and Mulago to check against the clinic log books used for patient registration and to aid in the identification of patients meeting eligibility criteria who may not have been included in the KCR database listing.
Exposure assessment
A cancer patient was classified as HIV-infected at the time of cancer diagnosis if he/she met any of the following criteria: documented positive HIV antibody test, indication of HIV infection in the medical history, or evidence of treatment at a local HIV clinic. If a cancer patient did not have specific evidence of HIV infection, the patient was classified as HIV-uninfected.
Outcome assessment
The primary outcome was vital status at 1 year following cancer diagnosis, with death from any cause classified as an event. Three possible vital status outcomes existed for each cancer patient: alive – medical record included a clinic visit dated at least 1 year after cancer diagnosis; dead – medical record noted the patient died during the year following cancer diagnosis; and unknown/lost – last clinic visit date in the medical record was less than 1 year after cancer diagnosis and medical record did not specifically note the patient died.
Cancer patients at the UCI and Mulago are often transferred to the national hospice system (Hospice Africa Uganda) for palliative care, and the national hospice system conducts active follow-up for enrollees. Therefore, for any cancer patient with unknown vital status at 1 year, linkage to the national hospice system database was conducted.
Survival time for each cancer patient was calculated beginning at the earlier of: date of first presentation, listed on the medical record intake form, or biopsy result date, listed on the pathology report confirming a histological diagnosis. The survival time end date was date of death listed in the medical record for deceased cancer patients. Survival time was censored on the last date of clinical contact listed in the medical record or last date listed in the national hospice system database for cancer patients who were not deceased. For 1-year survival analyses, cancer patients with survival time greater than 1 year were administratively censored at 1 year, and for 2-year survival, patients with survival time greater than 2 years were administratively censored at 2 years.
Covariates and extent of disease assessment
Patient characteristics at cancer diagnosis were abstracted directly from the medical record, including age, sex, duration of symptoms, history of smoking, family history of cancer, and prior medical conditions (diabetes, tuberculosis, cardiovascular disease). Reproductive history, including parity, contraception, menarche, and menopausal status, was generally recorded in the clinical notes for female patients. Information on BMI, measured for the purpose of chemotherapy dosage, was available for lymphoma patients, and hemoglobin was noted for lymphoma and cervical cancer patients.
Cancer stage was abstracted from the medical chart. If an initial clinical stage was not specifically noted, a consulting physician reviewed the medical record and adjudicated stage based on available data. If stage was assigned based on the TNM Classification of Malignant Tumors system, the T, N, and M values were used to assign a categorical value ranging from stage I to IV, with stages I and II considered early disease and stages III and IV considered advanced disease.
Statistical analysis
Kaplan–Meier product-limit survival estimates were generated comparing differences in time to death in the year following cancer diagnosis according to HIV infection using the log-rank test. Cox proportional hazards regression was utilized to evaluate the association between HIV infection at cancer diagnosis and 1-year cancer survival. Multivariable regression models included the following covariates selected a priori: age, calendar year of cancer diagnosis, and cancer stage.
Etiologically meaningful subgroups of cancers were defined as: cancers with an infectious cause (NHL, HL, cervical) and cancers without an established infectious cause (breast, esophageal). Regression models were evaluated among NHL and cervical cancer patients with further adjustment for variables with P-values less than 0.10 in Kaplan–Meier analyses, including BMI (NHL) and hemoglobin (NHL, cervical cancer).
HIV-infected patients were subdivided according to year of cancer diagnosis, which served as a surrogate for estimated ART availability in Uganda: 2003–2005 (ART coverage: 5–25%), 2006–2008 (ART coverage: 30–45%), and 2009–2010 (ART coverage ≥50%) [26]. Risk of death during the year after cancer diagnosis was compared between HIV-uninfected cancer patients and HIV-infected cancer patient subgroups. To avoid over-adjustment, year of diagnosis was not included in this model.
Results
The cohort (N = 802) included the following cancer diagnoses: breast cancer (n =220), cervical cancer (n = 316), NHL (n = 134), HL (n = 63), and esophageal cancer (n = 69) (Table 1, Fig. 1). Approximately one-third of cancer patients were HIV-infected at cancer diagnosis, although the proportion differed by cancer type. Among patients diagnosed with a cancer without an established infectious cause, HIV prevalence was much lower (breast: 11%; esophageal: 6%) than among patients diagnosed with infection-related cancers (NHL: 57%; cervical: 42%; HL 56%).
Table 1.
Cancer patient cohort characteristics.
| Total cohort (N = 802) |
HIV-negative (n = 528) |
HIV-positive (n = 274) |
Chi-square P-valuesa | ||||
|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | ||
| Cancer type | |||||||
| Breast cancer | 220 | 27.4 | 196 | 37.1 | 24 | 8.8 | |
| Cervical cancer | 316 | 39.4 | 182 | 34.5 | 134 | 48.9 | |
| Non-Hodgkin lymphoma | 134 | 16.7 | 57 | 10.8 | 77 | 28.1 | |
| Hodgkin lymphoma | 63 | 7.9 | 28 | 5.3 | 35 | 12.8 | |
| Esophageal cancer | 69 | 8.6 | 65 | 12.3 | 4 | 1.5 | <0.01 |
| Sex of patientb | |||||||
| Female | 125 | 47.0 | 62 | 41.3 | 63 | 54.3 | |
| Male | 141 | 53.0 | 88 | 58.7 | 53 | 45.7 | 0.04 |
| Age (years) | |||||||
| 18–35 | 211 | 26.3 | 103 | 19.5 | 108 | 39.4 | |
| 36–44 | 188 | 23.4 | 93 | 17.6 | 95 | 34.7 | |
| 45–55 | 210 | 26.2 | 159 | 30.1 | 51 | 18.6 | |
| 56–86 | 185 | 23.1 | 169 | 32.0 | 16 | 5.8 | <0.01 |
| Year of cancer diagnosis | |||||||
| 2003–2005 | 120 | 15.0 | 81 | 15.3 | 39 | 14.2 | |
| 2006–2008 | 367 | 45.8 | 244 | 46.2 | 123 | 44.9 | |
| 2009–2010 | 315 | 39.3 | 203 | 38.5 | 112 | 40.9 | 0.78 |
| BMIc | |||||||
| <18.5 | 73 | 37.2 | 34 | 40.5 | 39 | 34.8 | |
| 18.5–24.99 | 66 | 33.7 | 23 | 27.4 | 43 | 38.4 | |
| 25.0–29.99 | 19 | 9.7 | 11 | 13.1 | 8 | 7.1 | |
| 30+ | 7 | 3.6 | 4 | 4.8 | 3 | 2.7 | 0.32 |
| Missing | 31 | 15.8 | 12 | 14.3 | 19 | 17.0 | |
| Anemia at presentationd | |||||||
| No | 126 | 24.6 | 77 | 28.8 | 49 | 19.9 | |
| Yes: ≤12 g/dl | 221 | 43.1 | 100 | 37.5 | 121 | 49.2 | |
| Severe: ≤7 g/dl | 70 | 13.6 | 32 | 12.0 | 38 | 15.4 | 0.01 |
| Missing | 96 | 18.7 | 58 | 21.7 | 38 | 15.4 | |
| Tumor stage | |||||||
| Stage I | 31 | 3.9 | 19 | 3.6 | 12 | 4.4 | |
| Stage II | 105 | 13.1 | 49 | 9.3 | 56 | 20.4 | |
| Stage III | 377 | 47.0 | 262 | 49.6 | 115 | 42.0 | |
| Stage IV | 160 | 20.0 | 94 | 17.8 | 66 | 24.1 | <0.01 |
| Missing | 129 | 16.1 | 104 | 19.7 | 25 | 9.1 | |
| Parity | |||||||
| Nulliparous | 31 | 4.7 | 19 | 4.4 | 12 | 5.4 | |
| Parous | 535 | 81.4 | 348 | 79.8 | 187 | 84.6 | 0.67 |
| Missing | 91 | 13.9 | 69 | 15.8 | 22 | 10.0 | |
| Menopausal statuse | |||||||
| Post-menopausal | 183 | 27.9 | 165 | 37.8 | 18 | 8.1 | |
| Pre-menopausal | 369 | 56.2 | 190 | 43.6 | 179 | 81.0 | <0.01 |
| Missing | 105 | 16.0 | 81 | 18.3 | 24 | 10.9 | |
| Vital status at 1 year | |||||||
| Alive | 322 | 40.2 | 234 | 44.3 | 88 | 32.1 | |
| Dead | 152 | 19.0 | 79 | 15.0 | 73 | 26.6 | |
| Lost to follow-up | 328 | 40.9 | 215 | 40.7 | 113 | 41.2 | <0.01 |
Chi-square P-values for difference according to HIV infection status for each covariate of interest.
Numbers and percentages for sex restricted to lymphoma and esophageal cancer patients (n=266).
Numbers and percentages for BMI restricted to lymphoma patients (n=197).
Numbers and percentages for anemia restricted to lymphoma and cervical cancer patients (n=513).
Numbers and percentages for parity and menopausal status restricted to female patients (n=657).
Fig. 1. Ascertainment source and vital status at 1 year of cohort patients, according to cancer diagnosis.
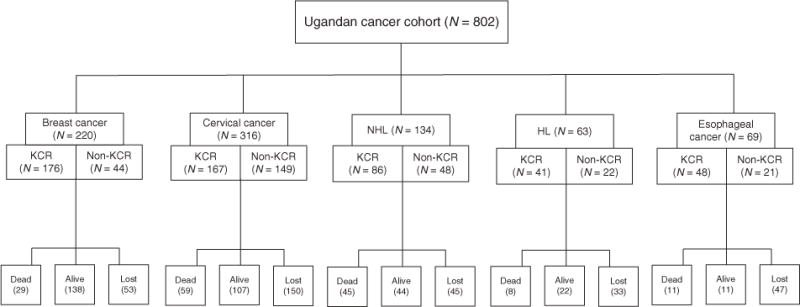
Ascertainment sources include KCR: medical records retrieved by providing the list of names from the KCR database to the records officers, and non-KCR: medical records retrieved by the records officers scanning clinic log books for additional cases who met eligibility criteria. KCR, Kampala Cancer Registry.
Only 322 of the 802 cohort members were alive 1 year after cancer diagnosis. Among those who died in the year following cancer diagnosis (n=152), more than two-thirds died in the first 6 months. For patients with unknown vital status (n = 328), over half were lost to follow-up by 3 months, indicative of loss occurring almost immediately after cancer diagnosis. Risk of death was significantly related to specific cancer type (log rank P-value <0.01), regardless of HIV status. Breast cancer patients had the best prognosis, with nearly two-thirds confirmed as alive at 1 year, whereas 1-year survival ranged from 33 to 35% for cancers with an infectious cause (Table 2). Only 16% of esophageal cancer patients were confirmed as alive at 1 year, although most patients (70%) were lost to follow-up in that first year.
Table 2.
Association between HIV and 1-year cancer survival.
| Total/deaths | Percentage alive at 1 year | Model 1a
|
Model 2b
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| All cancer patients (N = 802) | ||||||
| HIV-uninfected | 528/79 | 44.3 | 1.00 | 1.00 | ||
| HIV-infected | 274/73 | 32.1 | 2.08 (1.47–2.94) | <0.01 | 2.28 (1.61–3.23) | <0.01 |
| Cancers with infectious cause (n = 513) | ||||||
| HIV-uninfected | 267/48 | 36.7 | 1.00 | 1.00 | ||
| HIV-infected | 246/64 | 30.5 | 1.52 (1.01–2.28) | 0.04 | 1.56 (1.04–2.34) | 0.03 |
| NHL (n = 134) | ||||||
| HIV-uninfected | 57/19 | 42.1 | 1.00 | 1.00 | ||
| HIV-infected | 77/26 | 26.0 | 1.16 (0.63–2.14) | 0.65 | 1.33 (0.71–2.47) | 0.37 |
| HL (n = 63) | ||||||
| HIV-uninfected | 28/3 | 35.7 | 1.00 | 1.00 | ||
| HIV-infected | 35/5 | 34.3 | 1.71 (0.36–8.07) | 0.50 | 1.74 (0.36–8.33) | 0.49 |
| Cervical cancer (n = 316) | ||||||
| HIV-uninfected | 182/26 | 35.2 | 1.00 | 1.00 | ||
| HIV-infected | 134/33 | 32.1 | 1.59 (0.88–2.85) | 0.12 | 1.71 (0.96–3.04) | 0.07 |
| Cancers without infectious cause (n = 289) | ||||||
| HIV-uninfected | 261/31 | 52.1 | 1.00 | 1.00 | ||
| HIV-infected | 28/9 | 46.4 | 2.64 (1.19–5.86) | 0.02 | 2.68 (1.20–5.99) | 0.02 |
| Breast cancer (n = 220) | ||||||
| HIV-uninfected | 196/23 | 63.8 | 1.00 | 1.00 | ||
| HIV-infected | 24/6 | 54.2 | 1.98 (0.74–5.28) | 0.17 | 2.04 (0.76–5.47) | 0.16 |
| Esophageal cancer (n = 69) | ||||||
| HIV-uninfected | 65/8 | 16.9 | 1.00 | 1.00 | ||
| HIV-infected | 4/3 | 0.0 | 4.63 (0.95–22.6) | 0.06 | 4.09 (0.80–20.86) | 0.09 |
CI, confidence interval; HL, Hodgkin lymphoma; HR, hazard ratio; NHL, non-Hodgkin lymphoma.
Model includes: age, year of cancer diagnosis.
Model includes: Model 1 + stage at presentation (categorical: stage I/II, stage III/IV, stage unknown).
The majority of cohort members were women due to inclusion of breast and cervical cancer diagnoses; among lymphoma and esophageal cancer patients, approximately 45–55% were females. HIV-infected cancer patients were significantly younger than HIV-uninfected patients. For example, 41% of HIV-infected cervical cancer patients were 18–35 years old at diagnosis, compared to only 13% for HIV-uninfected cervical cancer patients.
Esophageal cancer, the malignancy with the lowest proportion of HIV-infected cases, was the only cancer for which the majority of patients (64%) were 56–86 years old at diagnosis. The stage of disease at presentation also varied according to HIV status (Fig. 2). HIV-infected cervical cancer patients were uniformly diagnosed at earlier stages: 38% of HIV-infected cervical cancer cases were diagnosed with stage I–II disease, compared to 21% of HIV-uninfected cervical cancer patients. In contrast, HIV-infected HL patients were diagnosed with advanced disease more often than HIV-uninfected patients. There was no substantive difference observed for NHL or breast cancer patients, who were uniformly diagnosed with advanced disease, regardless of HIV status. Stage at presentation was undetermined for 56 of the 69 esophageal cancer patients.
Fig. 2.
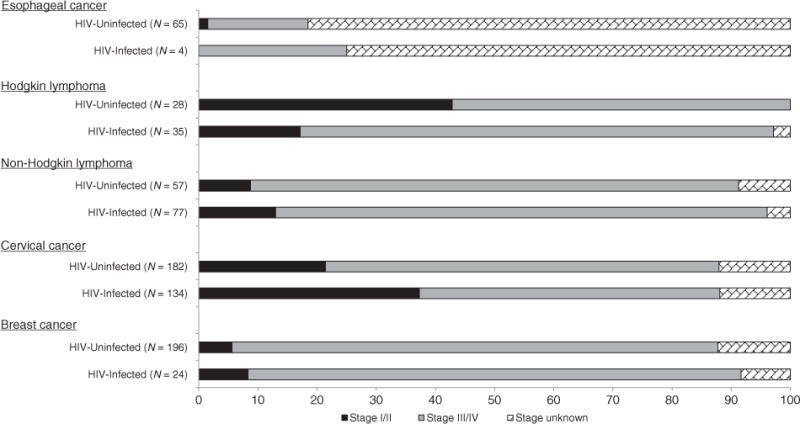
Percentage distribution of stage of disease at presentation, according to cancer diagnosis and HIV status.
HIV-infected cancer patients were more than twice as likely to die during the year following cancer diagnosis compared with HIV-uninfected cancer patients (hazard ratio 2.28; 95% CI 1.61–3.23) (Table 2). Importantly, this association between HIV infection at cancer diagnosis and poorer 1-year cancer survival was observed for both cancers with and without an infectious cause. Specifically, HIV-infected patients diagnosed with infection-related cancers had greater than 50% higher risk of death during the year following cancer diagnosis (hazard ratio 1.56; 95% CI 1.04–2.34), and HIV-infected patients diagnosed with cancers without an infectious cause also experienced significantly higher risk of death (hazard ratio 2.68; 95% CI 1.20–5.99). Risk of dying with NHL was not different from the results shown in Table 2 after further adjustment for sex, hemoglobin, and BMI, and risk of dying with cervical cancer was not different after adjustment for hemoglobin.
Although most deaths occurred in the first year after cancer diagnosis, an additional 42 patients died during the second year. Utilizing this additional follow-up showed 2-year outcomes consistent with those reported for 1 year (Supplemental Table 1, http://links.lww.com/QAD/A389). To investigate the impact of HIV therapy on 1-year cancer survival, we compared HIV-uninfected to HIV-infected cancer patients diagnosed during different periods of estimated ARTavailability in Uganda [26]. HIV-infected cases diagnosed prior to widespread ART availability (2003–2005) experienced the poorest 1-year cancer survival compared to HIV-uninfected patients (hazard ratio 2.75; 95% CI 1.43–5.28) (Fig. 3). Risk of death during the year after cancer diagnosis remained elevated in HIV-infected patients diagnosed in more recent years.
Fig. 3. Association between HIV and 1-year cancer survival, according to HIV treatment availability.
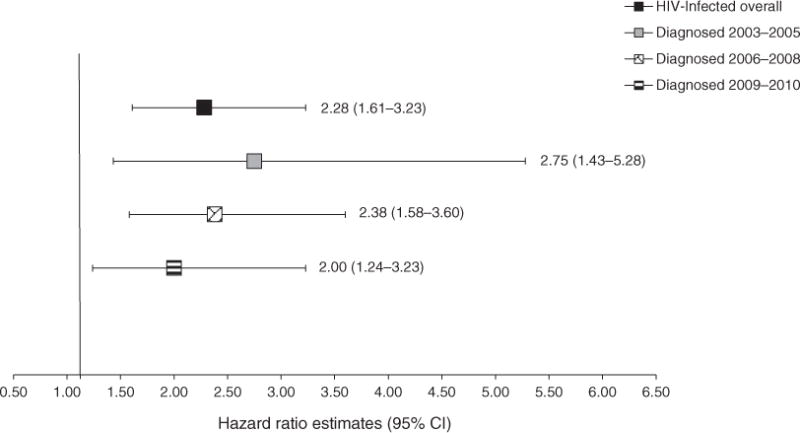
Estimated HIV treatment availability in Uganda [26]: 2003–2005: 5–25%; 2006–2008: 30–45%; 2009–2010: assumed to have increased above 50%.
Approximately 40% of patients classified as HIV-uninfected lacked direct confirmation of HIV-uninfected status in the medical record, defined as either a negative HIV antibody test result or HIV-uninfected status recorded in clinical notes. However, no differences in 1-year cancer survival were observed between confirmed and unconfirmed HIV-uninfected cases (hazard ratio 0.87; 95% CI 0.54–1.40). Results for 1-year cancer survival comparing only confirmed HIV-uninfected to HIV-infected cancer patients were consistent with results shown in Table 2 (hazard ratio 2.18; 95% CI 1.50–3.18).
Discussion
This is the largest and most comprehensive study of the association between HIV and cancer survival in sub-Saharan Africa to date. We observed that HIV-infected cancer patients in Uganda experienced a more than twofold increase in risk of death in the year following cancer diagnosis compared to HIV-uninfected cancer patients. This association between HIV status at cancer diagnosis and poorer cancer survival was observed for both cancers with and without an infectious cause, regardless of stage at diagnosis. These findings extend the established relationship between HIV and excess cancer risk to include a role for HIV in cancer outcomes.
Only one prior cancer survival study has been reported after the introduction of ART in Uganda. The UCI-based study observed that HIV-infected NHL patients receiving ART experienced comparable 1-year cancer survival to HIV-uninfected patients with NHL [24]. One pre-ART study examined outcomes 3 years after cervical cancer diagnosis and reported marginally poorer survival for HIV-infected women at 1 year, although differences according to HIV status did not persist among women surviving 3 years after cervical cancer diagnosis [27].
Our findings are consistent with the limited data available from the US on HIV-related immunosuppression and cancer survival. The largest study to date, a New York-based registry study, observed that the survival disadvantage after cancer diagnosis in HIV-infected relative to HIV-uninfected cancer patients decreased with the introduction of HAART [28]. However, the inclusion of data from 1980 to 2000 complicates interpretation of these results since cancer patient mortality could have been largely influenced by drastic changes in the rate of AIDS-related deaths across different HIV therapy decades. Only one large US cancer survival study to date has been conducted using data solely from the HAART era [29]. After accounting for cancer stage and cancer treatment among HIV-infected patients treated with combination ART, researchers observed lower risk of death after cancer diagnosis for HIV-infected patients with higher levels of immune competence and well controlled HIV viremia.
It is accepted that immunosuppression is associated with increased cancer risk, corroborated by observations in both organ transplant recipients [30–34] and HIV-infected patients [11,20,35]. Specifically, cancers attributable to infectious agents are most commonly elevated in immunosuppressed individuals [36–40]. We observed that a relationship between HIV infection and cancer survival exists not only for infection-related cancers but also for cancers without an established infectious cause. This suggests that once a tumor is present, mechanisms may exist whereby immunosuppression can alter tumor growth for cancers with distinct causes.
For infection-related cancers, immune dysfunction results in an inability to control oncogenic viruses. Both the number and functional capacity of antiviral, Epstein-Barr virus (EBV)-specific CD8+ T cells are impaired in AIDS patients who progress to NHL [41–43], providing a biologically plausible mechanism for the association between HIV-related immunosuppression and NHL risk. Antiviral immunity can also influence tumor progression; studies have observed that HIV-infected KS patients with clinical improvement had lower levels of replicating human herpesvirus 8 (HHV8), the causative agent for KS in the peripheral blood and superior CD8+ T-cell responses specific to HHV8 [44,45].
CD8+ T-cell dysfunction is a hallmark of HIV pathogenesis that occurs as a result of the destruction of CD4+ T cells by HIV and chronic immune activation that stems from persistent, long-term HIV infection and prevalent co-infections such as cytomegalovirus [46–51]. A key implication of chronic immune activation is premature ageing of the immune system, known as immune senescence [52–55]. This immune dysfunction has implications for antitumor surveillance among all cancer diagnoses since CD8+ T cells can develop cytotoxic responses to proteins on the surfaces of tumor cells, and both experimental and population studies have illustrated the importance of these lymphocytes in the prevention of tumor formation and in altering tumor prognosis [56–67].
Another direct implication of persistent immune activation in the context of HIV is chronic inflammation [68,69]. Levels of pro-inflammatory cytokines are significantly higher in HIV-infected versus HIV-uninfected individuals, and population studies have consistently found that inflammation correlates with the degree of HIV disease severity [70–73]. Elevated inflammatory signaling could alter tumor promotion since inflammation is considered to be an underlying hallmark of cancer [74–77]. Ultimately, the tumor environment may be affected by the presence of HIV infection, regardless of tumor cause. The exhaustion of key lymphocyte populations could diminish the capacity of the immune system to control the metastatic potential of that tumor, whereas high levels of inflammation can promote tumor growth.
Our findings also highlight clinically relevant differences between HIV-infected and HIV-uninfected cancer patients. The results of this study suggest that vigilance is indicated in the management of HIV-infected cancer patients as they often present at a high stage of disease. Interestingly, among cervical cancer patients, HIV-infected women were actually diagnosed at earlier stages of disease at twice the rate of HIV-uninfected women, suggesting that women currently in the HIV care system may be under increased surveillance.
In agreement with earlier findings from the UCI for NHL patients [24], we also observed evidence that management of HIV-infected cancer patient immunosuppression is important for 1-year cancer outcomes. Although individual calendar year did not impact cancer survival among all study participants, HIV-infected patients diagnosed in the subset of years with limited ART availability in Uganda had a more pronounced risk of death after cancer diagnosis compared to HIV-infected patients diagnosed after more widespread ART rollout. Future studies that collect sensitive information on immunosuppression, including cancer patient CD4+ T-cell counts, are needed to confirm these clinically relevant findings in this patient setting.
Strengths of this study include restriction of eligibility to recent years, avoiding vast differences that would be expected across different decades in overall survival because of changes in patterns of deaths due to AIDS. In addition, data on stage at presentation were collected, decreasing the potential for confounding bias in survival comparisons. The inclusion of HIV-uninfected cancer patients provided a true unexposed comparison group, and we were able to enroll adequate numbers of cancer patients to estimate type-specific effects for certain cancers. Future studies could combine data from extant cancer registries across sub-Saharan Africa and include additional years of cancer registration in order to increase event numbers and improve statistical power to detect differences for rarer malignancies. This study is not without limitations, including significant loss to followup. Approximately 40% of cancer patients were lost from vital status follow-up prior to 1 year. Reassuringly, however, the difference in loss to follow-up did not differ by HIV status. Another limitation was the lack of specific ART information. We examined the data by year of cancer diagnosis as a proxy for HIV treatment availability and found that survival appeared somewhat improved in more recent years. It is possible that cancer diagnostics and treatment improved with increasing calendar years, making it impossible to directly attribute the increased survival in more recent calendar years to availability of ART alone. Future studies should consider individual ART data over time across a range of baseline CD4+ T-cell counts to confirm that improved immune status is associated with altered cancer patient survival.
Uganda does not have nationwide death registration to provide cause of death information. This precluded an examination of cancer-specific survival. Instead, overall survival during the year following cancer diagnosis was used as a verifiable outcome. Our confidence that the survival differences observed reflected not just HIV-related causes of death but also cancer-specific death is strengthened by the proximity of the majority of events to the date of cancer diagnosis, a trend consistent irrespective of HIV status. We were also unable to ensure complete case ascertainment; cases lost to follow-up prior to histological diagnosis or with lost medical records were not available. Although this limitation does not threaten internal validity of the study, it may limit generalizability.
The study demonstrated a role for HIV in cancer patient survival for cancers with and without an infectious cause in a resource-limited, HIV-endemic setting. To extend our findings, future investigations should include prospective evaluation of cancer-specific outcomes and active mortality follow-up. The collection of more sensitive, longitudinal, HIV-related measures such as HIV RNA level and CD4+ T-cell counts may identify characteristics of HIV-infected cancer patients that could explain observed survival differences. The question of the role of immunosuppression in cancer patient outcomes will become increasingly important as the number of HIV-infected patients diagnosed with cancer continues to grow in both resource-rich and resource-limited settings.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute at the National Institutes of Health (T32 CA09168, R03 CA153371).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ray M, Logan R, Sterne JA, Hernandez-Diaz S, Robins JM, Sabin C, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasamba I, Baisley K, Mayanja BN, Maher D, Grosskurth H. The impact of antiretroviral treatment on mortality trends of HIV-positive adults in rural Uganda: a longitudinal population-based study, 1999–2009. Trop Med Int Health. 2012;17:e66–e73. doi: 10.1111/j.1365-3156.2012.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155:209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 4.Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ. Trends in Kaposi’s sarcoma and non-Hodgkin’s lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;94:1204–1210. doi: 10.1093/jnci/94.16.1204. [DOI] [PubMed] [Google Scholar]
- 5.Mbulaiteye SM, Biggar RJ, Goedert JJ, Engels EA. Immune deficiency and risk for malignancy among persons with AIDS. J Acquir Immune Defic Syndr. 2003;32:527–533. doi: 10.1097/00126334-200304150-00010. [DOI] [PubMed] [Google Scholar]
- 6.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 7.Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med. 2010;170:1337–1345. doi: 10.1001/archinternmed.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedimo RJ, McGinnis KA, Dunlap M, Rodriguez-Barradas MC, Justice AC. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr. 2009;52:203–208. doi: 10.1097/QAI.0b013e3181b033ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 10.Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. 2009;27:884–890. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- 11.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casper C. The increasing burden of HIV-associated malignancies in resource-limited regions. Annu Rev Med. 2011;62:157–170. doi: 10.1146/annurev-med-050409-103711. [DOI] [PubMed] [Google Scholar]
- 13.Parkin DM, Nambooze S, Wabwire-Mangen F, Wabinga HR. Changing cancer incidence in Kampala, Uganda, 1991–2006. Int J Cancer. 2010;126:1187–1195. doi: 10.1002/ijc.24838. [DOI] [PubMed] [Google Scholar]
- 14.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer. 2000;82:1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mbulaiteye SM, Katabira ET, Wabinga H, Parkin DM, Virgo P, Ochai R, et al. Spectrum of cancers among HIV-infected persons in Africa: the Uganda AIDS-Cancer Registry Match Study. Int J Cancer. 2006;118:985–990. doi: 10.1002/ijc.21443. [DOI] [PubMed] [Google Scholar]
- 16.Martin JBN, Bwama M, Muyindike W, Amerson E, Musick B, Wois-Kaloustain K. Prospective evaluation of the impact of ART on the incidence of KS–IDEA Consortium. 2012 [Google Scholar]
- 17.Parkin DM, Wabinga H, Nambooze S, Wabwire-Mangen F. AIDS-related cancers in Africa: maturation of the epidemic in Uganda. AIDS. 1999;13:2563–2570. doi: 10.1097/00002030-199912240-00010. [DOI] [PubMed] [Google Scholar]
- 18.Semeere AS, Busakhala N, Martin JN. Impact of antiretroviral therapy on the incidence of Kaposi sarcoma in resource-rich and resource-limited settings. Curr Opin Oncol. 2012;24:522–530. doi: 10.1097/CCO.0b013e328355e14b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedimo R, Chen RY, Accortt NA, Raper JL, Linn C, Allison JJ, et al. Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1989–2002. Clin Infect Dis. 2004;39:1380–1384. doi: 10.1086/424883. [DOI] [PubMed] [Google Scholar]
- 20.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99:962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 21.Crum-Cianflone N, Hullsiek KH, Marconi V, Weintrob A, Ganesan A, Barthel RV, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of anti-retroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 23.Chaabna K, Bray F, Wabinga HR, Chokunonga E, Borok M, Vanhems P, et al. Kaposi sarcoma trends in Uganda and Zimbabwe: a sustained decline in incidence? Int J Cancer. 2013;133:1197–1203. doi: 10.1002/ijc.28125. [DOI] [PubMed] [Google Scholar]
- 24.Bateganya MH, Stanaway J, Brentlinger PE, Magaret AS, Wald A, Orem J, et al. Predictors of survival after a diagnosis of non-Hodgkin lymphoma in a resource-limited setting: a retrospective study on the impact of HIV infection and its treatment. J Acquir Immune Defic Syndr. 2011;56:312–319. doi: 10.1097/QAI.0b013e31820c011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkin DM, Wabinga H, Nambooze S. Completeness in an African cancer registry. Cancer Causes Control. 2001;12:147–152. doi: 10.1023/a:1008966225984. [DOI] [PubMed] [Google Scholar]
- 26.Goldman JS, Mutyaba I, Okuku F, Nambooze S, Wabinga H, Kristal A, et al. Measurement of the impact of antiretroviral therapy coverage on incidence for AIDS-defining malignancies in sub-Saharan Africa. Lancet. 2011 published online March 14. [Google Scholar]
- 27.Wabinga H, Ramanakumar AV, Banura C, Luwaga A, Nambooze S, Parkin DM. Survival of cervix cancer patients in Kampala, Uganda: 1995–1997. Br J Cancer. 2003;89:65–69. doi: 10.1038/sj.bjc.6601034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biggar RJ, Engels EA, Ly S, Kahn A, Schymura MJ, Sackoff J, et al. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr. 2005;39:293–299. doi: 10.1097/01.qai.0000164033.02947.e3. [DOI] [PubMed] [Google Scholar]
- 29.Achenbach CJ, Cole SR, Kitahata MM, Casper C, Willig JH, Mugavero MJ, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2010 doi: 10.1097/QAD.0b013e3283437f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohme I, Forsberg B, et al. Cancer risk after renal transplantation in the Nordic countries, 1964–1986. Int J Cancer. 1995;60:183–189. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- 31.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 32.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 33.van Leeuwen MT, Grulich AE, Webster AC, McCredie MR, Stewart JH, McDonald SP, et al. Immunosuppression and other risk factors for early and late non-Hodgkin lymphoma after kidney transplantation. Blood. 2009;114:630–637. doi: 10.1182/blood-2009-02-202507. [DOI] [PubMed] [Google Scholar]
- 34.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. J Am Med Assoc. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverberg MJ, Chao C, Leyden WA, Xu L, Horberg MA, Klein D, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–2559. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 37.Busnach G, Piselli P, Arbustini E, Baccarani U, Burra P, Carrieri MP, et al. Immunosuppression and cancer: a comparison of risks in recipients of organ transplants and in HIV-positive individuals. Transplant Proc. 2006;38:3533–3535. doi: 10.1016/j.transproceed.2006.10.144. [DOI] [PubMed] [Google Scholar]
- 38.Cobucci RN, Saconato H, Lima PH, Rodrigues HM, Prudencio TL, Junior JE, et al. Comparative incidence of cancer in HIV-AIDS patients and transplant recipients. Cancer Epidemiol. 2012 doi: 10.1016/j.canep.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Serraino D, Piselli P, Busnach G, Burra P, Citterio F, Arbustini E, et al. Risk of cancer following immunosuppression in organ transplant recipients and in HIV-positive individuals in southern Europe. Eur J Cancer. 2007;43:2117–2123. doi: 10.1016/j.ejca.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Vajdic CM, van Leeuwen MT. What types of cancers are associated with immune suppression in HIV? Lessons from solid organ transplant recipients. Curr Opin HIV AIDS. 2009;4:35–41. doi: 10.1097/coh.0b013e328319bcd1. [DOI] [PubMed] [Google Scholar]
- 41.Kersten MJ, Klein MR, Holwerda AM, Miedema F, van Oers MH. Epstein-Barr virus-specific cytotoxic T cell responses in HIV-1 infection: different kinetics in patients progressing to opportunistic infection or non-Hodgkin’s lymphoma. J Clin Invest. 1997;99:1525–1533. doi: 10.1172/JCI119315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piriou E, van Dort K, Nanlohy NM, van Oers MH, Miedema F, van Baarle D. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2005;106:3166–3174. doi: 10.1182/blood-2005-01-0432. [DOI] [PubMed] [Google Scholar]
- 43.van Baarle D, Hovenkamp E, Callan MF, Wolthers KC, Kostense S, Tan LC, et al. Dysfunctional Epstein–Barr virus (EBV)-specific CD8(+) T lymphocytes and increased EBV load in HIV-1 infected individuals progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2001;98:146–155. doi: 10.1182/blood.v98.1.146. [DOI] [PubMed] [Google Scholar]
- 44.Bihl F, Berger C, Chisholm JV, 3rd, Henry LM, Bertisch B, Trojan A, et al. Cellular immune responses and disease control in acute AIDS-associated Kaposi’s sarcoma. AIDS. 2009;23:1918–1922. doi: 10.1097/QAD.0b013e3283300a91. [DOI] [PubMed] [Google Scholar]
- 45.Bihl F, Mosam A, Henry LN, Chisholm JV, 3rd, Dollard S, Gumbi P, et al. Kaposi’s sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi’s sarcoma. AIDS. 2007;21:1245–1252. doi: 10.1097/QAD.0b013e328182df03. [DOI] [PubMed] [Google Scholar]
- 46.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 47.Catalfamo M, Wilhelm C, Tcheung L, Proschan M, Friesen T, Park JH, et al. CD4+ and CD8+ T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. J Immunol. 2011;186:2106–2116. doi: 10.4049/jimmunol.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lempicki RA, Kovacs JA, Baseler MW, Adelsberger JW, Dewar RL, Natarajan V, et al. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proc Natl Acad Sci U S A. 2000;97:13778–13783. doi: 10.1073/pnas.250472097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popovic M, Tenner-Racz K, Pelser C, Stellbrink HJ, van Lunzen J, Lewis G, et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2005;102:14807–14812. doi: 10.1073/pnas.0506857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mens H, Kearney M, Wiegand A, Shao W, Schonning K, Gerstoft J, et al. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol. 2010;84:12971–12981. doi: 10.1128/JVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Baarle D, Tsegaye A, Miedema F, Akbar A. Significance of senescence for virus-specific memory T cell responses: rapid ageing during chronic stimulation of the immune system. Immunol Lett. 2005;97:19–29. doi: 10.1016/j.imlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Effros RB, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997;18:450–454. doi: 10.1016/s0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- 55.Jin HT, Jeong YH, Park HJ, Ha SJ. Mechanism of T cell exhaustion in a chronic environment. BMB Rep. 2011;44:217–231. doi: 10.5483/BMBRep.2011.44.4.217. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 58.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 60.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 61.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 62.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 63.Wang RF, Rosenberg SA. Human tumor antigens recognized by T lymphocytes: implications for cancer therapy. J Leukoc Biol. 1996;60:296–309. doi: 10.1002/jlb.60.3.296. [DOI] [PubMed] [Google Scholar]
- 64.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 65.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 66.Tsuchikawa T, Ikeda H, Cho Y, Miyamoto M, Shichinohe T, Hirano S, et al. Association of CD8(+) T cell infiltration in oesophageal carcinoma lesions with human leucocyte antigen (HLA) class I antigen expression and survival. Clin Exp Immunol. 2011;164:50–56. doi: 10.1111/j.1365-2249.2010.04311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 68.Deeks SG. HIV Infection, Inflammation, Immunosenescence, and Aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Decrion AZ, Dichamp I, Varin A, Herbein G. HIV and inflammation. Curr HIV Res. 2005;3:243–259. doi: 10.2174/1570162054368057. [DOI] [PubMed] [Google Scholar]
- 70.Keating SM, Golub ET, Nowicki M, Young M, Anastos K, Crystal H, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of US women. AIDS. 2011 doi: 10.1097/QAD.0b013e3283489d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bastard JP, Soulie C, Fellahi S, Haim-Boukobza S, Simon A, Katlama C, et al. Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir Ther. 2012 doi: 10.3851/IMP2093. [DOI] [PubMed] [Google Scholar]
- 73.Lau B, Sharrett AR, Kingsley LA, Post W, Palella FJ, Visscher B, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166:64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 74.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 75.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


