Abstract
BACKGROUND
Clinical trials have demonstrated benefit for cardiac resynchronization therapy (CRT) and implantable cardioverter-defibrillator (ICD) therapies in patients with heart failure with reduced ejection fraction (HFrEF); yet, questions have been raised with regard to the benefit of device therapy for minorities.
OBJECTIVES
The purpose of this study was to determine the clinical effectiveness of CRT and ICD therapies as a function of race/ethnicity in outpatients with HFrEF (ejection fraction ≤35%).
METHODS
Data from IMPROVE HF (Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting) were analyzed by device status and race/ethnicity among guideline-eligible patients for mortality at 24 months. Multivariate Generalized Estimating Equations analyses were conducted, adjusting for patient and practice characteristics.
RESULTS
The ICD/cardiac resynchronization defibrillator (CRT-D)–eligible cohort (n = 7,748) included 3,391 (44%) non-Hispanic white, 719 (9%) non-Hispanic black, and 3,638 (47%) other racial/ethnic minorities or race-not-documented patients. The cardiac resynchronization pacemaker (CRT-P)/CRT-D–eligible cohort (n = 1,188) included 596 (50%) non-Hispanic white, 99 (8%) non-Hispanic black, and 493 (41%) other/not-documented patients. There was clinical benefit associated with ICD/CRT-D therapy (adjusted odds ratio: 0.64, 95% confidence interval: 0.52 to 0.79, p = 0.0002 for 24-month mortality), which was of similar proportion in white, black, and other minority/not-documented patients (device–race/ethnicity interaction p = 0.7861). For CRT-P/CRT-D therapy, there were also associated mortality benefits (adjusted odds ratio: 0.55, 95% confidence interval: 0.33 to 0.91, p = 0.0222), and the device–race/ethnicity interaction was not significant (p = 0.5413).
CONCLUSIONS
The use of guideline-directed CRT and ICD therapy was associated with reduced 24-month mortality without significant interaction by racial/ethnic group. Device therapies should be offered to eligible heart failure patients, without modification based on race/ethnicity.
Keywords: cardiac resynchronization therapy, clinical effectiveness, heart failure, mortality, race/ethnicity
Implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy (CRT) are shown to improve clinical outcomes in selected patients with heart failure with reduced ejection fraction (HFrEF) in multiple clinical trials (1–5). For appropriate patients, guidelines endorse Class I recommendations for device therapies in the primary prevention of sudden cardiac death and CRT for functional improvement and risk reduction of heart failure (HF) events regardless of race (6,7). However, the under-representation of racial/ethnic minorities in prior trials has raised the question as to whether the benefits extend to these patient populations (8). Although analysis of the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) has shown similar event rates and mortality benefit for primary prevention in African Americans, uncertainty has remained regarding whether these findings can be generalized to real-world clinical practice (9). Furthermore, multiple studies have highlighted the disparate use of device therapies for racial/ethnic minorities and the complex combination of systemic factors that contribute to healthcare disparities (10–12). Although some recent studies suggest that the racial/ethnicity gaps for African Americans and Hispanics may be narrowing, opportunities to improve use in eligible patients remain (13,14). The uncertainty of whether minority groups derive similar benefit from device therapies in clinical trials and in real-world clinical practice may have contributed to their differential use in practice.
The IMPROVE HF (Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting) cohort provides an opportunity to evaluate the benefit of device therapy in real-world clinical practice among minority outpatients with HF. The purpose of this study was to determine the clinical effectiveness of cardiac resynchronization defibrillator (CRT-D) and ICD therapy as a function of racial/ethnic classification of out-patients with HFrEF (left ventricular ejection fraction [LVEF] ≤35%).
METHODS
This pre-specified analysis of the IMPROVE HF registry is a prospective, observational cohort study of 15,177 patients diagnosed with HF (or prior myocar-dial infarction [MI]) and reduced LVEF being treated at outpatient cardiology (including multispecialty) practices. The primary objective of the IMPROVE HF registry was to evaluate the effects of a practice-specific performance improvement initiative on adherence to guideline-recommended therapies. The methods and primary results of the IMPROVE HF registry were previously reported (15).
Briefly, community and university outpatient cardiology/multispecialty practices were invited to participate. All sites were required to obtain institutional review board approval or waivers prior to enrollment. Patients with a primary or secondary diagnosis of HF or prior MI with reduced ejection fraction who were >18 years of age at the time of the most recent office visit were eligible for enrollment. Study participants were required to have an LVEF ≤35%, as measured by the most recent echo-cardiogram, nuclear multigated acquisition (scan, contrast ventriculogram, cardiac magnetic resonance, or qualitative assessment of left ventricular function indicative of moderate-to-severe dysfunction). Patients who met the guideline-specified eligibility criteria for each individual therapy, with no contra-indications, intolerance, or other documented reasons for not receiving the therapy, were eligible for inclusion in the analyses for that measure. For this analysis, study participants were required to be eligible for ICD/CRT-D or CRT-P/CRT-D therapy. Eligibility for ICD therapy was based on a primary prevention indication, and CRT eligibility criteria were based on the American College of Cardiology/ American Heart Association/Heart Rhythm Society guidelines from 2005, 2008, and 2009 (16–19). Documentation of QRS duration and New York Heart Association (NYHA) functional class consistent with guideline specifications was required to be considered eligible for ICD/CRT-D or CRT-P/CRT-D therapy; thus, only patients with QRS duration documented were included in analyses for CRT-P/CRT-D therapy. The primary endpoint was vital status (alive/dead) at 24-month follow-up.
Patients were not eligible to participate in the IMPROVE HF registry if they were not expected to survive for 12 months due to medical conditions other than HF, or had undergone heart transplant surgery. NYHA functional class IV was an exclusion criterion for ICD-only therapy.
Medical records of eligible patients were selected at random to yield an average of approximately 90 patients per participating practice. A total of 34 trained chart-review specialists extracted baseline demographics, clinical characteristics, and diagnostic and laboratory findings from patient charts. Patient race and ethnicity were collected to evaluate subgroup differences. The administrative and/or medical staff at participating practices were instructed to record patients’ self-assigned race/ethnicity. Each case report form included the following options for race: American Indian or Alaskan Native, Asian, black or African American, Native Hawaiian or Pacific Islander, white, or undocumented. Ethnicity was recorded as Hispanic (yes/no) or not documented. A rigorous methodology was utilized in the IMPROVE HF study design to ensure the quality and accuracy of data. Data collection was centrally performed by Outcome Sciences Inc. (Cambridge, Massachusetts). The average inter-rater reliability between chart reviewers was 0.82 (κ statistic). An average of 1.7 automated data quality checks were performed for each data field to ensure that all values met pre-specified ranges, formats, and units. Source data verification was randomly performed for 20% of the entire patient sample for 10% of participating practices. Additionally, monthly data quality reports were provided to the steering committee.
STATISTICAL ANALYSIS
Descriptive statistics for patient baseline characteristics and practice characteristics by race were calculated within the ICD/ CRT-D and CRT-P/CRT-D cohorts. This included mean and SD for continuous variables and frequency and percentage for categorical variables.
Generalized Estimating Equations (GEE) modeling was used to estimate unadjusted and adjusted relationships between device treatment and patient-level mortality in the first 24 months and to investigate if the clinical effectiveness of ICD/CRT-D or CRT-P/ CRT-D therapy in improving 24-month mortality would vary by race/ethnic group. An exchangeable within-practice correlation matrix was used in the modeling to account for correlation of patients from the same cardiology practice. The clinical benefit of being treated with devices at baseline on 24-month mortality was evaluated using univariate GEE models for each cohort and then for race/ethnicity subgroups: non-Hispanic white; non-Hispanic black; and other race, Hispanic ethnicity, or race/ethnicity undocumented within each cohort. In each model, vital status (dead/alive) at 24 months was the outcome and treatment with the device at baseline (yes/no) was the predictor. Univariate analysis produced the unadjusted odds ratio (OR) of death and its 95% confidence interval (CI) for device therapy per cohort and per race/ethnicity-specific subcohort. Furthermore, multivariate GEE models were performed in ICD/CRT-D and CRT-P/CRT-D cohorts separately to determine if the clinical effectiveness of device therapy on 24-month mortality would be different between patients classified as non-Hispanic white; non-Hispanic black; and other race group, Hispanic ethnicity, or race/ethnicity undocumented, controlling for other baseline patient and practice characteristics. We first screened the characteristics through univariate GEE analysis and included those with p values ≤0.10 as the covariates for device therapy in initial multivariate GEE models. We then eliminated the covariates with p values ≥0.05 using backward selection and added race/ ethnicity (if it was not in the reduced models yet) and race/ethnicity by device interaction terms into the reduced models. The adjusted OR, 95% CI, and p value for device therapy were reported, as well as the p value for device by race/ethnicity interaction. In sensitivity analyses, univariate and multivariate GEE analyses also were conducted in the ICD-only cohort. To verify the findings on interaction effect, additional device by race/ethnicity interaction testing was performed for each cohort by subgrouping the entire sample into non-Hispanic white and black patients, which excluded other race and race/ethnicity undocumented patients. Similarly, interaction testing was performed on the patients classified as non-Hispanic white and other race and race/ethnicity undocumented, which excluded non-Hispanic black patients. As part of a post-hoc analysis, given our observational sample size and OR estimated from the multivariate GEE analysis, we estimate a 75% power in the ICD/CRT-D cohort, a 41% power in the ICD-only cohort, and 15% power in the CRT-P/CRT-D cohort to detect a device by race/ ethnicity interaction at a significance level of 0.05.
During the first 12 months of the performance initiative, some patients who were eligible for a device, yet not treated at baseline, crossed over and had the device implanted. The crossover patients were included in the descriptive analysis of baseline characteristics. However, they were excluded from all of the GEE analyses to assess the pure relationship between use of device therapy at baseline and vital status at 24 months.
All statistical inference testing was 2-sided, with results considered statistically significant at p < 0.05. Analyses were completed using SAS statistical software version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
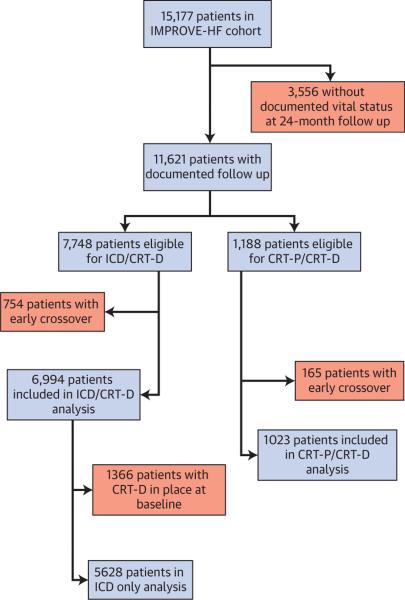
A total of 15,177 patients from 167 U.S. outpatient cardiology practices were evaluated at baseline and included in the longitudinal cohort. A median of 90 patient records per practice were entered. There was documentation of vital status at the 24-month follow-up in 11,621 (76.6%) patients. Of patients with vital status data at follow-up, 7,748 were eligible for ICD/CRT-D and 1,188 for CRT-P/CRT-D therapy at baseline. During the first 12 months of the performance initiative, 754 ICD/CRT-D–eligible and 165 CRT-P/CRT-D–eligible patients who did not have such devices at baseline had them implanted. After excluding these early crossovers, this analysis included a total of 6,994 patients from the ICD/CRT-D cohort and 1,023 patients from the CRT-P/CRT-D cohort (Fig. 1). In the analyses for the ICD-only cohort, 1,366 patients who had CRT-D in place at baseline were excluded from the 6,994 ICD/CRT-D patients, leaving 5,628 patients for these ICD-only analyses.
FIGURE 1. Patient Enrollment and Study Eligibility.
Flow diagram of patient enrollment and study eligibility by device type. CRT-D = cardiac resynchronization de fibrillator; CRT-p = cardiac resynchronization pacemaker; ICD = implantable cardioverter-defibrillator; IMPROVE HF = Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting.
Baseline patient and practice site characteristics among the ICD/CRT-D–eligible cohort were stratified by race/ethnicity and are listed in Tables 1 and 2. The ICD/CRT-D–eligible cohort (n = 7,748) patients were 44% (n = 3,391) non-Hispanic white, 9% (n = 719) non-Hispanic black, and 47% (n = 3,638) other race, Hispanic ethnicity, or race/ethnicity undocumented. Within the other race, Hispanic ethnicity, or race/ ethnicity undocumented cohort (n = 3,638), the patients were 3.9% (n = 142) Hispanic, 0.4% (n = 13) non-Hispanic American Indian/Native American, 1.1% (n = 39) non-Hispanic Asian, 0.3% (n = 9) non-Hispanic Native Hawaiian/Other Pacific Islander, 1.3% (n = 48) other race/ethnicity, and 93% (n = 3,387) race/ethnicity undocumented. Notably, non-Hispanic black patients were much younger, and the proportion of them having HF attributed to ischemic heart disease was 32% lower in absolute values compared with non-Hispanic white patients and patients in the other/undocumented race/ethnicity group. The proportion of women among black patients was 15% higher in absolute values than the proportion of women in the white and other or race/ethnicity undocumented groups. Rates of hypertension and diabetes were higher for black patients. The rates of prior MI, coronary artery bypass graft surgery, percutaneous coronary intervention, and atrial fibrillation were much lower for black patients as compared with white patients and other or race/ethnicity undocumented groups. A higher proportion of black patients had NYHA functional class III or IV. Black patients had the shortest QRS duration and lowest B-type natriuretic peptides, whereas white patients had the longest QRS duration and largest B-type natriuretic peptide value. Mean LVEF, sodium, blood and urea nitrogen were similar among the 3 groups. Of patients eligible for ICD/CRT-D therapy, 1,810 of 3,391 (53%) non-Hispanic white, 362 of 719 (50%) non-Hispanic black, and 1,755 of 3,638 (48%) other race, Hispanic ethnicity, or race/ethnicity undocumented patients had an ICD/CRT-D device in place. Of patients eligible for CRT-P/CRT-D therapy, 228 of 596 (38%) non-Hispanic white, 38 of 99 (38%) non-Hispanic black, and 182 of 493 (37%) other race, Hispanic ethnicity, or race/ethnicity undocumented patients had a CRT-P/CRT-D device in place.
TABLE 1.
Patient Characteristics of the ICD- or CRT-D-Eligible Cohort by Race/Ethnicity*
| Non-Hispanic White (n = 3,391) | Non-Hispanic Black (n = 719) | Other Race, Hispanic Ethnicity, or Not Documented (n = 3,638) | |
|---|---|---|---|
| Age, yrs | 69.9 ± 12 | 59.4 ± 14.9 | 69.7 ± 12.6 |
| Women | 932 (27.5) | 306 (42.6) | 1,023 (28.1) |
| Insurance | |||
| Medicare | 2,193 (64.7) | 364 (50.6) | 2,218 (61) |
| Other | 1,023 (30.2) | 317 (44.1) | 1,146 (31.5) |
| Not documented | 175 (5.2) | 38 (5.3) | 274 (7.5) |
| Ischemic heart failure | 2,488 (73.4) | 299 (41.6) | 2,652 (72.9) |
| History of atrial fibrillation | 1,131 (33.4) | 171 (23.8) | 1,154 (31.7) |
| History of diabetes | 1,198 (35.3) | 299 (41.6) | 1,240 (34.1) |
| History of hypertension | 2,165 (63.8) | 554 (77.1) | 2,143 (58.9) |
| Previous myocardial infarction | 1,777 (52.4) | 211 (29.3) | 1,834 (50.4) |
| History of chronic obstructive pulmonary disease | 713 (21) | 90 (12.5) | 568 (15.6) |
| History of coronary artery bypass grafting | 1,250 (36.9) | 92 (12.8) | 1,255 (34.5) |
| History of percutaneous coronary intervention | 1,051 (31) | 116 (16.1) | 998 (27.4) |
| History of peripheral vascular disease | 447 (13.2) | 72 (10) | 425 (11.7) |
| NYHA functional class | |||
| I and II | 2,372 (69.9) | 454 (63.1) | 2,600 (71.5) |
| III and IV | 1,019 (30.1) | 265 (36.9) | 1,038 (28.5) |
| Left ventricular ejection fraction, % | 24.8 ± 6.8 | 23.1 ± 7.3 | 25.1 ± 6.8 |
| Systolic blood pressure, mm Hg | 119.3 ± 18.4 | 121.2 ± 19.7 | 119.4 ± 18.5 |
| Diastolic blood pressure, mm Hg | 69.3 ± 10.7 | 73 ± 12.2 | 69.4 ± 11 |
| Heart rate at rest, beats/min | 72.1 ± 11.1 | 74.6 ± 12.6 | 71.7 ± 11.1 |
| Edema | 725 (21.4) | 171 (23.8) | 717 (19.7) |
| Sodium, mEq/l | 139.1 ± 3.3 | 138.9 ± 3.4 | 139.5 ± 3.4 |
| Serum urea nitrogen, mg/dl | 26.2 ± 14.4 | 23.5 ± 16.4 | 25.9 ± 14.4 |
| Creatinine, mg/dl | 1.4 ± 0.7 | 1.6 ± 1.2 | 1.4 ± 0.7 |
| Potassium, mEq/l | 4.4 ± 0.5 | 4.2 ± 0.5 | 4.4 ± 0.5 |
| B-type natriuretic peptide, pg/ml | 697.8 ± 896.4 | 643 ± 815.2 | 688.7 ± 870.4 |
| QRS duration, ms | 135.8 ± 38.4 | 123.5 ± 35.3 | 131 ± 37.2 |
| QRS missing | 997 (29.4) | 184 (25.6) | 1,231 (33.8) |
| QRS duration >120 ms | 1,430 (42.2) | 230 (32) | 1,280 (35.2) |
Values are mean ± SD or n (%).
Early crossovers included.
CRT-D = cardiac resynchronization defibrillator; ICD = implantable cardioverter-defibrillator; NYHA = New York Heart Association.
TABLE 2.
Practice Characteristics of the ICD- or CRT-D-Eligible Cohort by Race/Ethnicity*
| Non-Hispanic White (n = 3,391) | Non-Hispanic Black (n = 719) | Other Race, Hispanic Ethnicity, or Not Documented (n = 3,638) | |
|---|---|---|---|
| Census region | |||
| South | 1,374 (40.5) | 378 (52.6) | 1,370 (37.7) |
| West | 384 (11.3) | 66 (9.2) | 616 (16.9) |
| Central | 733 (21.6) | 104 (14.5) | 656 (18) |
| Northeast | 900 (26.5) | 171 (23.8) | 996 (27.4) |
| Outpatient practice setting | |||
| Nonuniversity, nonteaching | 2,182 (64.3) | 299 (41.6) | 2,615 (71.9) |
| Nonuniversity, teaching | 880 (26) | 208 (28.9) | 802 (22) |
| University, teaching | 329 (9.7) | 212 (29.5) | 221 (6.1) |
| Multispecialty | 706 (20.8) | 245 (34.1) | 957 (26.3) |
| Electronic health record | |||
| Paper | 1,647 (48.6) | 403 (56.1) | 1,466 (40.3) |
| Mixed | 632 (18.6) | 158 (22) | 685 (18.8) |
| Electronic | 1,112 (32.8) | 158 (22) | 1,487 (40.9) |
| Heart failure nurses | |||
| >1 | 1,412 (41.6) | 259 (36) | 1,655 (45.5) |
| ≤1 | 1,909 (56.3) | 428 (59.5) | 1,877 (51.6) |
| Missing | 70 (2.1) | 32 (4.5) | 106 (2.9) |
| Number of electrophysiologists in practice | 1.6 ± 1.7 | 2.1 ± 1.7 | 2 ± 2 |
| Number of interventionalists in practice | 4.9 ± 3.3 | 4.9 ± 2.8 | 5.3 ± 3.3 |
| Number of heart failure clinics in practice | 1.5 ± 0.5 | 1.3 ± 0.5 | 1.5 ± 0.5 |
| Number of cardiologists in practice | 13.1 ± 12.1 | 13.4 ± 9.2 | 16.3 ± 13.5 |
The practice characteristics of the ICD/CRT-D– eligible cohort are shown in Table 2. The majority of the patients were from nonuniversity, nonteaching (5,096 of 7,748, 66%) and nonmultispecialty clinics (1,890 of 7,748, 24%). Less than 40% (2,757 of 7,748) of patients had electronic medical health records. The percentage of non-Hispanic black patients that were treated at university-based teaching institutions was much higher than that of the other 2 race/ethnicity-specific subgroups (Table 2).
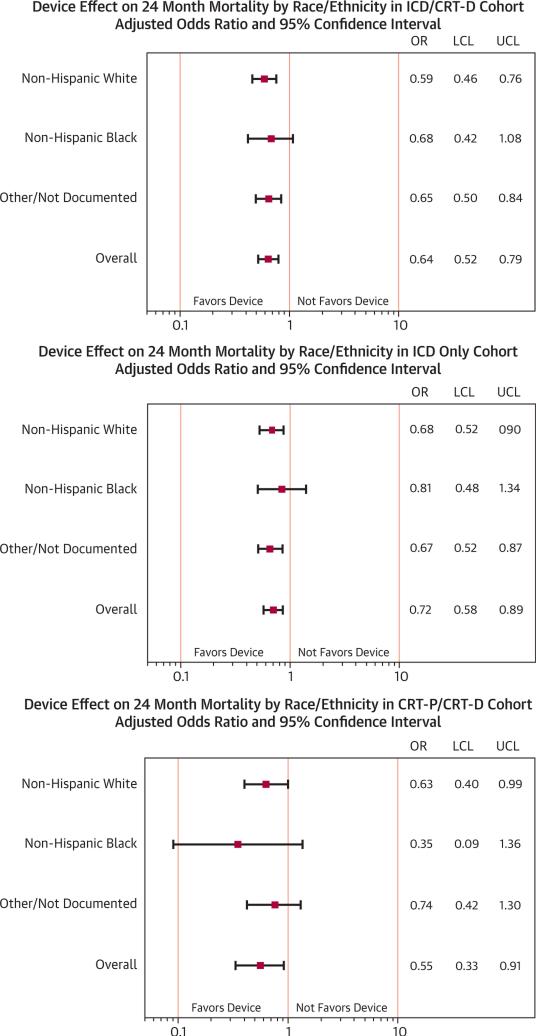
Table 3 demonstrates 24-month mortality rates and unadjusted/adjusted ORs for the ICD/CRTD–eligible cohort and race groups within the cohort. Study patients having ICD or CRT-D at baseline were 34% less likely to die at 24 months compared with those who did not have a device at baseline (20.4% vs. 27.8%, unadjusted OR: 0.66, 95% CI: 0.58 to 0.74, p < 0.0001). On multivariate analysis controlling for age; sex; race; heart failure etiology; comorbid conditions including diabetes, myocardial infarction, chronic obstructive pulmonary disease, and peripheral vascular disease; NYHA functional class; edema; LVEF; systolic blood pressure; sodium; blood urea nitrogen; and creatinine, these findings remained significant (adjusted OR: 0.64, 95% CI: 0.52 to 0.79, p = 0.0002). The test for device by race/ethnicity interaction was not significant (p = 0.7861). The proportional risk reductions were similar across each of the race/ethnicity groups according to the multivariate GEE analysis. The overlapping of their 95% CIs is consistent with the finding of nonsignificant interaction between device and race/ethnicity. However, statistical significance was not detected in all individual race/ethnicity groups according to the multivariate GEE analysis.
TABLE 3.
Rates and Unadjusted and Adjusted Odds Ratios for 24-Month Mortality for ICD/CRT-D Therapy
| Subjects | ICD or CRT-D at Baseline | n | Mortality at 24 Months, n(%) | Unadjusted Odds Ratio (95% CI) | p Value | Adjusted Odds Ratio (95% CI) | p Value | pValue (Device-Race/Ethnicity Interaction) |
|---|---|---|---|---|---|---|---|---|
| ICD/CRT-D eligible | Yes | 3,927 | 801 (20.4) | 0.66 (0.58-0.74) | <0.0001 | 0.64 (0.52-0.79) | 0.0002 | 0.7861 |
| No | 3,067 | 852 (27.8) | ||||||
| Non-Hispanic white | Yes | 1,810 | 371 (20.5) | 0.65 (0.55-0.77) | <0.0001 | 0.59 (0.46-0.76) | <0.0001 | – |
| No | 1,294 | 365 (28.2) | ||||||
| Non-Hispanic black | Yes | 362 | 74 (20.4) | 0.74 (0.54-1.03) | 0.0717 | 0.68 (0.42-1.08) | 0.1023 | 0.3558* |
| No | 294 | 77 (26.2) | ||||||
| Other race, Hispanic ethnicity, or not documented | Yes | 1,755 | 356 (20.3) | 0.66 (0.55-0.80) | <0.0001 | 0.65 (0.50-0.84) | 0.0011 | 0.5152* |
| No | 1,479 | 410 (27.7) |
Interaction testing was repeated for subsets of the entire population: non-Hispanic white and non-Hispanic black; non-Hispanic white and other race/Hispanic ethnicity/undocumented. Variables used for adjustment are noted in the Results section.
CI = confidence interval; other abbreviations as in Table 1.
Table 4 demonstrates 24-month mortality rates and unadjusted/adjusted ORs for the ICD-only cohort and race/ethnicity groups within the cohort. Study patients with baseline ICD therapy were less likely to die at 2 years when compared with those without ICD therapy (20.2% vs. 27.8%), and after multivariable analysis, this finding remained significant (adjusted OR: 0.72, 95% CI: 0.58 to 0.89, p = 0.0046). Again, the device by race/ethnicity interaction was not significant in this ICD-only cohort (p = 0.8225).
TABLE 4.
Ratesand Unadjusted andAdjustedOddsRatios for 24-Month Mortality for ICD-Only Therapy
| Subjects | ICD at Baseline |
n | Mortality at 24 Months n (%) |
Unadjusted Odds Ratio (95% CI) |
p Value | Adjusted Odds Ratio (95% CI) |
p Value | p Value (Device-Race/ Ethnicity Interaction) |
|---|---|---|---|---|---|---|---|---|
| ICD-only eligible | Yes | 2,561 | 518 (20.2) | 0.65 (0.57-0.74) | <0.0001 | 0.72 (0.58-0.89) | 0.0046 | 0.8225 |
| No | 3,067 | 852 (27.8) | ||||||
| Non-Hispanic white | Yes | 1,152 | 241 (20.9) | 0.67 (0.56-0.81) | <0.0001 | 0.68 (0.52-0.90) | 0.0075 | – |
| No | 1,294 | 365 (28.2) | ||||||
| Non-Hispanic black | Yes | 235 | 50 (21.3) | 0.78 (0.53-1.14) | 0.2001 | 0.81 (0.48-1.34) | 0.4109 | 0.3151* |
| No | 294 | 77 (26.2) | ||||||
| Other race, Hispanic ethnicity, or not documented | Yes | 1,174 | 227 (19.3) | 0.62 (0.52-0.75) | <0.0001 | 0.67 (0.52-0.87) | 0.0031 | 0.9550* |
| No | 1,479 | 410 (27.7) |
Table 5 demonstrates 24-month mortality rates and unadjusted/adjusted ORs for the CRT-P/CRTD–eligible cohort and race/ethnicity groups within the cohort. Study patients with CRT-D therapy at baseline were less likely to die at 2 years compared with those without CRT-P/CRT-D (28.8% vs. 38.3%), and on multivariable analyses, these findings were also significant (adjusted OR: 0.55, 95% CI: 0.33 to 0.91, p = 0.0222). Similar to the ICD/CRT-D–eligible cohort and ICD-only cohort, the device by race/ethnicity interaction effect was not significant (p = 0.5413). Figure 2 plots the adjusted OR of mortality for device therapy by race/ethnicity groups.
TABLE 5.
Rates and Unadjusted and Adjusted Odds Ratios for 24-Month Mortality for CRT-P/CRT-D Therapy
| Subjects | CRT-P/CRT-D at Baseline | n | Mortality at 24 Months, n (%) | Unadjusted Odds Ratio (95% CI) | p Value | Adjusted Odds Ratio (95% CI) | p Value | p Value (Device-Race/Ethnicity Interaction) |
|---|---|---|---|---|---|---|---|---|
| CRT-P/CRT-D eligible | Yes | 448 | 129 (28.8) | 0.63 (0.48-0.84) | 0.0017 | 0.55 (0.33-0.91) | 0.0222 | 0.5413 |
| No | 575 | 220 (38.3) | ||||||
| Non-Hispanic white | Yes | 228 | 69 (30.3) | 0.62 (0.43-0.90) | 0.0122 | 0.63 (0.40-0.99) | 0.0435 | – |
| No | 278 | 107 (38.5) | ||||||
| Non-Hispanic black | Yes | 38 | 4 (10.5) | 0.32 (0.11-0.99) | 0.0487 | 0.35 (0.09-1.36) | 0.1306 | 0.2977* |
| No | 55 | 18 (32.7) | ||||||
| Other race, Hispanic ethnicity, or not documented | Yes | 182 | 56 (30.8) | 0.70 (0.44-1.13) | 0.1444 | 0.74 (0.42-1.30) | 0.2930 | 0.4549* |
| No | 242 | 95 (39.3) |
Interaction testing was repeated for subsets of the entire population: non-Hispanic white and non-Hispanic black; non-Hispanic white and other race/Hispanic ethnicity/undocumented. Variables used for adjustment are noted in the Results section.
FIGURE 2. Plots of Adjusted ORs for 24-Month Mortality by Device Type and Race/Ethnicity.
Adjusted odds ratios (ORs) and 95% confidence intervals for 24-month mortality by device type by each race/ethnic group and overall. LCL = lower confidence limit; UCL = upper confidence limit; other abbreviations as in Figure 1.
DISCUSSION
Among the 6,994 patients with HFrEF treated at outpatient cardiology/multispecialty practices who were eligible for ICD/CRT-D and 1,023 patients eligible for CRT-P/CRT-D without crossover, the clinical benefit associated with ICD or CRT was substantial. Study patients with device therapy at baseline had a lower likelihood of death at 24 months compared with those without baseline device therapy (Central Illustration). Importantly, ICD and CRT therapy was associated with significant survival benefits overall, and the analyses of device and race/ethnicity interactions were not statistically significant (p = 0.7861 for ICD/CRT-D, p = 0.5413 for CRT-P/CRT-D), suggesting that the clinical benefit of device therapy is not driven by race/ethnicity among outpatients with HF. Although individual race/ethnicity groups did not meet statistical significance, p values for significance among subgroups may not be reliable evidence for lack of benefit, and interaction testing is preferred for generaliz-ability (19). In addition, the mortality benefit from device therapy was not driven by CRT alone, as the ICD-only analysis yielded a clinically and statistically significant reduction in events. Multivariate analysis revealed that the mortality benefit of baseline device therapy persisted after adjusting for baseline characteristics.
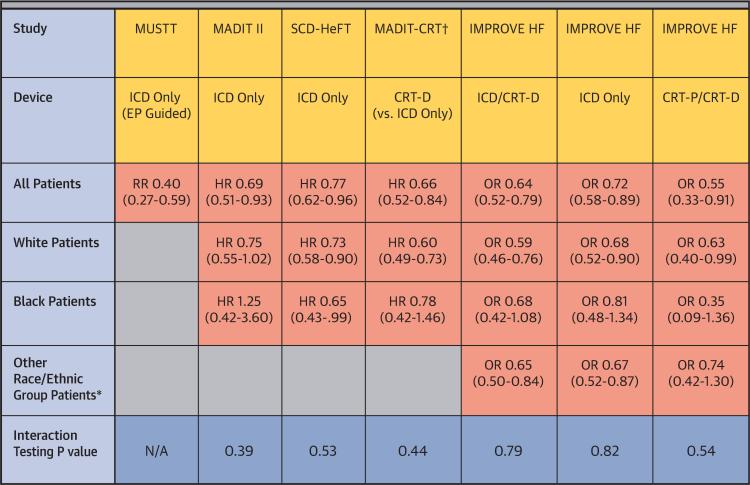
CENTRAL ILLUSTRATION. Clinical Outcomes Comparison of Racial/Ethnic Groups With CRT and ICD Therapies.
Cardiac resynchronization therapy (CRT) and implantable cardioverter-defibrillator (ICD) therapy has proven to enhance survival in randomized clinical trials. In the MUSTT (Multicenter Unsustained Tachycardia Trial), black patients (n = 61) did not do as well with electrophysiology (EP)-guided ICD placement as white patients. Analysis of MADIT II (Multicenter Automatic Defibrillator Implantation Trial) suggested there were different outcomes with ICD therapy among black (n = 102) and white patients. SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) showed similar mortality benefit with ICD compared to placebo in black (n = 425) and white patients. MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy) showed no heterogeneity in clinical outcomes for black (n = 143) and white patients. This new study using data from IMPROVE HF (Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting) provides evidence of real-world clinical effectiveness with CRT and ICD therapy in patients with heart failure, with no significant heterogeneity in mortality benefits among race/ethnic groups. These findings suggest device therapy should be offered to all eligible heart failure patients without consideration of race or ethnicity. Relative risks, hazard ratios, or odds ratios together with 95% confidence intervals are shown. *Includes patients were race/ethnicity were not documented. †Primary outcome composite of death or HF events. CRT-D = cardiac resynchronization defibrillator; CRT-p = cardiac resynchronization pacemaker; HR = hazard ratio; OR = odds ratio; RR = risk ratio.
Prior studies have shown mortality benefit for ICD and CRT therapies, although questions have remained as to whether there are differences in the efficacy and effectiveness of device therapy by race and ethnicity. In a subgroup analysis of black patients in the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy), black patients experienced a similar magnitude of risk reduction in HF events and death. In this subgroup analysis, CRT and race did not contribute a statistically significant interaction to primary outcome, although the primary endpoint within the race subset was not statistically significant secondary to small sample sizes (Central Illustration). An analysis of the racial subgroups in the MUSTT (Multicenter Unsustained Tachycardia Trial) found that black patients did not do as well when randomized to electrophysiologic-guided therapy for ICD placement, although the number of black patients in the subgroup analysis was small (n = 61) (20) (Central Illustration). Similarly, a subgroup analysis of black patients in the MADIT-II trial was not found to have a mortality benefit, but was limited by small patient numbers (n = 102) (21) (Central Illustration). As previously mentioned, results from the SCD-HeFT trial, which included 425 black patients, found similar mortality benefits without evidence that minorities were less willing to accept device therapies (9). Our findings are consistent with the SCDHeFT subgroup analysis, but provide evidence of real-world clinical effectiveness. Nevertheless, priority must be given for future research and clinical trials to improve the representation of racial and ethnic minorities to avoid lingering questions regarding generalizability.
With the expanding evidence base provided by clinical trials, the number of guideline-recommended HF therapies has increased. This increase has placed additional burdens on patients in terms of adherence and on physicians and health systems in terms of resource allocation. As racial/ethnic minorities have historically been under-represented in clinical trials of CRT/ICD therapy, there are questions regarding whether this patient population benefits to a similar degree with device therapy as white patients. The under-representation of minorities in clinical trials is a larger issue that likely reflects systemic factors rather than patient-specific factors (22,23). Although disparities in the use of CRT and ICD therapy among minorities appears to be diminishing, these disparities still exist among a proportion of patients receiving CRT and ICD therapy (24).
To our knowledge, the present study is among the largest studies to address the question of race/ ethnicity-specific benefits of ICD or CRT therapies in real-world clinical practice. Our data support the need for race- and ethnicity-specific outcome reporting and refute any meaningful differences in clinical effectiveness as a function of race/ethnicity for either ICD or CRT-D therapy. These findings reinforce current Class I recommendations from the American College of Cardiology/American Heart Association HF guidelines that selected HF patients without racial/ethnic differentiation should, in the absence of specific evidence to treat otherwise, have clinical screening and therapy in a manner identical to that provided to the broader HF population. Given the known existence of racial/ethnic disparities in the use of both CRT and ICD therapies, these data demonstrating the similarity of benefit elevate to the highest tier the need to eliminate racial/ethnic disparities in device-based therapy for HF.
STUDY LIMITATIONS
The design of the IMPROVE HF registry has some inherent limitations that may affect the interpretation of findings. Specifically, patient data were collected by medical chart review, which is dependent on the accuracy and completeness of documentation. Some patients considered eligible for treatment who were not treated may have had contraindications or other reasons that prevented treatment but were not documented in the medical record. Although practices were instructed to record self-assigned race and ethnicity, these characteristics cannot be confirmed, making misclassification possible. The number of patients in race/ethnicity cohorts other than non-Hispanic white and non-Hispanic black were too small to analyze independently and were included with patients lacking racial/ ethnicity identification data. The cohort of other minorities and those lacking racial identification may not reflect a readily-identifiable patient group in the literature or practice. In addition, the study had only modest power to detect device–race/ethnicity interactions if they are truly significant, and thus, additional well-powered studies are needed. Patients receiving devices for secondary prevention, who are at higher baseline mortality risk, were not distinguished and may have biased the results in favor of no device therapy at baseline. Follow-up on vital status was not achieved for all patients. This analysis was confined to patients with complete follow-up at 24 months, and patients with early crossover to treatment were excluded from the analysis, which may have also introduced bias. We did not assess health-related quality of life, symptom control, functional capacity, patient satisfaction, hospitalization rates, or other clinical outcomes that may be of interest. As with all observational studies, the possibility for residual measured or unmeasured confounding exists, potentially leading to overestimation or underestimation of treatment effects. The associations of device use with outcomes do not determine causality and may reflect treatment selection bias. We could not adjust for socioeconomic factors. The majority of patients who received CRT received a CRT-D device, preventing analysis of the association of CRT-P with outcomes. The guidelines for CRT have been recently revised and stipulate criteria that differ in some ways from those in place during the study. Although patients in the IMPROVE HF registry were selected from a representative sample from each practice, enrollment required documented left ventricular function and at least 2 office visits with a cardiologist in the last 2 years, which may have introduced some ascertainment bias. These findings may not apply to practices that differ in patient-case mix, baseline care patterns, motivation, resources, and other factors from those that agreed to participate in the IMPROVE HF registry.
CONCLUSIONS
In this large outpatient cohort of chronic HF patients treated at cardiology/multispecialty practices participating in a performance improvement initiative, the use of guideline-directed CRT and ICD therapy was associated with substantially reduced 24-month mortality in eligible HFrEF patients without significant interaction by racial/ethnic group. Our data did not show any meaningful differences in clinical effectiveness as a function of race/ethnicity for either ICD or CRT therapy, but additional research is warranted. These findings may have important clinical implications and indicate that CRT-D and ICD therapies should be offered to all eligible patients with HFrEF without modification based on race/ ethnicity, pending further studies.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: ICDs and CRT reduce the risks of death and cardiac arrhythmic events in appropriately-selected patients with heart failure, but racial/ethnic minorities were under-represented in the pivotal trials that support current clinical practice guideline recommendations.
TRANSLATIONAL OUTLOOK: While additional studies are needed to more clearly define the genetic, environmental, and lifestyle factors most closely associated with the benefit of device-based therapies for patients with ischemic and nonischemic forms of advanced cardiac disease, this study suggests that device therapy should be offered to all eligible heart failure patients without consideration of race or ethnicity.
Acknowledgments
The IMPROVE HF registry and this study are supported by Medtronic, Inc. Outcome Sciences performed data abstraction and checks, stored site-specific and aggregate data, and provided benchmarked quality-of-care reports to participating sites with funding from Medtronic. This paper was submitted to Medtronic prior to submission for publication, and all authors have read and agreed to the written content. Dr. Ziaeian's brother is a clinical specialist for Biotronik. Dr. Zhang is an employee of Medtronic, Inc. Dr. Curtis has received research honoraria from Medtronic, Inc. and St. Jude Medical; and has served on the advisory board of Sanofi-Aventis, St. Jude Medical, Biosense Webster, Janssen Pharmaceuticals, Bristol-Myers-Squibb, Pfizer, Inc., and Daiichi Sankyo. Dr. Mehra has served as a consultant to Thoratec, Medtronic, Inc., Johnson & Johnson, St. Jude Medical, Boston Scientific, and Abbott Vascular; has received grants/research support from the National Institutes of Health/National Heart, Lung, and Blood Institute and American Board of Internal Medicine; and serves as editor-in-chief of the Journal of Heart and Lung Transplantation. Dr. Gheorghiade has served as a consultant to Abbott Laboratories, Astellas, AstraZeneca, Bayer Schering Pharma AG, CorThera, Inc., Cytokinetics, Inc., Debio-Pharm SA, Errekappa Terapeutici, GlaxoSmithKline, Johnson & Johnson, Medtronic, Inc., Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, and Takeda. Dr. Heywood has received research grants from Biosite, Medtronic, Inc., and St. Jude Medical; has served on the Speaker's Bureau of or received honoraria from GlaxoSmithKline, Medtronic, AstraZeneca, Novartis, Actelion, St. Jude Medical, Otsuka, Biotronik, and Boston Scientific; and has served as a consultant to or on the advisory board of Emerge, Medtronic, Inc., Biotronik, and Actelion. Dr. O'Connor has served as a consultant to Forest, Medtronic, Inc., Amgen, Medpace, Impulse Dynamics, Actelion, Cytokinetics, Roche, and Trevena. Dr. Reynolds has received research grants from Medtronic and Biotronik; has served on the Speakers’ Bureau of Medtronic and Sorin; and has served as a consultant to Medtronic. Dr. Walsh has served on the scientific advisory board for United HealthCare; and has served as a consultant to Eli Lilly, United HealthCare, and Novartis. Dr. Fonarow has received research support from the National Institutes of Health and AHRQ; and has served as a consultant to Medtronic, Novartis, Johnson & Johnson, Takeda, The Medicines Company, and Gambro. Drs. Albert and Yancy have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CI
confidence interval
- CRT
cardiac resynchronization therapy
- CRT-D
cardiac resynchronization defibrillator
- CRT-P
cardiac resynchronization pacemaker
- GEE
generalized estimating equations
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- ICD
implantable cardioverter-defibrillator
- LVEF
left ventricular ejection fraction
- OR
odds ratio
REFERENCES
- 1.Moss AJ, Hall WJ, Cannom DS, et al. Multi-center Automatic Defibrillator Implantation Trial Investigators Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JGF, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 3.Anand IS, Carson P, Galle E, et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation. 2009;119:969–77. doi: 10.1161/CIRCULATIONAHA.108.793273. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 5.Tang ASL, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 6.Epstein AE, DiMarco JP, Ellenbogen K, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline). J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2012;60:1297–313. doi: 10.1016/j.jacc.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Redberg RF. Disparities in use of implantable cardioverter-defibrillators: moving beyond process measures to outcomes data. JAMA. 2007;298:1564–6. doi: 10.1001/jama.298.13.1564. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell JE, Hellkamp AS, Mark DB, et al. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J. 2008;155:501–6. doi: 10.1016/j.ahj.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas KL, Al-Khatib SM, Kelsey RC, et al. Racial disparity in the utilization of implantable-cardioverter defibrillators among patients with prior myocardial infarction and an ejection fraction of < or = 35%. Am J Cardiol. 2007;100:924–9. doi: 10.1016/j.amjcard.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–32. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- 12.Farmer SA, Kirkpatrick JN, Heidenreich PA, et al. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm. 2009;6:325–31. doi: 10.1016/j.hrthm.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Al-Khatib SM, Hellkamp AS, Hernandez AF, et al. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125:1094–101. doi: 10.1161/CIRCULATIONAHA.111.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds D, Albert NM, Curtis AB, et al. Race and improvements in the use of guideline-recommended therapies for patients with heart failure: findings from IMPROVE HF. J Natl Med Assoc. 2012;104:287–98. doi: 10.1016/s0027-9684(15)30156-5. [DOI] [PubMed] [Google Scholar]
- 15.Fonarow GC, Yancy CW, Albert NM, et al. Improving the use of evidence-based heart failure therapies in the outpatient setting: the IMPROVE HF performance improvement registry. Am Heart J. 2007;154:12–38. doi: 10.1016/j.ahj.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline). J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:1343–82. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 20.Russo AM, Hafley GE, Lee KL, et al. Racial differences in outcome in the Multicenter UnSustained Tachycardia Trial (MUSTT): a comparison of whites versus blacks. Circulation. 2003;108:67–72. doi: 10.1161/01.CIR.0000078640.59296.6F. [DOI] [PubMed] [Google Scholar]
- 21.Vorobiof G, Goldenberg I, Moss AJ, et al. Effectiveness of the implantable cardioverter defibrillator in blacks versus whites (from MADIT-II). Am J Cardiology. 2006;98:1383–6. doi: 10.1016/j.amjcard.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 22.Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3:e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang T, Tsang W, Wijeysundera HC, Ko DT. Reporting and representation of ethnic minorities in cardiovascular trials: a systematic review. Am Heart J. 2013;166:52–7. doi: 10.1016/j.ahj.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Eapen ZJ, Al-Khatib S, Lopes RD, et al. Are racial/ethnic gaps in the use of cardiac resynchronization therapy narrowing? An analysis of 107,096 patients from the National Cardiovascular Data Registry's ICD Registry. J Am Coll Cardiol. 2012;60:1577–8. doi: 10.1016/j.jacc.2012.06.024. [DOI] [PubMed] [Google Scholar]