Abstract
Background
We prospectively assessed anxiety, depression, and behavior in children with standard risk acute lymphoblastic leukemia (SR-ALL) during the first year of therapy and identified associated risk factors.
Methods
Cohort study of 159 children (age 2–9.99 years) with SR-ALL enrolled on Children’s Oncology Group protocol AALL0331 at 31 sites. Parents completed the Behavior Assessment System for Children, the Family Assessment Device-GF, and the Coping Health Inventory for Parents at ~1, 6 and 12 months after diagnosis.
Results
Overall, mean scores for anxiety, depression, aggression and hyperactivity were similar to population norms. However, more children scored in the at risk/clinical range for depression than the expected 15% at one month (21.7%, p=0.022), six months (28.6%, p<0.001), and twelve months (21.1%, p=0.032). For anxiety, more scored in the at risk/clinical range at one month (25.2% vs. 15%, p=0.001), but then reverted to expected levels. In adjusted analysis, unhealthy family functioning predicted anxiety (OR=2.24, p=0.033) and depression (OR=2.40, p=0.008). Hispanic ethnicity was associated with anxiety (OR=3.35, p=0.009). Worse physical functioning (p=0.049), unmarried parents (p=0.017), and less reliance on social support (p=0.004) were associated with depression. Emotional distress at one month predicted anxiety (OR=7.11, p=0.002) and depression (OR=3.31, p=0.023) at twelve months.
Conclusion
Anxiety is a significant problem in a subpopulation of SR-ALL patients immediately after diagnosis, while depression remains a significant problem for at least one year. Children of Hispanic ethnicity or with unhealthy family functioning may be particularly vulnerable. These data suggest that clinicians should screen for anxiety and depression throughout the first year of therapy.
Keywords: childhood acute lymphoblastic leukemia, anxiety, depression, family functioning
Introduction
Over 90% of children with acute lymphoblastic leukemia (ALL) with standard risk (SR) features will be cured.1 The improved survival rate is, in part, due to more intensive therapies of 2.5–3.5 years duration.2 This therapy involves multiple different chemotherapeutic agents that can affect the child’s emotional functioning. In particular, corticosteroids, a key component of ALL therapy, affect mood, behavior, and cognition.3
This is the first prospective, longitudinal study of emotional and behavioral functioning in a large sample of children on active treatment for standard-risk ALL who did not receive cranial radiation, to our knowledge.4 Information about the behavioral and emotional health of children with cancer is largely based on studies of children after therapy, rather than on active treatment.5 Those studies have utilized cross-sectional designs and yielded mixed results regarding emotional distress in ALL patients.4,6,7 The few longitudinal studies that are available were small, included diverse cancer populations, and/or excluded ALL patients younger than 5 years.8–10 Variables associated with children’s psychosocial adjustment include older age,11,12 female sex,13,14 lower household income,14 parental distress,14 and more intensive treatment.12
The contribution of family functioning and coping behaviors to children’s emotional functioning is of particular interest because there are emerging data that suggest these factors may be modifiable. For example, a randomized clinical trial for adolescent survivors of childhood cancer and their families found that a one-day family-based intervention improved symptoms of posttraumatic stress.15 Additionally, there is compelling evidence of the efficacy of family interventions in other childhood illness populations such as in Type 1 Diabetes.16
We prospectively evaluated the emotional and behavioral functioning of a large, representative sample of children with ALL enrolled on a frontline Children’s Oncology Group (COG) therapeutic study during the first year of therapy. We sought to: (1) Describe the longitudinal trajectory of the psychological adjustment of children during their first year of therapy by measuring symptoms of anxiety, depression and behavioral disturbances, and (2) Identify factors associated with worse psychological functioning in children, including potentially modifiable variables related to family functioning and coping that could be targeted in future interventions.
Methods
Study population
We conducted a prospective, longitudinal study of emotional and behavioral outcomes in children with SR-ALL who were enrolled on COG AALL0331 between 2005–2009 at 31 sites. We focused on a subset of children who were classified as average risk ALL, defined as standard risk by National Cancer Institute criteria (initial white blood cell count <50,000/microliter and age 1.0–9.99 years) without central nervous system or testicular leukemia who had a good early response to therapy based on bone marrow morphology and minimal residual disease burden at end induction, and other variables.17,18 Additional eligibility requirements for this study of emotional and behavioral outcomes included age ≥2 years and at least one parent with reading comprehension of English or Spanish, the languages for which validated surveys exist. The participating sites were chosen from all COG sites to include a combination of community-based and tertiary care centers with available staffing for this ancillary study.
Patients received a three-drug, four-week induction with vincristine, PEG-asparaginase, dexamethasone (6 mg/m2/day × 28 days) and intrathecal chemotherapy. No patient received cranial radiation. There were two therapeutic randomizations: (1) standard Consolidation vs. intensified Consolidation that added two doses of cyclophosphamide and peg-asparaginase, and (2) standard Interim Maintenance with oral methotrexate vs. augmented Interim Maintenance with escalating intravenous methotrexate as post-consolidation therapy. In 2008, the second randomization was halted based upon the results of the CCG 1991 SR-ALL trial.2
One hundred ninety four patients enrolled in AALL0331 at the participating sites met the eligibility criteria for this ancillary study. Of these, 24 declined and 170 consented to participate. Of those who consented, 4 withdrew from AALL0331 before the first required survey evaluations and 7 were not given the evaluations because of error at the study sites. The 159 participants (82% of eligible) were similar to the 35 eligible nonparticipants in terms of age at diagnosis and sex, with some differences in ethnicity (Table 1).
Table 1.
Comparison of participants to eligible nonparticipants
| Participants, (n = 159) |
Eligible nonparticipants, (n = 35) |
P-value | |
|---|---|---|---|
| Age group at diagnosis, no. (%) | 0.134 | ||
| Pre-school (ages 2–4) | 86 (54.1%) | 24 (68.6%) | |
| School-age (ages 5–9) | 73 (45.9%) | 11 (31.4%) | |
| Sex, no. (%) | 0.576 | ||
| Female | 76 (47.8%) | 19 (54.3%) | |
| Male | 83 (52.2%) | 16 (45.7%) | |
| Child ethnicity, no. (%) | 0.011 | ||
| White, non-Hispanic | 108 (67.9%) | 16 (45.7%) | |
| Black, non-Hispanic | 11 (6.9%) | 1 (2.9%) | |
| Hispanic | 26 (16.4%) | 9 (25.7%) | |
| Other | 14 (8.8%) | 9 (25.7%) | |
| Marital status of parents, no. (%) | |||
| Married | 105 (66.0%) | ||
| Not Married | 45 (28.3%) | ||
| Missing | 9 (5.7%) | ||
| Maternal highest level of education, no. (%) | |||
| Less than college | 92 (57.9%) | ||
| At least some college | 55 (34.6%) | ||
| Missing | 9 (5.7%) | ||
| Family Income, no. (%) | |||
| Less than $50,000 | 72 (45.3%) | ||
| $50,000–$79,999 | 25 (15.7%) | ||
| $80,000 or more | 30 (18.9%) | ||
| Missing | 32 (20.1%) | ||
| Therapeutic randomization, no. (%) | |||
| Standard CS/standard IM-DI1 | 42 (26.42%) | ||
| Intensified CS/standard IM-DI1 | 51 (32.08%) | ||
| Standard CS/augmented IM-DI1 | 37 (23.27%) | ||
| Intensified CS/augmented IM-DI1 | 29 (18.24%) | ||
CS = consolidation, IM = Interim Maintenance, DI= Delayed Intensification
Procedures
The institutional review board of each participating center as well as the Yale University Human Investigation Committee approved the current study. Informed consent, and assent when indicated, was obtained for all participants. The identified primary caregiver (the child’s mother in 84% of instances) completed surveys at three selected time-points during the first year of therapy: day 1 of Consolidation (approximately 1 month after diagnosis), the end of the Delayed Intensification (approximately 6 months after diagnosis), and six months after starting Maintenance (approximately 12 months after diagnosis). Of the 159 participants, 145, 131, and 136 completed the evaluations at the first, second, and third timepoints, respectively.
Measures
Emotional and behavioral functioning was assessed by the Behavioral Assessment System for Children, Second Edition: Parent Report Scale (BASC-2 PRS), a valid and reliable instrument that has been used successfully in pediatric oncology populations.19,20 Children 8 years of age and older also completed the BASC-2 Self Report of Personality. However, only 17 (10.7%) of children in our study were at least 8 years old at diagnosis, so there were inadequate data to compare their self-report outcomes. The BASC-2 PRS yields standardized T-scores on a variety of clinical scales. Scores of 60–69 represent the at-risk range and scores ≥70 represent the clinically significant range. The BASC-2 PRS has been standardized on normative data obtained from a random sample of 12,350 children who are representative of the United States population based on gender, ethnicity, socioeconomic status, geographic region and culture.21 Expected frequencies of elevated scores in the normative (i.e. healthy comparison) population of children are available in the BASC manual.19 For the current study, the hyperactivity, aggression, anxiety and depression scales were analyzed. Anxiety and depression are often comorbid conditions, but in this instrument, there are discrete scales for each.
Family functioning was evaluated using the General Functioning Scale of the Family Assessment Device (FAD-GF).22 In this 12-item scale, parents indicate the degree to which they feel each statement describes their family (e.g. “We are able to make decisions about how to solve problems”). Possible scores range from 1–4. Scores ≥2 reflect unhealthy family functioning.22
Family coping was assessed using the Coping Health Inventory for Parents (CHIP), which has been validated for children with a variety of chronic illnesses.23 In this 45-item checklist, parents rate how helpful a specific coping behavior is on a 4-point scale ranging from “not helpful” to “extremely helpful.” A higher score on each of the three subscales, (1) Maintaining family integration and optimism, (2) Maintaining social support and self-esteem, and (3) Understanding the medical situation, indicates a greater reliance on that particular coping pattern, but there are no normative scores.
Physical functioning was measured by the “pain and hurt” and “nausea” subscales of the Pediatric Quality of Life Inventory (PedsQL) 3.0 Cancer Module.24 In this questionnaire, parents rate how much of a problem each symptom has been in the past month. Scores are transformed on a scale from 0 (worst health) to 100 (best health).
A parent demographic survey included questions about ethnicity, household income, marital status, maternal education and family size.
Data Analysis
Patient characteristics, including age at diagnosis, gender, race, and ethnicity, were summarized and compared between participants and eligible nonparticipants using an exact chi-square test to evaluate the potential for response bias.
The primary outcomes of interest were the BASC-2 PRS subscales for anxiety and depression. The proportions of patients in the “at least at-risk” and clinical ranges were compared to the corresponding proportions in the normative population, a comparison group of healthy children, using a one-sided binomial exact test.
Both univariate and multivariate longitudinal analyses were conducted. For univariate analysis, a logistic regression model was tested with the dichotomized BASC-2 PRS scores (i.e. elevated vs. not elevated scores) for anxiety and depression as dependent variables, taking into consideration the dependence of repeated measurements at 3 timepoints for each subject. The following independent variables were analyzed: age at diagnosis, gender, race and ethnicity, household income, maternal education, marital status, family size and pain and hurt subscales, as well as repeated measures of general family functioning and coping behaviors. The multivariate regression modeling included the patient and family factors that were associated with elevated anxiety and depression scores by univariate analysis at p<0.1. All analyses were performed using SAS software, version 9.2.
Results
Participants
The participants had a mean age of 4.9±2.2 years at diagnosis; 16.4% were of Hispanic ethnicity and 66.0% had married parents (Table 1).
Frequency of emotional and behavioral problems
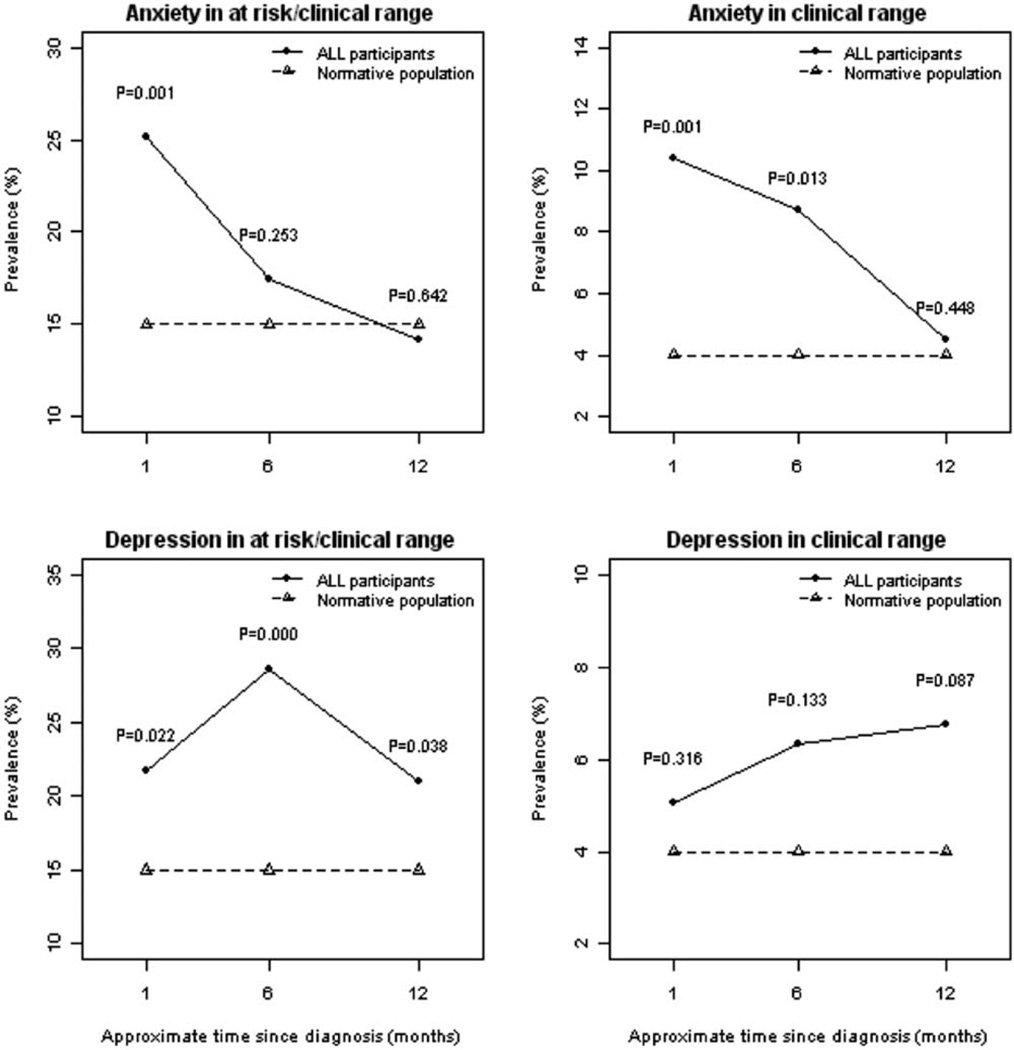
Mean scores for parental report of anxiety, depression, aggression, and hyperactivity symptoms were stable and in the average range at all three timepoints. However, the frequency of elevated anxiety and depression scores in the at-risk or clinically significant range was greater than expected compared to the normative population of children (Figure 1). At one month after diagnosis, a greater percentage of children scored in the at-risk or clinically significant range for anxiety (25.2% vs. 15%, p=0.001) than the normative population, but then reverted to expected levels at six (17.5% vs. 15%, p=0.253) and twelve months (14.2% vs. 15%, p=0.542) after diagnosis. The frequency of anxiety scores that were elevated to the clinically significant range was greater than expected at one month (10.4% vs. 4%, p=0.001) and six months (8.7% vs. 4%, p=0.013) after diagnosis, but then declined to expected levels by twelve months after diagnosis (4.5% vs. 4%, p=0.448).
Figure 1.
Prevalence of elevated anxiety and depression scores in the first year after diagnosis of acute lymphoblastic leukemia are shown.
For depression, a higher percentage of children had scores in the at-risk or clinically significant range than expected throughout the first year of therapy: one month (21.7% vs. 15%, p=0.022), six months (28.6% vs. 15%, p<0.001), and twelve months (21.1% vs. 15%, p=0.038). However, the frequency of depression scores in the clinically significant range was not significantly different from expected levels at any timepoint: one month (5% vs. 4%, p=0.316), six months (6.4% vs. 4%, p=0.133), and twelve months (6.8% vs. 4%, p=0.087).
At the three timepoints, the proportion of children with both anxiety and depression scores in the at-risk/clinically significant range was 12.6%, 15.1%, and 8.3%, respectively. The frequency of elevated hyperactivity and aggression scores was similar to that expected in a normative population (data not shown).
Longitudinal analysis
Compared to children with anxiety scores in the average range, those with anxiety scores in the at-risk/clinically significant range one month after diagnosis were 7.70 (95% CI, 2.39–24.85; p<0.001) times as likely to have elevated scores six months and 7.11 times (95% CI, 2.08–24.30; p=0.002) as likely to have elevated scores twelve months after diagnosis. Children with scores in the at-risk/clinically significant range six months after diagnosis were 20.64 (95% CI, 6.02–70.74; p<0.001) times as likely to have scores in the at-risk/clinically significant range twelve months after diagnosis.
Compared to children with depression scores in the average range, those with depression scores in the at-risk/clinically significant range one month after diagnosis were 3.51 (95% CI, 1.33–9.26; p=0.015) times as likely to have scores in the at-risk/clinically significant range six months and 3.31 (95% CI, 1.20–9.10; p=0.023) times as likely to have scores in the at-risk/clinically significant range twelve months after diagnosis. Children with scores in the at-risk/clinically significant range six months after diagnosis were 5.11 (95% CI, 1.94–13.48; p<0.001) times as likely to have scores in the at-risk/clinically significant range twelve months after diagnosis (data not displayed).
Predictors of anxiety and depression
Table 2 displays the results of the univariate analysis adjusted for time elapsed since diagnosis. Significant predictors of anxiety and depressive symptoms by parental report included unhealthy family functioning and less reliance of each of the three coping patterns measured by the CHIP. Hispanic ethnicity was associated with worse anxiety symptoms, but not depressive symptoms. Conversely, worse physical functioning as measured by the pain and hurt subscale of the PedsQL was associated with depression, but not anxiety. There were no differences detected among the four treatment groups.
Table 2.
Univariate association of patient and family factors with anxiety and depression
| Anxiety | Depression | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age group at diagnosis | ||||
| Pre-school (ages 2–4) | Reference group | Reference group | ||
| School-age (ages 5–12) | 0.62 (0.37,1.05) | 0.076 | 0.78 (0.49,1.26) | 0.314 |
| Gender | ||||
| Male | Reference group | Reference group | ||
| Female | 1.62 (0.97,2.72) | 0.067 | 1.27 (0.79,2.02) | 0.325 |
| Race/Ethnicity | ||||
| White, non-Hispanic | Reference group | Reference group | ||
| Hispanic | 3.32 (1.80,6.15) | 0.000 | 1.36 (0.73,2.53) | 0.335 |
| Black, non-Hispanic | 0.83 (0.23,2.96) | 0.769 | 1.22 (0.46,3.25) | 0.696 |
| Other | 1.61 (0.68,3.83) | 0.277 | 1.59 (0.75,3.37) | 0.226 |
| Annual family income | ||||
| ≥$50,000 | Reference group | Reference group | ||
| <$50,000 | 1.11 (0.63,1.98) | 0.720 | 1.17 (0.68,2.01) | 0.564 |
| Maternal education | ||||
| At least some college | Reference group | Reference group | ||
| Less than college | 1.15 (0.64,2.04) | 0.642 | 1.24 (0.74,2.09) | 0.410 |
| Marital status of parents | ||||
| Married | Reference group | Reference group | ||
| Not married | 1.30 (0.74,2.29) | 0.354 | 1.65 (0.99,2.75) | 0.054 |
| General Family Functioning1 | ||||
| Healthy family functioning | Reference group | Reference group | ||
| Unhealthy family functioning | 3.01 (1.76,5.15) | <0.001 | 2.37 (1.45,3.85) | 0.001 |
| Maintaining family integration coping behaviors2 | 0.97 (0.94,0.99) | 0.009 | 0.96 (0.94,0.98) | 0.001 |
| Maintaining social support coping behaviors3 | 0.96 (0.94,0.99) | 0.005 | 0.95 (0.93,0.97) | <0.001 |
| Understanding the medical situation coping behaviors4 | 0.95 (0.90,1.00) | 0.038 | 0.93 (0.89,0.98) | 0.003 |
| Pain and hurt by parental report5 | 0.99 (0.98,1.00) | 0.062 | 0.99 (0.98,1.00) | 0.016 |
| Nausea by parental report6 | 0.99 (0.97,1.01) | 0.186 | 0.99 (0.98,1.01) | 0.230 |
| Therapeutic randomization7 | ||||
| SC/SIM-SDI | Reference group | Reference group | ||
| IC/SIM-SDI | 1.20 (0.60,2.40) | 0.598 | 0.95 (0.50,1.78) | 0.862 |
| SC/AIM-ADI | 1.23 (0.59,2.57) | 0.585 | 1.00 (0.51,1.95) | 0.991 |
| IC/AIM-ADI | 1.02 (0.45,2.28) | 0.970 | 1.14 (0.56,2.33) | 0.713 |
Measured by the FAD-GF.
Measured by the CHIP subscale 1.
Measured by the CHIP subscale 2.
Measured by the CHIP subscale
Measured by the PedsQL pain and hurt scale
Measured by the PedsQL nausea scale
SC = Standard Consolidation, IC = Intensified Consolidation, SIM-SDI = Standard Interim Maintenance and Standard Delayed Intensification, AIM-ADI = Augmented Interim Maintenance and Augmented Delayed Intensification
Table 3 displays the results of a multivariate model, which included the patient and family factors that were at least marginally significant (p≤0.1) by univariate analysis. In this adjusted analysis, Hispanic ethnicity (OR=3.35; 95% CI, 1.36–8.24) and unhealthy family functioning (OR=2.24; 95% CI, 1.07–4.70) remained significant predictors of worse anxiety symptoms.
Table 3.
Multivariate analysis of the association of patient and family factors with anxiety and depression
| Anxiety | Depression | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age group at diagnosis | ||||
| Pre-school (ages 2–4) | Reference group | Reference group | ||
| School-age (ages 5–12) | 0.49 (0.24,1.01) | 0.053 | 0.77 (0.42,1.40) | 0.387 |
| Gender | ||||
| Male | Reference group | Reference group | ||
| Female | 1.57 (0.79,3.16) | 0.206 | 1.24 (0.68,2.25) | 0.478 |
| Race/Ethnicity | ||||
| White, non-Hispanic | Reference group | Reference group | ||
| Hispanic | 3.35 (1.36,8.24) | 0.009 | 0.52 (0.20,1.39) | 0.192 |
| Black, non-Hispanic | 0.85 (0.16,4.54) | 0.846 | 0.86 (0.25,2.99) | 0.815 |
| Other | 1.39 (0.42,4.52) | 0.592 | 1.10 (0.42,2.87) | 0.849 |
| Marital status of parents | ||||
| Married | Reference group | Reference group | ||
| Not married | 1.15 (0.49,2.56) | 0.797 | 2.36 (1.17,4.75) | 0.017 |
| General Family Functioning1 | ||||
| Healthy family functioning | Reference group | Reference group | ||
| Unhealthy family functioning | 2.24 (1.07,4.70) | 0.033 | 2.40 (1.26,4.56) | 0.008 |
| Maintaining family integration coping behaviors2 | 0.99 (0.94,1.05) | 0.771 | 1.04 (0.99,1.10) | 0.085 |
| Maintaining social support coping behaviors3 | 0.98 (0.94,1.03) | 0.366 | 0.94 (0.91,0.98) | 0.004 |
| Understanding the medical situation coping behaviors4 | 1.00 (0.90,1.10) | 0.964 | 0.95 (0.88,1.04) | 0.283 |
| Pain and hurt by parental report5 | 0.99 (0.98,1.00) | 0.152 | 0.99 (0.98,1) | 0.049 |
Measured by the FAD-GF.
Measured by the CHIP subscale 1.
Measured by the CHIP subscale 2.
Measured by the CHIP subscale
Measured by the PedsQL pain and hurt scale
The significant predictors of worse depressive symptoms by adjusted analysis were unhealthy family functioning (OR= 2.40; 95% CI, 1.26–4.56), unmarried parents (OR=2.36; 95% CI, 1.17–4.75), worse physical functioning (p=0.049), and less reliance on maintaining social support coping behaviors (p=0.004).
Discussion
This is the first prospective, longitudinal study of emotional and behavioral functioning in a large sample of children on active treatment for standard-risk acute lymphoblastic leukemia who did not receive cranial radiation, to our knowledge. Though most children had anxiety and depression scores similar to controls, a significant subpopulation of children displayed symptoms that merit intervention. We found that depressive symptoms were a significant problem from the end of the first month of therapy to 12 months after diagnosis. In contrast, the frequency of anxiety was elevated at the end of the first month of therapy, but then declined to levels expected in a normative population at 6 and 12 months after diagnosis. Anxiety and depression scores at one month after diagnosis significantly predicted persistence of symptoms throughout the first year of therapy. In adjusted analysis, unhealthy family functioning and self-reported Hispanic ethnicity were the variables that had the strongest association with emotional functioning. Children with unhealthy family functioning were 2.24 times as likely to have anxiety symptoms and 2.40 times as likely to have depressive symptoms. Hispanic children were 3.35 times as likely as white, non-Hispanic children to have anxiety symptoms, but were not at increased risk for depressive symptoms. Age, gender, family socioeconomic status, and therapeutic randomization did not predict emotional functioning. We did not find behavioral changes to be a significant problem.
Our study is unique in that our sample was a homogeneous group of ALL patients with a high expected cure rate who were also enrolled on a randomized clinical trial. Thus, we were able to account for any differences due to therapeutic randomization. Since we enrolled patients at 31 sites from a range of community and tertiary-care centers and rural and urban regions, our study can be generalized more than single institution studies. Furthermore, we had a high participation rate (82% of eligible), which greatly reduced the potential for selection bias.
Limited published data are available regarding the longitudinal psychosocial functioning of children currently receiving chemotherapy. Our results can be most closely compared with the prospective, cohort study of 38 patients by Sawyer et al., who also found that children experience considerable emotional distress in the immediate post-diagnosis period.9,25 But in contrast to our study, Sawyer et al concluded that, by one year after diagnosis, children treated for cancer had similar psychological functioning as children in the community. This other study had a smaller sample size and included a heterogeneous group of childhood cancer patients. A more recent study by Furlong et al. prospectively assessed quality of life in children with standard-risk and high-risk ALL, some of whom received cranial radiation. Though their outcomes included global quality of life health utilities instead of clinical measures of psychological functioning, Furlong et al. found that meaningful distress improved throughout therapy in a pattern similar to our results.10 This study also differed from ours in that the instrument used (the Health Utilities Index) was only validated for children ≥5 years old, which excluded over 50% of the SR-ALL sample. The most recent longitudinal report measured the prevalence and distress of cancer-related symptoms in children throughout therapy.8 This study, which included parents of 89 children with different cancer diagnoses, also found that psychological distress improved throughout therapy, but was still an issue for a subset of children at the end of therapy.
We found that family functioning was an important predictor of emotional functioning of children with cancer. Most previous research has focused on family functioning as an outcome variable. Maurice-Stam et al. studied off-therapy children and found both positive and negative correlations between family functioning and quality of life, depending on the age of the child.26 In pediatric asthma patients, family dysfunction has been associated with children’s mental health.27 Our study used the FAD-GF, which measures perceived family cohesion and ability of family members to communicate with each other. Our results suggest that families who demonstrate worse cohesion and communication should be considered higher-risk and be offered more psychosocial support. There are available psychosocial risk screening measures such as the validated Psychosocial Assessment Tool (PAT2.0), which can integrate assessments of family functioning.28 Furthermore, family functioning may be a modifiable variable, and thus, our results support developing family-based interventions that target family functioning.
An important conclusion of our study is that anxiety and depression at one month after diagnosis significantly predict persistence of symptoms throughout the first year of therapy. Clinicians have long advocated the importance of addressing acute symptoms as part of multidisciplinary supportive care during cancer therapy.29 Our results further highlight the importance of early identification and intervention of distressed children to avoid long-term emotional distress. Previous studies in the general population suggest that anxiety in children may result in the later development of depressive disorders and substance abuse.30 Studies in non-cancer pediatric populations have also found that there are inherited factors that contribute to individual risk of responding to early-life adversity with depression and anxiety.31 Such stressors can result in changes in the corticotropin-releasing factor (CRF) system in genetically predisposed individuals.
Although anxiety and depressive symptoms may lessen throughout therapy, it is important to recognize the distress they cause and provide appropriate psychosocial interventions. The NIH consensus statement on cancer symptoms states: “All patients with cancer should have optimal symptom [includes pain, depression, and fatigue] control from diagnosis throughout the course of illness, irrespective of personal and cultural characteristics.”32 A wealth of psychosocial interventions exists for children with cancer, including cognitive-behavioral therapy, social-recreational activities, and psychoeducational interventions.33 Many interventions use family-based methods, which have been associated with beneficial outcomes for children.34 Understanding the efficacy of various interventions is an ongoing area of research.33
The association of Hispanic ethnicity with anxiety is novel in children with ALL. To make sure this was not due to a methodological error in using an inappropriate comparison group, we verified that the frequencies of Hispanic children in the BASC-2 PRS normative comparison group (16.5–20%) were similar to that in our study population group (16.4%).19 There is a single report that showed poorer emotional functioning in Hispanic children with cancer, but it only addressed the off-treatment period.35 There are other differences between Hispanic and non-Hispanic children with ALL, including inferior survival rates in Hispanic children.1,36 We do not have the data available to explain how Hispanic ethnicity leads to worse psychological functioning, but given these differences, this area of study deserves further attention.
The current study has some methodological characteristics that should be considered in interpreting the results. First, some patients enrolled in the study did not complete the evaluations at all the required timepoints due to withdrawals from the therapeutic study, administrative errors at study sites, and/or incomplete forms. Second, only parent-report was available for the majority (89.3%) of children in our study because there was no self-report option of the BASC-2 available for children under the age of eight. In fact, there are no validated, objective self-report assessment tools for emotional functioning for children under age 5 years and few for those between the ages of 5 and 8 years. Young children may lack the language abilities to report on and/or the cognitive capacity to reflect upon one’s own behaviors or feelings. Children as old as 11 years tend to report fewer psychiatric symptoms and are unreliable in reporting about time factors, such as duration or frequency of symptoms;37 thus, collateral information is emphasized.38 Assessing preschool age children is typically accomplished through parent-report and observation.39 In addition, we were able to association family functioning with emotional functioning, but we were unable to determine the direction of the association from our data. It may be that children with better emotional functioning lessen the burden on their families.
From this large, multisite, cohort study of children treated for SR-ALL, we conclude that symptoms of depression and anxiety are a significant problem in the immediate post-diagnosis period. While anxiety symptoms lessen after the first month of therapy, depressive symptoms persist throughout at least the first year. We also found that we can identify children at one month after diagnosis who are substantially more likely to have worse psychological functioning throughout the first year of therapy. These results are highly relevant to pediatric oncologists who should be screening for clinical levels of anxiety and depression starting in the early post-diagnosis period for at least one year after diagnosis. Furthermore, our results highlight high-risk groups who should receive additional psychosocial support, including children of Hispanic ethnicity or with parent-reported unhealthy family functioning. Further studies are needed to develop evidence-based interventions, including those targeting family functioning and patient subgroups at greatest risk, to prevent and treat anxiety and depression in children with acute lymphoblastic leukemia leukemia.
Acknowledgements
Funding Sources: This research was supported by grants from the National Institutes of Health to the COG including CA13539, CA98543, and a Community Cancer Oncology Program (CCOP) grant from the National Cancer Institute Division of Cancer Prevention to the Children’s Oncology Group. Dr. Hunger is the Ergen Family Chair in Pediatric Cancer. Dr. Kadan-Lottick is supported in part by American Cancer Society Scholar Grant 119700-RSGHP-10-107-01-CPHPS and a Team Brent St. Baldrick’s Foundation Scholar award.
Footnotes
Financial Disclosures: None
Contributor Information
Regina M Myers, Section of Pediatric Hematology/Oncology, Yale University School of Medicine and Yale Comprehensive Cancer Center, New Haven, CT, USA.
Lyn Balsamo, Section of Pediatric Hematology/Oncology, Yale University School of Medicine and Yale Comprehensive Cancer Center, New Haven, CT, USA.
Xiaomin Lu, Department of Biostatistics, Colleges of Medicine, Public Health & Health Professions, University of Florida, Gainesville, FL, USA.
Meenakshi Devidas, Department of Biostatistics, Colleges of Medicine, Public Health & Health Professions, University of Florida, Gainesville, FL, USA.
Stephen P. Hunger, Department of Pediatrics, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora, CO, USA.
William L. Carroll, New York University Cancer Institute, New York, NY, USA.
Naomi J. Winick, Division of Pediatric Hematology/Oncology, University of Texas Southwestern School of Medicine, Dallas, TX, USA.
Kelly W. Maloney, Department of Pediatrics, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora, CO, USA.
Nina S. Kadan-Lottick, Section of Pediatric Hematology/Oncology, Yale University School of Medicine and Yale Comprehensive Cancer Center, New Haven, CT, USA.
References
- 1.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2011;118:243–251. doi: 10.1182/blood-2010-12-322909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochhauser CJ, Lewis M, Kamen BA, et al. Steroid-induced alterations of mood and behavior in children during treatment for acute lymphoblastic leukemia. Support Care Cancer. 2005;13:967–974. doi: 10.1007/s00520-005-0882-8. [DOI] [PubMed] [Google Scholar]
- 4.Sung L, Yanofsky R, Klaassen RJ, et al. Quality of life during active treatment for pediatric acute lymphoblastic leukemia. Int J Cancer. 2011;128:1213–1220. doi: 10.1002/ijc.25433. [DOI] [PubMed] [Google Scholar]
- 5.Pickard AS, Topfer LA, Feeny DH. A structured review of studies on health-related quality of life and economic evaluation in pediatric acute lymphoblastic leukemia. J Natl Cancer Inst Monogr. 2004:102–125. doi: 10.1093/jncimonographs/lgh002. [DOI] [PubMed] [Google Scholar]
- 6.Shankar S, Robison L, Jenney ME, et al. Health-related quality of life in young survivors of childhood cancer using the Minneapolis-Manchester Quality of Life-Youth Form. Pediatrics. 2005;115:435–442. doi: 10.1542/peds.2004-0649. [DOI] [PubMed] [Google Scholar]
- 7.Waters EB, Wake MA, Hesketh KD, et al. Health-related quality of life of children with acute lymphoblastic leukaemia: comparisons and correlations between parent and clinician reports. Int J Cancer. 2003;103:514–518. doi: 10.1002/ijc.10815. [DOI] [PubMed] [Google Scholar]
- 8.Heden L, Poder U, von Essen L, et al. Parents' Perceptions of Their Child's Symptom Burden During and After Cancer Treatment. J Pain Symptom Manage. 2013;46:366–375. doi: 10.1016/j.jpainsymman.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer M, Antoniou G, Toogood I, et al. Childhood cancer: a 4-year prospective study of the psychological adjustment of children and parents. J Pediatr Hematol Oncol. 2000;22:214–220. doi: 10.1097/00043426-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Furlong W, Rae C, Feeny D, et al. Health-related quality of life among children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59:717–724. doi: 10.1002/pbc.24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meeske K, Katz ER, Palmer SN, et al. Parent proxy-reported health-related quality of life and fatigue in pediatric patients diagnosed with brain tumors and acute lymphoblastic leukemia. Cancer. 2004;101:2116–2125. doi: 10.1002/cncr.20609. [DOI] [PubMed] [Google Scholar]
- 12.Landolt MA, Vollrath M, Niggli FK, et al. Health-related quality of life in children with newly diagnosed cancer: a one year follow-up study. Health Qual Life Outcomes. 2006;4:63. doi: 10.1186/1477-7525-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mounir GM, Abolfotouh MA. Assessment of health related quality of life among school children with cancer in Alexandria. J Egypt Public Health Assoc. 2007;82:219–238. [PubMed] [Google Scholar]
- 14.Klassen AF, Anthony SJ, Khan A, et al. Identifying determinants of quality of life of children with cancer and childhood cancer survivors: a systematic review. Support Care Cancer. 2011;19:1275–1287. doi: 10.1007/s00520-011-1193-x. [DOI] [PubMed] [Google Scholar]
- 15.Kazak AE, Alderfer MA, Streisand R, et al. Treatment of posttraumatic stress symptoms in adolescent survivors of childhood cancer and their families: a randomized clinical trial. J Fam Psychol. 2004;18:493–504. doi: 10.1037/0893-3200.18.3.493. [DOI] [PubMed] [Google Scholar]
- 16.Chesla CA. Do family interventions improve health? J Fam Nurs. 2010;16:355–377. doi: 10.1177/1074840710383145. [DOI] [PubMed] [Google Scholar]
- 17.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Myers RBL, Carroll W, Hunger S, Winick M, Devidas X, Lu X, Maloney K, Kadan-Lottick N. Emotional and behavioral functioning in the first year after diagnosis of standard risk (SR) acute lymphoblastic leukemia (ALL): a report from children's oncology group (COG) AALL0331. Presented at the American Society of Pediatric Hematology/Oncology; New Orleans, LA. 2012. [Google Scholar]
- 19.Reynolds CR, Kamphaus RW. Behavior assessment system for children. 2nd ed. Circle Pine, MN: American Guidance Service; 2004. [Google Scholar]
- 20.Wolfe-Christensen C, Mullins LL, Stinnett TA, et al. Use of the Behavioral Assessment System for Children 2nd Edition: Parent Report Scale in pediatric cancer populations. J Clin Psychol Med Settings. 2009;16:322–330. doi: 10.1007/s10880-009-9174-7. [DOI] [PubMed] [Google Scholar]
- 21.Carpentieri SC, Meyer EA, Delaney BL, et al. Psychosocial and behavioral functioning among pediatric brain tumor survivors. J Neurooncol. 2003;63:279–287. doi: 10.1023/a:1024203323830. [DOI] [PubMed] [Google Scholar]
- 22.Miller IW, Bishop DS, Epstein NB, et al. The Mcmaster Family Assessment Device - Reliability and Validity. Journal of Marital and Family Therapy. 1985;11:345–356. [Google Scholar]
- 23.McCubbin H, McCubbin M, Nevin R, et al. Coping Health Inventory for Parents. In: H M, A T, editors. Family Assessment Inventories for Research and Practice. Madison, WI: University of Wisconsin-Madison; 1987. [Google Scholar]
- 24.Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94:2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 25.Sawyer M, Antoniou G, Toogood I, et al. Childhood cancer: a two-year prospective study of the psychological adjustment of children and parents. J Am Acad Child Adolesc Psychiatry. 1997;36:1736–1743. doi: 10.1097/00004583-199712000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Maurice-Stam H, Grootenhuis MA, Brons PP, et al. Psychosocial indicators of health-related quality of life in children with cancer 2 months after end of successful treatment. J Pediatr Hematol Oncol. 2007;29:540–550. doi: 10.1097/MPH.0b013e3181256b66. [DOI] [PubMed] [Google Scholar]
- 27.Sawyer MG, Spurrier N, Whaites L, et al. The relationship between asthma severity, family functioning and the health-related quality of life of children with asthma. Qual Life Res. 2000;9:1105–1115. doi: 10.1023/a:1016655511879. [DOI] [PubMed] [Google Scholar]
- 28.Pai AL, Patino-Fernandez AM, McSherry M, et al. The Psychosocial Assessment Tool (PAT2.0): psychometric properties of a screener for psychosocial distress in families of children newly diagnosed with cancer. J Pediatr Psychol. 2008;33:50–62. doi: 10.1093/jpepsy/jsm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Wetering MD, Schouten-van Meeteren NY. Supportive care for children with cancer. Semin Oncol. 2011;38:374–379. doi: 10.1053/j.seminoncol.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Kendall PC, Safford S, Flannery-Schroeder E, et al. Child anxiety treatment: outcomes in adolescence and impact on substance use and depression at 7.4-year follow-up. J Consult Clin Psychol. 2004;72:276–287. doi: 10.1037/0022-006X.72.2.276. [DOI] [PubMed] [Google Scholar]
- 31.Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- 32.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: pain, depression, and fatigue, July 15–17, 2002. J Natl Cancer Inst Monogr. 2004:9–16. doi: 10.1093/jncimonographs/djg014. [DOI] [PubMed] [Google Scholar]
- 33.Pai AL, Drotar D, Zebracki K, et al. A meta-analysis of the effects of psychological interventions in pediatric oncology on outcomes of psychological distress and adjustment. J Pediatr Psychol. 2006;31:978–988. doi: 10.1093/jpepsy/jsj109. [DOI] [PubMed] [Google Scholar]
- 34.Meyler E, Guerin S, Kiernan G, et al. Review of family-based psychosocial interventions for childhood cancer. J Pediatr Psychol. 2010;35:1116–1132. doi: 10.1093/jpepsy/jsq032. [DOI] [PubMed] [Google Scholar]
- 35.Meeske KA, Patel SK, Palmer SN, et al. Factors associated with health-related quality of life in pediatric cancer survivors. Pediatr Blood Cancer. 2007;49:298–305. doi: 10.1002/pbc.20923. [DOI] [PubMed] [Google Scholar]
- 36.Goggins WB, Lo FF. Racial and ethnic disparities in survival of US children with acute lymphoblastic leukemia: evidence from the SEER database 1988–2008. Cancer Causes Control. 2012;23:737–743. doi: 10.1007/s10552-012-9943-8. [DOI] [PubMed] [Google Scholar]
- 37.Schwab-Stone M, Fallon T, Briggs M, et al. Reliability of diagnostic reporting for children aged 6–11 years: a test-retest study of the Diagnostic Interview Schedule for Children-Revised. Am J Psychiatry. 1994;151:1048–1054. doi: 10.1176/ajp.151.7.1048. [DOI] [PubMed] [Google Scholar]
- 38.Chrisman A, Egger H, Compton SN, et al. Assessment of childhood depression. Child and Adolescent Mental Health. 2006;11:111–116. doi: 10.1111/j.1475-3588.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 39.Luby J, Tandon M. Assessing the preschool-age child. In: Dulcan M, editor. Dulcan's Textbook of Child and Adolescent Psychiatry. Arlington, VA: American Psychiatric Publishing; 2010. [Google Scholar]