Abstract
Exaggerated beta range (15-30 Hz) oscillatory activity is observed in the basal ganglia of Parkinson's disease (PD) patients during implantation of deep brain stimulation electrodes. This activity has been hypothesized to contribute to motor dysfunction in PD patients. However, it remains unclear how these oscillations develop and how motor circuits become entrained into a state of increased synchronization in this frequency range after loss of dopamine. It is also unclear whether this increase in neuronal synchronization actually plays a significant role in inducing the motor symptoms of this disorder. The hemiparkinsonian rat has emerged as a useful model for investigating relationships between loss of dopamine, increases in oscillatory activity in motor circuits and behavioral state. Chronic recordings from these animals show exaggerated activity in the high beta/low gamma range (30-35 Hz) in the dopamine cell-lesioned hemisphere. This activity is not evident when the animals are in an inattentive rest state, but it can be stably induced and monitored in the motor cortex and basal ganglia when they are engaged in an on-going activity such as treadmill walking. This review discusses data obtained from this animal model and the implications and limitations of this data for obtaining further insight into the significance of beta range activity in PD.
Introduction
Functional neurosurgery has provided an opportunity to record directly from the basal ganglia in the human. This opportunity most commonly arises in patients with Parkinson's disease (PD) undergoing implantation of electrodes in the subthalamic nucleus (STN) or the globus pallidus interna (GPi) for therapeutic deep brain stimulation at high frequency (DBS) [1]. The availability of direct recordings of basal ganglia activity from PD patients has led to new ideas about mechanisms underlying motor dysfunction associated with PD. Most notably, excessive synchronization of neuronal activity in the beta (12–30 Hz) frequency range has been reported in the STN and GPi of PD patients [2-7]. This activity has been hypothesized to be responsible for the akinesia and bradykinesia characteristic of PD [8-12], and it has been suggested that DBS alleviates motor symptoms in PD, to some extent , by disrupting this overly synchronized activity [13-19].
Human studies have their limitations when it comes to examination of these hypotheses. The fact that all basal ganglia recordings are performed in patients with some type of neurological disorder does not allow a clear comparison of the normal state with the diseased state before and after treatment. In addition, it is difficult to examine the relationship between the onset and progression of exaggerated oscillatory activity and the emergence of motor symptoms in PD patients as neurosurgical intervention is reserved for specific target sites and advanced disease states.
Animal models of PD provide an opportunity to explore these issues. In vitro as well as in vivo experiments allow researchers to focus on specific mechanisms from cell to network levels. Research in PD has been facilitated by the fact that many aspects of this disorder can be modeled in rodents as well as primates. The main motor symptoms are believed to be due to the loss of dopamine neurons, a cell type which is selectively sensitive to certain neurotoxins. In the late 1960's, Ungerstedt described the use of 6-hydroxydopamine (6-OHDA) as a research tool for inducing dopamine cell death in rats [20, 21] and in the 1980's1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was found to have a similar effect in primates and mice [22-29]
The availability of 6-OHDA led to the widespread use of the hemiparkinsonian rat model for biochemical as well as neurophysiological studies of the effects of dopamine cell death on basal ganglia circuitry. A relatively extensive dopamine cell lesion can be obtained by infusion of 6-OHDA into the medial forebrain bundle (MFB), where it is transported into the axons of the dopamine neurons as they travel from the substantia nigra to their terminals, most prominently in the striatum and, to a lesser extent, in other basal ganglia sites and the motor cortex. Because the dopamine neurons in the basal ganglia project unilaterally, lesioning the dopamine neurons in one hemisphere induces only indirect, presumably minor effects on the contralateral hemisphere. Since the dopamine system in one hemisphere remains intact, the animals are able to eat and move around in spite of having severe dopamine depletion in the lesioned hemisphere. The motor symptoms that emerge are mainly evident on the side of the body contralateral to the 6-OHDA lesion. This review will discuss recent studies investigating the utility of the awake, behaving hemiparkinsonian rat for obtaining insight into the relationships between motor symptoms, behavioral state and synchronized activity in basal ganglia and motor cortex in PD.
The treadmill walking task
A significant challenge in chronic recording studies in animal models of PD is how to control the animal's behavior so that tasks performed are relevant to PD but still capable of being expressed by both control and lesioned rats. An effective strategy has involved the use of a circular treadmill with a track just wide enough to allow a rat to walk forward without turning around (Fig 1a) [30]. The treadmill, designed in collaboration with the National Institutes of Health's Section on Instrumentation, rotates at a relatively slow speed (1-1.5 sec between steps) and the presence of a stationary paddle encourages the rat to maintain continuous locomotion. Before the 6-OHDA-induced dopamine cell lesion, rats are trained to walk in both directions on the circular treadmill. After unilateral dopamine cell lesion, the hemiparkinsonian rats retain their ability to walk steadily in the direction ipsiversive to the lesion, with their affected paws on the outside of the circular path. However, the ability of the rat to walk contraversive to the lesion, with their affected paws on the inside of the circular path, is dramatically impaired. They have considerable difficulty making steady progress, and generally freeze or rear and attempt to turn around (Fig 1b). This arrangement allows comparisons of basal ganglia activity in the intact and lesioned hemispheres in the hemiparkinsonian rats as they walk ipsiversively on the treadmill. Similar recordings can be performed in control rats walking at the same speed. The marked difficulty the rats experience with contraversive walking provides an index of disability, as contraversive walking is notably improved by dopamine replacement with levodopa (L-DOPA) [30, 31] or dopaminergic agonists such as apomorphine. After treadmill walking epochs, rats tend to remain inactive for a period of time and often engage in inattentive rest. Thus, comparisons can be made between the rest and walk states.
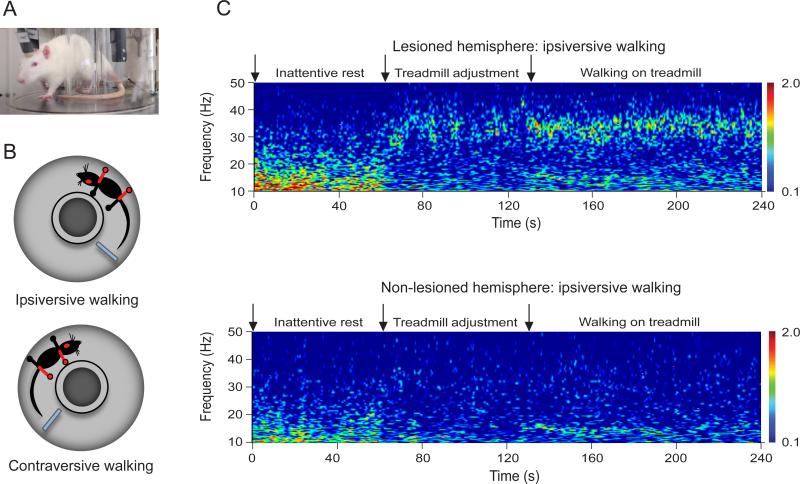
Figure 1. Representative example of treadmill walking and SNpr LFP activity in lesioned and non-lesioned hemispheres.
A. Rat walking on the treadmill with chronically implanted electrodes. B. Schematic representation of the rat walking ipsiversively and contraversively to the lesion hemisphere (red dot) with the impaired paws (red) outside and inside the treadmill, respectively. C. Wavelet based scalogram of simultaneously recorded SNpr LFP activity from the lesioned hemisphere (top) and the non-lesioned hemisphere (bottom) during one epoch of inattentive rest, treadmill adjustment and treadmill walking. During the treadmill adjustment period, low beta LFP power (12-18 Hz) was reduced in the SNpr in both intact and lesioned hemispheres compared to inattentive rest epochs. Meanwhile, SNpr LFP power increased in the 25-30 Hz range in the lesioned hemisphere. When the rats began to walk, robust 30-35 Hz high beta/low gamma range activity became quite prominent in the SNpr of the lesioned hemisphere (data taken in part from Avila et al., 2010 [31]).
Local field potential (LFP) and spike synchronization in basal ganglia output in the hemiparkinsonian rat
The first studies in the awake behaving rats using this model ([30, 31] focused on comparisons between activity in the substantia nigra pars reticulata (SNpr), the principal basal ganglia output nucleus, in the intact and dopamine cell-lesioned hemispheres (dopamine cell loss >90%, as indicated by tyrosine hydroxylase staining) during epochs of rest and treadmill walking in the ipsiversive direction. During epochs identified as inattentive rest, SNpr LFP power in the 12-18 Hz low beta range was significantly greater in the dopamine-depleted hemispheres than in control hemispheres (either contralateral to the 6-OHDA lesions in bilateral recordings or in control rats with no lesion). Also notable was the intermittent presence of prominent high voltage spindles in the LFPs recorded during inattentive rest epochs, consisting of 3-5 sec epochs, when the LFP displayed prominent oscillations at frequencies of 5-13 Hz. This phenomenon has been described as being considerably more evident in the basal ganglia and motor cortex of the 6-OHDA-lesioned rat than in the control rat [32-34]. Interestingly, we noted that when a prominent high voltage spindle was present in the SNpr of the 6-OHDA lesioned hemisphere, a much smaller version, with shorter duration and lower amplitude could regularly be found occurring simultaneously in LFP recordings from the contralateral intact SNpr. Spiking activity in the SNpr was strongly phase-locked with these spindles in the lesioned hemisphere, but less so in the intact (I. Avila, data not shown).
At the end of each rest epoch the treadmill was turned on. As the rat was alerted by the activation of the treadmill, low beta LFP power (12-18 Hz) was reduced in the SNpr in both intact and lesioned hemispheres. As the rat began to move and adjust it's stepping to the speed of the treadmill, SNpr LFP power increased in the 25-40 Hz range in the lesioned hemisphere. When the rats began to walk at a constant speed in the direction ipsilateral to the lesion, 30-35 Hz high beta/low gamma activity became strikingly prominent in the SNpr of the lesioned hemisphere. Similar activity was also evident in the dopamine-lesioned hemisphere when the rats were oriented in the opposite (contraversive) direction on the treadmill where they showed greater difficulty walking in a steady manner [30, 31]. The observations of this abnormal activity in the lesioned hemisphere during orientation in the contraversive direction, as with orientation in the ipsilateral direction, is consistent with the fact that even though the animal was having difficulty walking steadily in the contraversive direction, he was frequently rearing or moving to try to turn around, and therefore still attempting to use both the impaired and intact paws. Thus, in the hemiparkinsonian rat, dopamine loss is associated with changes in synchronization of SNpr activity in low beta or high beta/low gamma ranges in the lesioned hemisphere depending on the behavioral state of the rat.
Spike triggered waveform averages were calculated to assess the extent to which the increases in exaggerated oscillatory LFP activity were associated with increases in phase-locked spiking. During rest periods in which low beta activity was prominent, and during periods of high beta/low gamma activity when rats were walking, spikes were significantly more phase locked to the dominant frequency expressed in the LFP in the dopamine cell-lesioned hemisphere than they were to the same frequency in the intact hemisphere. Interestingly, LDOPA administration reduced the synchronization of activity observed in the lesioned hemisphere in the hemiparkinsonian rats and improved their ability to walk in the contraversive direction on the treadmill. This was consistent with the efficacy of L-DOPA in reducing exaggerated synchronization in the beta range in in PD patients [30, 31].
These observations are relevant to a clinically pertinent issue regarding the increased oscillatory activity in the basal ganglia observed after loss of dopamine: the hypothesis that dysfunctional synchronization in basal ganglia, and basal ganglia output, plays a role in mediating motor symptoms in PD [8-12, 35, 36]. In the extensively lesioned hemiparkinsonian rat, the increase in synchronized spiking activity and high beta/low gamma LFP power, evident in the SNpr by 7 days post-lesion, is clearly associated with difficulties in gait and responsive to L-DOPA administration. However, a recent study has raised doubt about the relationship between increases in oscillatory activity and motor dysfunction in this animal model when dopamine cell lesion is less extensive. Quiroga-Varela and colleagues [37] recorded LFP and spike activity from the SNpr during the development of a progressive bilateral dopaminergic lesion mediated by intracerebral administration of 6-OHDA into the third ventricle over 7 days [38]. The results failed to show evidence of increases in synchronized activity in SNpr recordings during treadmill walking in spite of clear evidence of a reduction in motor function and bilateral loss of dopamine neurons of approximately 65%. Similarly, 6-OHDA MFB injections inducing a comparable partial loss of dopaminergic neurons also failed to induce increased oscillatory activity in the 30-35 Hz range in the SNpr [37]. One implication of these findings may be that relatively subtle and localized changes in neuronal synchronization and coherence in basal ganglia circuits are sufficient to provoke motor symptoms. Alternatively, it may be possible that the robust increase in synchronized activity observed in the parkinsonian rat represents a maladaptive compensatory process which emerges after substantial dopamine cell loss. It is interesting to consider that these observations in the rodent model may be relevant to reports of a lack of correlation between onset of motor symptoms and exaggerated beta range synchronization in the basal ganglia in PD patients and primate models of PD [7, 39-41].
High beta/low gamma LFP synchronization and coherence in motor cortex and basal ganglia during rest and walk in the hemiparkinsonian rat
Another area of controversy with respect to the synchronized activity observed in the basal ganglia after loss of dopamine is the source of these oscillations. This has proven a complex problem to address, even at the level of a rodent model of PD. Early studies in the anesthetized hemiparkinsonian rat focused on the effects of dopamine cell loss on striatal sensitivity to cortical input [42]. These studies lent support to the idea that dopamine depletion facilitates the transmission of cortical rhythms through the striatum to the basal ganglia. Indeed, a number of investigators have shown that the transmission of slow wave activity from cortex to downstream nuclei is markedly enhanced by dopamine cell lesion [42-48]. While it is appealing to think that faster cortical frequencies might similarly exert an enhanced effect on striatal output after loss of dopamine, the studies by Zold and colleagues [49, 50] show that oscillations with frequencies in the 20 Hz range and higher are not efficiently transmitted through the striatum in the rat after 6-OHDA lesions. This raises questions about the potential for cortical input to the basal ganglia to contribute to the emergence of synchronized 30-35 Hz activity observed in the SNpr during treadmill walking in the hemiparkinsonian rat, and calls attention to a role for the hyperdirect pathway from the cortex to the STN [51]. In addition, in vitro studies and in vivo studies in anesthetized rats have provided evidence for emergence of oscillatory activity in the beta range in the globus pallidus externa (GPe)-STN circuit after loss of dopamine [52-57]. These studies suggest that changes in the properties of the reciprocal connectivity between STN and GPe could bring the network into a regime that intrinsically oscillates in the beta range, adding another possibility to the ways in which loss of dopamine could promote entrainment of oscillatory activity in the basal ganglia in PD.
Relevant to these considerations, we examined whether LFPs in the motor cortex in the dopamine cell-lesioned hemisphere show increases in oscillatory activity in the same 30-35 Hz frequency range during treadmill walking as observed in the SNpr [31]. And indeed, motor cortex LFP activity recorded 1-4 weeks after dopamine depletion showed marked increases in the 30-35 Hz range during treadmill walking which was highly coherent with the SNpr LFP activity in the dopamine-lesioned hemisphere. While these observations are consistent with the idea that cortical activity contributes to the entraining of oscillatory activity in the basal ganglia after loss of dopamine, it is not clear how the dominant frequency becomes focused in the 30-35 Hz range in the dopamine-lesioned hemisphere. During treadmill walking, a broad band of modest LFP power in the 40-50 Hz range is present in the control rat. There is very little activity in the 30-35 Hz range in the motor cortex LFP prior to 6-OHDA lesion [31]. Thus, the emergence of the 30-35 Hz rhythm in the SNpr is not simply a potentiation of activity normally seen in the cortex during treadmill walking.
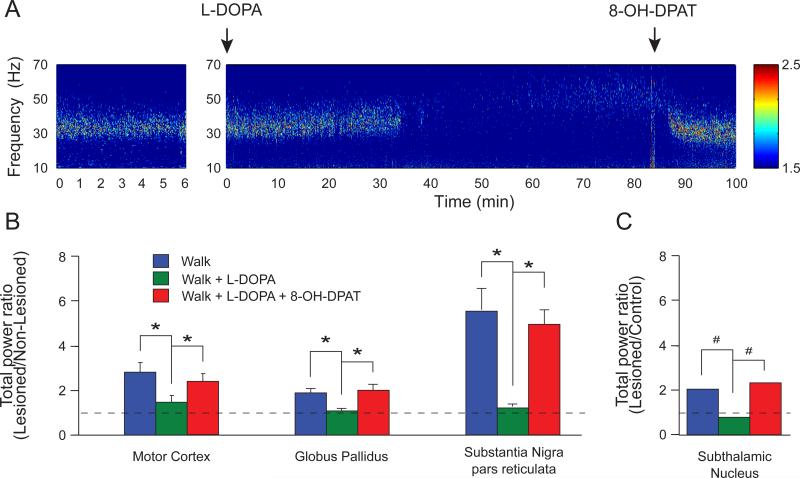
Consistent with the above observations, LFP recordings in the GPe and STN in the hemiparkinsonian rat also showed increases in oscillatory activity in the dopamine lesioned hemisphere, leaving open the possibility that reciprocal connections between GPe and STN could support the emergence of the high beta/low gamma oscillations. As in the motor cortex and SNpr, LFP activity in the GPe and STN nuclei was more entrained to the 30-35 Hz rhythm during treadmill walking in the dopamine cell-lesioned hemisphere relative to the control hemisphere, although, as shown in Fig 2, lesioned vs intact hemisphere ratios are greater for motor cortex and SNpr. The increase in 25-40 Hz range power in the lesioned hemisphere relative to the non-lesioned hemisphere is 2-fold in GPe and STN, 3-fold in motor cortex, and 5-fold in SNpr. Administration of L-DOPA significantly reduced the expression of LFP power in this range in all 4 areas, as seen with respect to beta range activity in the PD patients. Subsequent administration of 8-OH-DPAT, a serotonergic 5-HT1A agonist, reversed the effect of L-DOPA in the 25-40 Hz frequency range (Fig 2). This 5-HT1A agonist has been shown to reduce dopamine levels in the striatum after L-DOPA treatment [58].
Figure 2. Increases in the 25-40 Hz high beta/low gamma LFP activity in the motor cortex, GPe, STN and SNpr during treadmill walking after dopamine cell lesion.
A. Wavelet-based spectrogram of motor cortex LFP activity in the lesioned hemisphere during walking (left), after L-DOPA (t=0 min; 12 mg/kg combined with 15 mg/kg of benserazide, sc) and 8-OH-DPAT (t=85 min; 0.2 mg/kg, sc) injections. Note the prominent 30-35 Hz high beta/low gamma range activity during walking which is eliminated following L-DOPA treatment and was restored after 8-OHDPAT injection, a serotonergic 1A agonist known to reduce the release of dopamine via the serotoninergic terminals in the striatum and reverse the behavioral and pharmacological effects of L-DOPA [65]. B. Ratios between lesioned and non-lesioned hemispheres for total power in the 25-40 Hz in motor cortex, GPe and SNpr. All structures showed significantly greater high beta/low gamma power in the lesioned compared to the non-lesioned hemisphere (ratio > 1). These increases were reduced by L-DOPA (5 mg/kg sc with 15 mg/kg benserazide, sc) and restored by 8-OH-DPAT (*p<0.05, repeated measures one-way ANOVA). C. Ratio between the lesioned hemisphere in hemiparkinsonian rats and control hemisphere in intact rats for total power in the 25-40 Hz range in STN during walking. As in the structures described in (B), STN showed significantly higher power in 25-40 Hz frequency range in the lesioned animals compared to control (ratio >1). Also, these increases were reduced by L-DOPA (12 mg/kg combined with 15 mg/kg of benserazide, sc) and reversed by 8-OH-DPAT (#p<0.05, chi2 test).
The relatively larger increase in LFP power in the high beta/low gamma range during treadmill walking in the SNpr, as compared to increases in motor cortex, GPe, and STN in the hemiparkinsonian rat (Fig. 2), calls attention to the potential for the SNpr to exert downstream effects on the ventral medial (VM) thalamus, the principal target of SNpr innervation. This further suggests an additional mechanism which could contribute to increases in synchronized activity in the basal ganglia: SNpr-mediated entrainment of thalamocortical circuitry to the 30-35 Hz rhythm. To examine the possibility of entrainment of high beta/low gamma synchrony throughout the basal ganglia-thalamocortical circuit, cortical LFP waveforms were band-pass filtered between 25-40 Hz and used as a common temporal reference to establish the relationship between synchronized single cells in motor cortex and SNpr. The data showed that SNpr neurons – which were significantly phase-locked to the cortical LFP filtered in the 25-40 Hz frequency range with a 32 ms average cycle – spiked approximately 17 ms after the mean spiking of the cortical single units. Cortical neurons which were significantly phase-locked to the same cortical LFPs, in turn, fired around 15 ms after the SNpr neurons. These temporal lags are consistent with the possibility that the basal ganglia, VM thalamus, and cortical structures could be spiking in sequence to maintain the 30-35 Hz rhythm entrained within the basal gangliathalamocortical circuit. A preliminary report [59] of increased 30-35 Hz activity in the VM thalamus during treadmill walking in the hemiparkinsonian rat supports this idea. Other observations suggest that the network generating these oscillations undergoes some degree of long-term plasticity. The peak frequency of the high beta/low gamma activity seen during treadmill walking increased significantly over time, beginning at approximately 31 Hz at 7 days post-lesion and increasing about 1 Hz per week over the subsequent 3-4 weeks [31]. Together, these results suggest that another mechanism through which dopamine loss in the hemiparkinsonian rat may contribute to increased beta range synchronization is by facilitating entrainment of basal ganglia-thalamocortical network activity to a frequency compatible with the dynamic resonance of this circuit.
Implications and limitations of the hemiparkinsonian rat model
The changes in neuronal activity observed in motor circuits in the hemiparkinsonian rat, as described above, suggest that this animal model has the potential for providing insight into PD. The fact that the rat shows increases in oscillatory activity in the basal ganglia after loss of dopamine allows investigators to study the significance of this aberrant pattern of activity and its relationship to motor dysfunction. However, it remains to be determined whether the firing patterns emerging in the rodent after loss of dopamine are sufficiently comparable to those in PD patients to allow translational insight into increased LFP synchronization and underlying mechanisms in the patient.
One concern is that the oscillations prominent in the lesioned hemisphere in the rat during treadmill walking occur at a somewhat higher frequency than the range reported for PD patients. It has been suggested that this difference could be due to the fact that oscillatory activity is not being examined during comparable behavioral states in the hemiparkinsonian rat and PD patient. Patients are not commonly engaged in motor activity as vigorous as treadmill walking while being recorded. Although motor activity is increasingly being taken into consideration in studies with PD patients [60], it is still not clear exactly how firing patterns in the basal ganglia would be affected during effort to overcome difficulties in gait.
A related concern with respect to relationships between oscillatory activity and behavioral state has to do with whether increased activity in a particular frequency range is more effective than other ranges in promoting motor symptoms in PD patients. And if so, can a rat model of PD have translational value if the motor symptoms are expressed in conjunction with exaggerated activity in a different frequency range? The 20 Hz beta range activity observed in PD patients is believed to be correlated with a state of motor preparation in normal individuals [35]. This has led to the idea that increased power in the 20 Hz frequency range may prolong or “lock in” this stage of motor preparation and thus promote akinesia or bradykinesia. One might argue that species differences could exist such that the state of motor preparation and idling is facilitated by a higher 30-35 Hz rhythm in the rat. However, recent studies by Berke and coworkers [61] suggest that increases in LFP power in the 20 Hz range in the rat are indeed associated with the same type of behaviors as in the human: action programming, rather than on-going motor activity. Increases in synchronized activity in the parkinsonian primates are also found in frequency ranges different from those observed in the PD patient. After treatment with MPTP, synchronized spiking and LFP activity in primate basal ganglia is typically reported to be in the range of 7-13 Hz [62-64]. These frequencies are lower than those commonly reported in PD patients. One conclusion that could be drawn from these differences among species is that the increase in synchronization and/or coherence of spiking activity within the basal ganglia after loss of dopamine may be more critical to motor dysfunction than the particular frequency range of the synchronization.
Conclusion
In summary, the awake, behaving hemiparkinsonian rat is a useful resource for studying relationships between motor activity and network oscillations in the dopamine-depleted brain that models advanced PD. The excessive beta range oscillations observed in the basal ganglia nuclei in hemiparkinsonian rats resemble similar neuronal activity recorded from the basal ganglia of PD patients, though differences in the dominant frequency of network oscillations are important discrepancies to consider. Opportunities for future studies in this model include examination of the time course of the emergence of the oscillatory activity in the basal ganglia thalamocortical circuits and investigation of changes induced by this activity in specific downstream neuronal populations over time. In combination with data from in vitro studies, relationships between single cells and LFPs in cortical, thalamic and basal ganglia structures can illuminate mechanisms underlying the dynamic effects of loss of dopamine on motor circuits. These studies should facilitate identification of electrophysiological correlates of dysfunctional behavioral states and provide a bridge between cellular/synaptic levels and global phenomena such as oscillations, leading to insights into both normal and pathological function.
Acknowledgements
The Intramural Research Program of the NINDS, NIH supported this research. We wish to thank Tom Talbot, Daryl Bandy and Newlin Morgan in the Section on Instrumentation, NIMH/NINDS for design and fabrication of the rotary treadmill. Irene Avila's current address is: Neural Circuits and Cognition Unit, NIA, NIH, BG BRC RM 09C220251 Bayview Boulevard, Baltimore, MD 21224-6825
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: There are no financial disclosures or conflict of interest for any of the authors.
Reference List
- 1.Wichmann T, Delong MR. Deep-Brain Stimulation for Basal Ganglia Disorders. Basal Ganglia. 2011;1:65–77. doi: 10.1016/j.baga.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20:7766–75. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci. 2001;21:1033–8. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown P. Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18:357–63. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- 5.Priori A, Foffani G, Pesenti A, et al. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson's disease. Experimental Neurology. 2004;189:369–79. doi: 10.1016/j.expneurol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Alonso-Frech F, Zamarbide I, Alegre M, et al. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson's disease. Brain. 2006;129:1748–57. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger M, Mahant N, Hutchison WD, et al. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J Neurophysiol. 2006;96:3248–56. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- 8.Fogelson N, Kuhn AA, Silberstein P, et al. Frequency dependent effects of subthalamic nucleus stimulation in Parkinson's disease. Neurosci Lett. 2005;382:5–9. doi: 10.1016/j.neulet.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 9.Eusebio A, Brown P. Oscillatory activity in the basal ganglia. Parkinsonism Relat Disord. 2007;13(Suppl 3):S434–S436. doi: 10.1016/S1353-8020(08)70044-0. [DOI] [PubMed] [Google Scholar]
- 10.Chen CC, Litvak V, Gilbertson T, et al. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson's disease. Experimental Neurology. 2007;205:214–21. doi: 10.1016/j.expneurol.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Current Opinion in Neurobiology. 2007;17:656–64. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn AA KA. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci. 2006 doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- 13.Brown P, Eusebio A. Paradoxes of functional neurosurgery: Clues from basal ganglia recordings. Mov Disord. 2008;23:12–20. doi: 10.1002/mds.21796. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn AA, Kempf F, Brucke C, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci. 2008;28:6165–73. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn AA, Brucke C, Schneider GH, et al. Increased beta activity in dystonia patients after drug-induced dopamine deficiency. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta-band profile in Parkinson's disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol. 2009;215:20–8. doi: 10.1016/j.expneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Eusebio A, Thevathasan W, Doyle GL, et al. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry. 2011;82:569–73. doi: 10.1136/jnnp.2010.217489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosa M, Giannicola G, Servello D, et al. Subthalamic local field beta oscillations during ongoing deep brain stimulation in Parkinson's disease in hyperacute and chronic phases. Neurosignals. 2011;19:151–62. doi: 10.1159/000328508. [DOI] [PubMed] [Google Scholar]
- 19.Eusebio A, Cagnan H, Brown P. Does suppression of oscillatory synchronisation mediate some of the therapeutic effects of DBS in patients with Parkinson's disease? Front Integr Neurosci. 2012;6:47. doi: 10.3389/fnint.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5:107–10. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 21.Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24:485–93. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 22.Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983;80:4546–50. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sershen H, Reith ME, Hashim A, Lajtha A. Reduction of dopamine uptake and cocaine binding in mouse striatum by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Eur J Pharmacol. 1984;102:175–8. doi: 10.1016/0014-2999(84)90354-6. [DOI] [PubMed] [Google Scholar]
- 24.Langston JW, Forno LS, Rebert CS, Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Res. 1984;292:390–4. doi: 10.1016/0006-8993(84)90777-7. [DOI] [PubMed] [Google Scholar]
- 25.Chiueh CC, Burns RS, Markey SP, Jacobowitz DM, Kopin IJ. Primate model of parkinsonism: selective lesion of nigrostriatal neurons by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine produces an extrapyramidal syndrome in rhesus monkeys. Life Sci. 1985;36:213–8. doi: 10.1016/0024-3205(85)90061-x. [DOI] [PubMed] [Google Scholar]
- 26.Langston JW. MPTP neurotoxicity: an overview and characterization of phases of toxicity. Life Sci. 1985 1985 Jan 21;3636(3):201–6. doi: 10.1016/0024-3205(85)90059-1. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson G, Sundstrom E, Mefford I, et al. Electrophysiological and neurochemical correlates of the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in the mouse. Naunyn Schmiedebergs Arch Pharmacol. 1985;331:1–6. doi: 10.1007/BF00498844. [DOI] [PubMed] [Google Scholar]
- 28.Fox SH, Brotchie JM. The MPTP-lesioned non-human primate models of Parkinson's disease. Past, present, and future. Prog Brain Res. 2010;184:133–57. doi: 10.1016/S0079-6123(10)84007-5. [DOI] [PubMed] [Google Scholar]
- 29.Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism - selective destruction of dopaminergic-nurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA. 1983;80:4546–50. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avila I, Parr-Brownlie LC, Brazhnik E, Castaneda E, Bergstrom DA, Walters JR. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Exp Neurol. 2010;221:307–19. doi: 10.1016/j.expneurol.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brazhnik E, Cruz AV, Avila I, et al. State-Dependent Spike and Local Field Synchronization between Motor Cortex and Substantia Nigra in Hemiparkinsonian Rats. J Neurosci. 2012;32:7869–80. doi: 10.1523/JNEUROSCI.0943-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dejean C, Gross CE, Bioulac B, Boraud T. Dynamic changes in the cortex-basal ganglia network after dopamine depletion in the rat. J Neurophysiol. 2008;100:385–96. doi: 10.1152/jn.90466.2008. [DOI] [PubMed] [Google Scholar]
- 33.Dejean C, Nadjar A, Le MC, Bioulac B, Gross CE, Boraud T. Evolution of the dynamic properties of the cortex-basal ganglia network after dopaminergic depletion in rats. Neurobiol Dis. 2012;46:402–13. doi: 10.1016/j.nbd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Ge SN, Yang C, Li M, et al. Dopamine depletion increases the power and coherence of high-voltage spindles in the globus pallidus and motor cortex of freely moving rats. Brain Res. 2012;1465:66–79. doi: 10.1016/j.brainres.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34:611–8. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Little S, Pogosyan A, Kuhn AA, Brown P. Beta band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp Neurol. 2012;236:383–8. doi: 10.1016/j.expneurol.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quiroga-Varela A, Walters JR, Brazhnik E, Marin C, Obeso JA. What basal ganglia changes underlie the parkinsonian state? The significance of neuronal oscillatory activity. Neurobiol Dis. 2013;58:242–8. doi: 10.1016/j.nbd.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez M, Barroso-Chinea P, Abdala P, Obeso J, Gonzalez-Hernandez T. Dopamine cell degeneration induced by intraventricular administration of 6-hydroxydopamine in the rat: similarities with cell loss in parkinson's disease. Exp Neurol. 2001;169:163–81. doi: 10.1006/exnr.2000.7624. [DOI] [PubMed] [Google Scholar]
- 39.Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur J Neurosci. 2007;26:1701–13. doi: 10.1111/j.1460-9568.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- 40.Syed EC, Benazzouz A, Taillade M, et al. Oscillatory entrainment of subthalamic nucleus neurons and behavioural consequences in rodents and primates. Eur J Neurosci. 2012;36:3246–57. doi: 10.1111/j.1460-9568.2012.08246.x. [DOI] [PubMed] [Google Scholar]
- 41.Stein E, Bar-Gad I. beta oscillations in the cortico-basal ganglia loop during parkinsonism. Exp Neurol. 2013;245:52–9. doi: 10.1016/j.expneurol.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 42.Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci. 2001;21:6430–9. doi: 10.1523/JNEUROSCI.21-16-06430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belluscio MA, Kasanetz F, Riquelme LA, Murer MG. Spreading of slow cortical rhythms to the basal ganglia output nuclei in rats with nigrostriatal lesions. Eur J Neurosci. 2003;17:1046–52. doi: 10.1046/j.1460-9568.2003.02543.x. [DOI] [PubMed] [Google Scholar]
- 44.Murer MG, Tseng KY, Kasanetz F, Belluscio M, Riquelme LA. Brain oscillations, medium spiny neurons, and dopamine. Cell Mol Neurobiol. 2002;22:611–32. doi: 10.1023/A:1021840504342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walters JR, Hu D, Itoga CA, Parr-Brownlie LC, Bergstrom DA. Phase relationships support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience. 2007;144:762–76. doi: 10.1016/j.neuroscience.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aravamuthan BR, Bergstrom DA, French RA, Taylor JJ, Parr-Brownlie LC, Walters JR. Altered neuronal activity relationships between the pedunculopontine nucleus and motor cortex in a rodent model of Parkinson's disease. Exp Neurol. 2008;213:268–80. doi: 10.1016/j.expneurol.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–30. doi: 10.1016/s0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 48.Valencia M, Chavez M, Artieda J, Bolam JP, Mena-Segovia J. Abnormal functional connectivity between motor cortex and pedunculopontine nucleus following chronic dopamine depletion. J Neurophysiol. 2013 doi: 10.1152/jn.00555.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zold CL, Kasanetz F, Pomata PE, et al. Striatal gating through up states and oscillations in the basal ganglia: Implications for Parkinson's disease. J Physiol Paris. 2012;106:40–6. doi: 10.1016/j.jphysparis.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Zold CL, Escande MV, Pomata PE, Riquelme LA, Murer MG. Striatal NMDA receptors gate cortico-pallidal synchronization in a rat model of Parkinson's disease. Neurobiol Dis. 2012;47:38–48. doi: 10.1016/j.nbd.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 51.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal 'hyperdirect' pathway. Neuroscience Research. 2002;43:111–7. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 52.Baufreton J, Atherton JF, Surmeier DJ, Bevan MD. Enhancement of excitatory synaptic integration by GABAergic inhibition in the subthalamic nucleus. J Neurosci. 2005;25:8505–17. doi: 10.1523/JNEUROSCI.1163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen KZ, Johnson SW. Dopamine depletion alters responses to glutamate and GABA in the rat subthalamic nucleus. Neuroreport. 2005;16:171–4. doi: 10.1097/00001756-200502080-00021. [DOI] [PubMed] [Google Scholar]
- 54.Mallet N, Pogosyan A, Marton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian Beta Oscillations in the External Globus Pallidus and Their Relationship with Subthalamic Nucleus Activity. J Neurosci. 2008;28:14245–58. doi: 10.1523/JNEUROSCI.4199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holgado AJN, Terry JR, Bogacz R. Conditions for the Generation of Beta Oscillations in the Subthalamic Nucleus-Globus Pallidus Network. J Neurosci. 2010;30:12340–52. doi: 10.1523/JNEUROSCI.0817-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cruz AV, Mallet N, Magill PJ, Brown P, Averbeck BB. Effects of dopamine depletion on information flow between the subthalamic nucleus and external globus pallidus. J Neurophysiol. 2011;106:2012–23. doi: 10.1152/jn.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tachibana Y, Iwamuro H, Kita H, Takada M, Nambu A. Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia. Eur J Neurosci. 2011;34:1470–84. doi: 10.1111/j.1460-9568.2011.07865.x. [DOI] [PubMed] [Google Scholar]
- 58.Kannari K, Yamato H, Shen H, Tomiyama M, Suda T, Matsunaga M. Activation of 5-HT(1A) but not 5-HT(1B) receptors attenuates an increase in extracellular dopamine derived from exogenously administered L-DOPA in the striatum with nigrostriatal denervation. J Neurochem. 2001;76:1346–53. doi: 10.1046/j.1471-4159.2001.00184.x. [DOI] [PubMed] [Google Scholar]
- 59.Brazhnik E, Hatch CE, Novikov N, et al. Ventral medial thalamus contributes to increased high beta/low gamma coherence in the basal ganglia thalamocortical network in an awake behaving rodent model of Parkinson's disease. Soc Neurosci Abstr Online. 2011 Program No. 811.23. [Google Scholar]
- 60.Joundi RA, Brittain JS, Green AL, Aziz TZ, Brown P, Jenkinson N. Persistent suppression of subthalamic beta-band activity during rhythmic finger tapping in Parkinson's disease. Clin Neurophysiol. 2013;124:565–73. doi: 10.1016/j.clinph.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 61.Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73:523–36. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heimer G, Rivlin-Etzion M, Bar-Gad I, Goldberg JA, Haber SN, Bergman H. Dopamine replacement therapy does not restore the full spectrum of normal pallidal activity in the 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine primate model of Parkinsonism. J Neurosci. 2006;26:8101–14. doi: 10.1523/JNEUROSCI.5140-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–71. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, Delong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- 65.Tronci E, Carta M. 5-HT1 receptor agonists for the treatment of L-DOPA-induced dyskinesia: From animal models to clinical investigation. Basal Ganglia. 2013;3:9–13. [Google Scholar]