Abstract
The largest risk factor for age-related macular degeneration (ARMD) is advanced age. A prominent age-related change in the human retina is the accumulation of histochemically detectable neutral lipid in normal Bruch’s membrane (BrM) throughout adulthood. This change has the potential to have a major impact on physiology of the retinal pigment epithelium (RPE). It occurs in the same compartment as drusen and basal linear deposit, the pathognomonic extracellular, lipid-containing lesions of ARMD. Here we present evidence from light microscopic histochemistry, ultrastructure, lipid profiling of tissues and isolated lipoproteins, and gene expression analysis that this deposition can be accounted for by esterified cholesterol-rich, apolipoprotein B-containing lipoprotein particles constitutively produced by the RPE. This work collectively allows ARMD lesion formation and its aftermath to be conceptualized as a response to the retention of a sub-endothelial apolipoprotein B lipoprotein, similar to a widely accepted model of atherosclerotic coronary artery disease (CAD) (Tabas et al., 2007). This approach provides a wide knowledge base and sophisticated clinical armamentarium that can be readily exploited for the development of new model systems and the future benefit of ARMD patients.
Keywords: Age-related macular degeneration, Retinal pigment epithelium, Bruch’s membrane, Drusen, Basal deposits, Lipoproteins, Cholesterol, Retinyl ester, Apolipoprotein B
1. Statement of scope
Age-related macular degeneration (ARMD) is a major cause of vision loss in the elderly of the industrialized world. The largest risk factor for ARMD is advanced age. One of the most prominent age-related changes to the human retina is the accumulation of histochemically detectable neutral lipid in normal Bruch’s membrane (BrM) throughout adulthood (Pauleikhoff et al.,1990). Arguably one the most important observations relevant to ARMD pathobiology, the Pauleikhoff study indicated that a significant, universal, and previously unknown change to BrM occurs with aging. This change, the accumulation of lipid in BrM, has the potential to have a major impact on physiology of the retinal pigment epithelium (RPE), the cell layer that supports photoreceptors. Further, this lipid deposition occurs in the same BrM compartment in which the pathognomonic extracellular, lipid-containing lesions of ARMD later arise.
Our thinking about this phenomenon has been extensively influenced by the vast knowledge base available for atherosclerosis, a condition for which lipid deposition in vessel walls is a well-established causative agent. Others have sought similar connections between ARMD and vascular disease with an emphasis on hemodynamics (Friedman, 2004). Here we review recent work primarily from our laboratories establishing that BrM lipid accumulation can be accounted for by cholesterol-rich lipoproteins of intraocular origin. This work collectively lays a foundation for considering the RPE as a constitutive lipoprotein secretor and ARMD as exhibiting both key similarities and differences with atherosclerosis. After we examine the process of lipoprotein accumulation in BrM, we summarize briefly aspects of lipoprotein biology and atherosclerosis pathobiology. We then review the evidence supporting local production of lipoproteins and consider the possible pathological implications of lipoprotein accumulation in BrM. We conclude by examining implications of this work for future research in basic and clinical areas. Many ARMD-relevant topics not covered herein are reviewed elsewhere (Edwards and Malek, 2007; Grisanti and Tatar, 2008; Jackson et al., 2005; Montezuma et al., 2007).
2. Introduction to outer retina and choroid (Fig. 1)
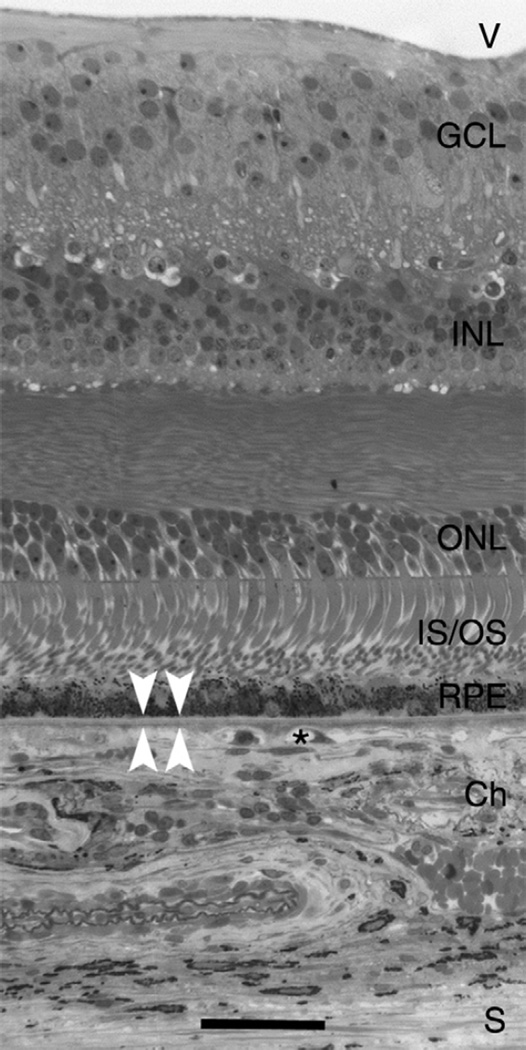
Fig. 1.
Chorioretinal anatomy in macula. V, vitreous; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; IS/OS, inner and outer segments of photo-receptors; RPE, retinal pigment epithelium; Ch, choroid; asterisk, choriocapillaris; white arrowheads, BrM; S, sclera. Bar, 50 µm.
The 100 million rod and cone photoreceptors located at the outer surface of the retinal sheet are supported by the RPE. This polarized monolayer serves diverse functions essential for optimal photoreceptor health, including daily phagocytosis of photore-ceptor outer segment tips, vitamin A metabolism, maintenance of retinal attachment, and coordination of cytokine-mediated immune protection. While inner retinal layers rely on the intrinsic retinal circulation, the photoreceptors and RPE depend on the choroid located external to them (Fig. 1) Branches of the ophthalmic artery enter through the sclera with the optic nerve and ramify to form the choroid and the choriocapillaris, a dense capillary bed. About 200–300 µm thick, the choroid has the highest blood flow per unit tissue perfused in the body, with 7-fold greater flow in the macula relative to the periphery. The innermost 2–4 µm of the choroid (subjacent to the RPE) is BrM (Figs. 1, 2A,D), a pentilaminar vessel wall that is laid out flat along one side of the choriocapillaris rather than wrapped around individual lumens. Unhindered transport across BrM of nutrients to and metabolites from the RPE is essential for normal vision by the photoreceptors (Hillenkamp et al., 2004; Marshall et al., 1998).
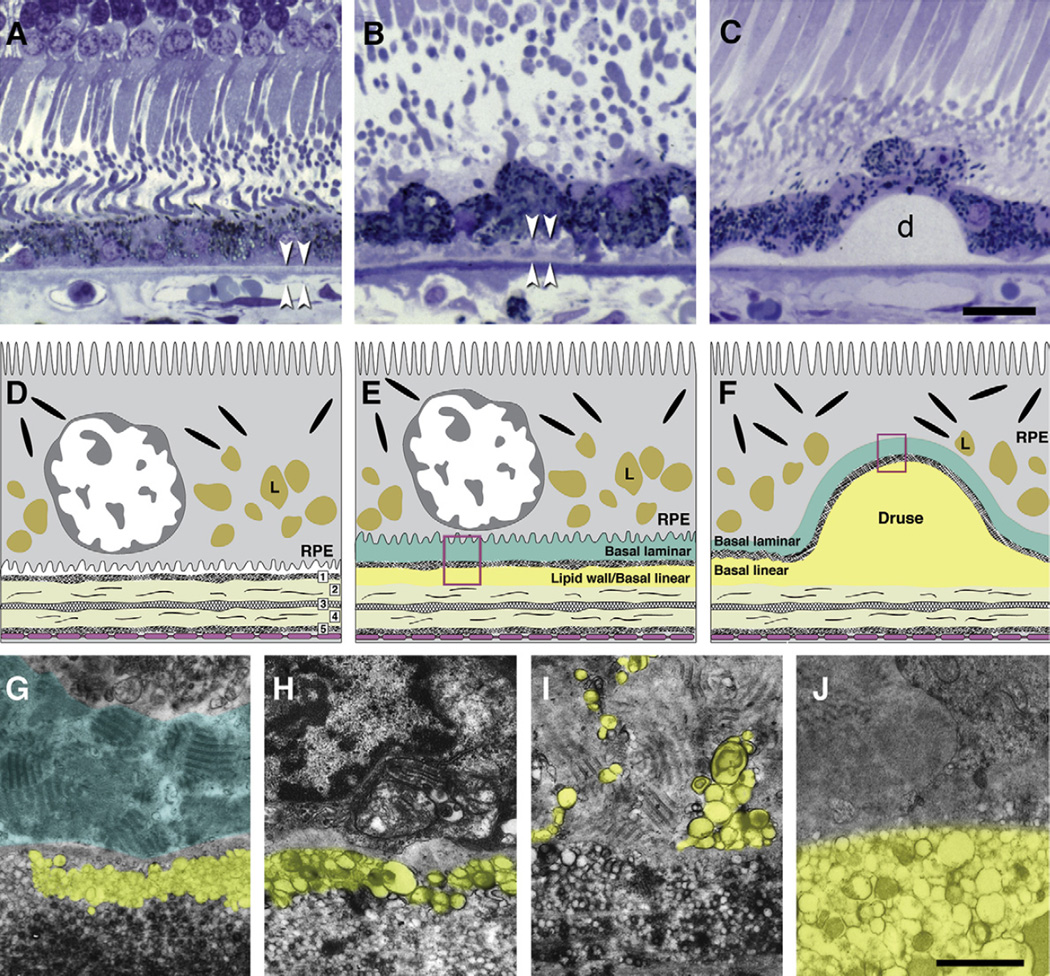
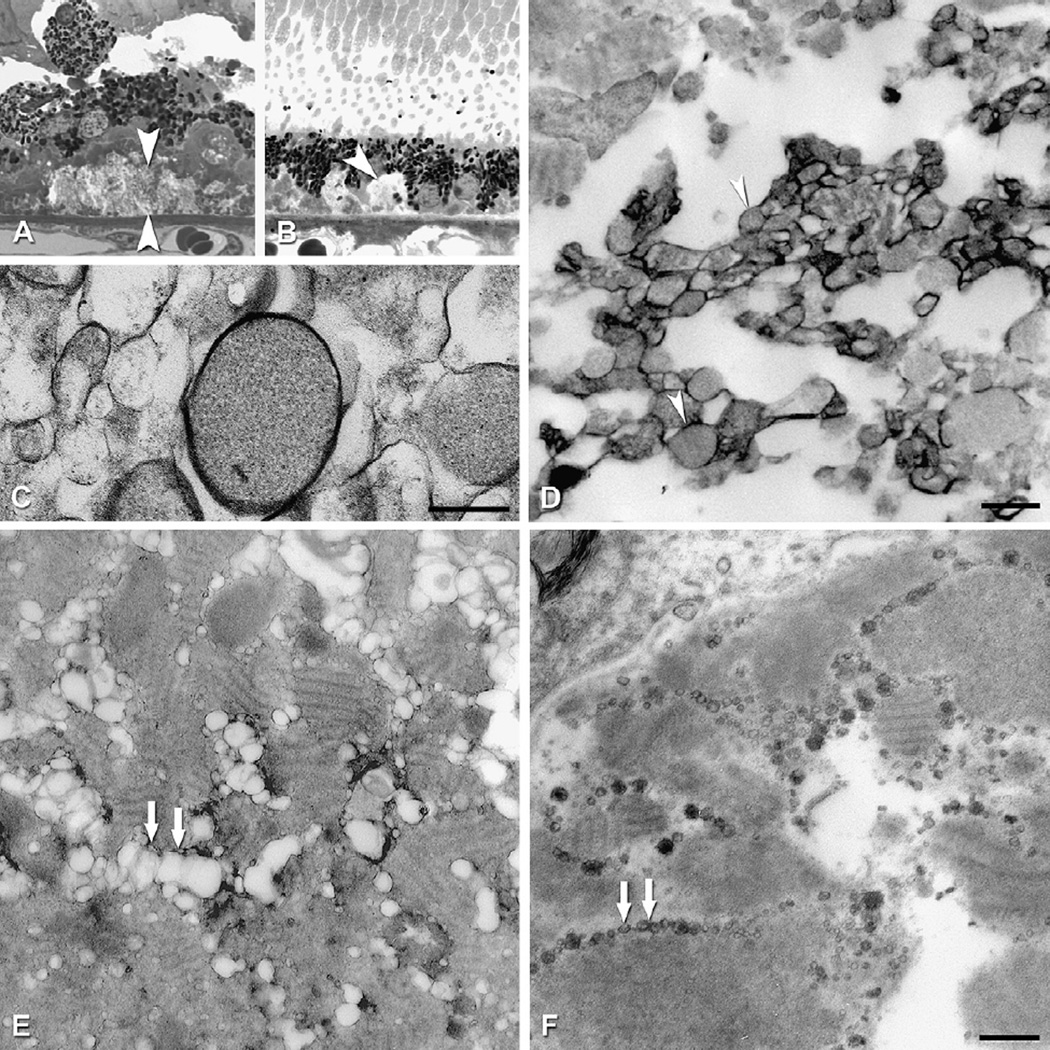
Fig. 2.
Bruch’s membrane and ARMD lesions. A–C: 1 µm sections, toluidine blue. Bar in C, 20 µm; A. Normal. RPE, BrM (arrowheads). B. Basal deposits (arrowheads) external to disrupted RPE. C: Druse (d). D–F: cartoons of extracellular lesions. D. BrM has 5 layers in a normal eye: 1, basal lamina of the RPE; 2, inner collagenous layer; 3, elastic layer; 4, outer collagenous layer; 5, basal lamina of the choriocapillary endothelium (fenestrated cells, pink). L, lipofuscin. E. An ARMD eye has basal laminar deposit (BlamD) and basal linear deposit (BlinD) and its precursor, the Lipid Wall. Boxed area is shown in panels G–I. F. Drusen, BlinD, and the Lipid Wall occupy the same plane. Boxed area is shown in panel J. G–J: colorized transmission electron micrographs. Aqua, basal laminar deposit (BlamD); yellow, BlinD, membranous debris, and Lipid Wall. Bar in J, 1 µm G. BlamD (aqua) and Lipid Wall (yellow). H. BlinD. I. Membranous debris crosses BlamD. J. Membranous debris within a large soft druse.
3. Introduction to ARMD
3.1. Epidemiology and risk factors
While responsible for high-acuity vision, the macula is also vulnerable to ARMD, the major cause of vision loss among elderly of European derivation living in industrialized societies (Congdon et al., 2004). RPE cell death across the macula (geographic atrophy) is a slow but devastating loss of vision (“dry” ARMD). Some patients at early to intermediate stages of dry ARMD can benefit from supplementation with an anti-oxidant mixture (2001a). Choroidal neovascularization, an invasion of choriocapillaries across BrM and under the RPE or retina (Grossniklaus and Green, 2004), is an urgent and sight-threatening complication (“wet” ARMD) of ARMD’s underlying degeneration. Recently, extraordinary progress has culminated in the development of highly specific inhibitors of vascular endothelial growth factor that, when injected intravitreally, not only slow vision loss but also improve vision in many of the 15% of ARMD patients afflicted with choroidal neovascularization (Ciulla and Rosenfeld, 2009). However, the majority of ARMD patients lack neovascularization and cannot be treated in this manner.
Of ARMD risk factors (Klein et al.,2004), advanced age and family history are strongly and consistently related to ARMD across multiple studies. Cigarette smoking, hypertension, and cataract surgery appear to increase the risk of progression to neovascular ARMD in most studies. Other risk factors, like inflammatory disease, obesity, atherosclerotic vascular disease, and hyperopia, have less consistent findings, and associations, if significant, are often weaker. No association exists between ARMD and either diabetes or plasma cholesterol levels (Section 9.2.2). Linkage studies and genome-wide scans have identified polymorphisms in complement factor H, HTRA1, ARMS2, and mitochondrial DNA polymorphism A4917G as risk factors, and complement factor B, C3, and apolipoprotein E4 (apoE4) as protective factors (Canter et al., 2008; Dewan et al., 2006; Gold et al., 2006; Hageman et al., 2005; Kanda et al., 2007; Souied et al., 1998; Yates et al., 2007).
3.2. Histopathology (Fig. 2)
A prominent histopathologic sign of ARMD is the finding of extracellular lesions in the RPE/BrM complex (basal deposits and drusen, Fig. 2B,C) that ultimately impact RPE and photoreceptor health (Green and Enger, 1993; Sarks, 1976). In addition to the lesions, ARMD involves multiple, temporally overlapping biological processes such as cell death, choroidal neovascularization, inflammation, and scar formation subsequent to vessel in-growth. This review focuses on the lesions, as they are pathognomonic for the disease, and they have not yet been faithfully reproduced in laboratory animals. New insights into the pathobiology of early ARMD lesions to inform the development of model systems and new treatments are therefore needed.
Drusen are yellow-white deposits seen in a retinal fundus examination and are associated with ARMD. They are typically classified as “hard” and “soft” at the level of the RPE by the characteristics of their borders, with the latter associated with higher risk for developing advanced disease (Davis et al., 2005; Klein et al., 1991, 2007; Sarks, 1980). Histologically, drusen are defined as focal, dome-shaped lesions between the RPE basal lamina and the inner collagenous layer of BrM. Drusen of 30–300 µm diameter are visible in the fundus by the intrinsic elevation of the dome and loss of pigment from the effaced overlying RPE (Sarks et al., 1999). Pale spots in the fundus have sometimes proven to be other entities by histological analysis (e.g., lipid-filled RPE cells, sub-retinal debris) (Anderson et al., 2006; Bressler et al., 1994; Raoul et al., 2008; Rudolf et al., 2008b). Very common in eyes of older persons, drusen are numerous in peripheral retina, with soft drusen (loosely packed with membranous material) confined primarily to the macula (Rudolf et al., 2008a).
The last decade has witnessed intense study of druse composition, particularly by Hageman, Anderson, and Johnson (Anderson et al., 2002). Many druse components are now identified, including vitronectin, tissue inhibitor of metalloproteinase 3 (TIMP-3), complement factor H, fibrillar and non-fibrillar amyloid, complement component C3, and zinc (Fariss et al., 1997; Hageman et al., 1999, 2005; Johnson et al., 2002; Johnson et al., 2001; Lengyel et al., 2007; Luibl et al., 2006). Cholesterol and apolipoproteins in lesions will be discussed in Section 8.1.
Basal deposits are two diffuse lesions associated with ARMD that have distinctly different size, composition, and significance (Fig. 2; (Sarks et al., 2007)). Basal laminar deposit (BlamD), between the RPE and its basement membrane, forms small pockets in many older eyes or a continuous layer as thick as 15 µm in eyes with ARMD. Ultrastructurally, BlamD resembles basement membrane material and contains laminin, fibronectin, and type IV and VI collagen (Knupp et al., 2000; Löffler and Lee, 1986; Marshall et al., 1992; Reale et al., 2008). Other proteins detected in thick, mature BlamD, such as vitronectin, MMP-7, TIMP-3, C3, and C5b-9 (Lommatzsch et al., 2008) are also found in drusen and may represent components in transit from the RPE to BrM. Basal linear deposit (BlinD), between the RPE basement membrane and the inner collagenous layer of BrM, is thin (0.4–2 µm) and contains mostly membrane-like coils and lipid pools. Because BlinD is located in the same plane and contains the same material as soft drusen, these lesions are plausibly considered alternate forms of the same entity by microscopists and clinicians alike (Bressler et al., 1994; Curcio and Millican, 1999).
In 1993, Green and Enger stated that understanding differences between BlamD and BlinD would provide important insight into ARMD pathogenesis (Green and Enger, 1993). Large, confluent, or soft (indistinct edges) drusen and BlinD are universally recognized as the specific lesions of ARMD (Curcio and Millican, 1999; Green and Enger, 1993; Sarks et al., 2007). The presence of BlinD with a continuous layer of BlamD is said to signify threshold ARMD (Sarks et al., 2007) and thick layers of Blam25
D indicate increased risk for ARMD advancement (Sarks, 1976; Spraul and Grossniklaus, 1997). According to Sarks et al, “BlamD is remarkably resilient, persisting even in areas of geographic atrophy and disciform scars. although not specific for ARMD, it is found in all eyes with disease, making it a .reliable marker for ARMD. In terms of the disease process, however, BlamD appears inert” (Sarks et al., 2007). Thus, understanding the origins and effects of BlinD should now be considered critical. Thin and flat like the RPE layer and BrM adjoining it, BlinD might require fewer formative processes than drusen, which involve three-dimensional expansion and participation of non-RPE cells (Hageman et al., 2001).
3.3. Approaches to new knowledge
Discovery of characteristic lesion components has been a successful means of identifying biological pathways perturbed by coronary artery disease (CAD) (Smith and Slater, 1972), Alzheimer disease (Selkoe et al., 1982), and ARMD (Mullins et al., 2000). We, with others (Handa et al., 1999; Kamei and Hollyfield, 1999), have expanded this approach to include BrM and its age changes, which by virtue of sharing the same compartment as the specific lesions, has great potential for direct involvement in forming them. Age-related changes in BrM include thickening, accumulation of cross-linked and electron-dense debris, reduced collagen solubility due to cross-linking, and the deposition of neutral lipids, AGEs, and TIMP-3 (Fariss et al., 1997; Feeney-Burns and Ellersieck, 1985; Handa et al., 1999; Karwatowski et al., 1995; Pauleikhoff et al., 1990). We chose to study lipid deposition (Section 4) because of its recognized role in initiating CAD (Section 5). We focused on using normal and ARMD human tissues provided by the Alabama Eye Bank. As ophthalmic records are not required by U.S. eye banks, we developed methods for identifying ARMD eyes via post-mortem fundus appearance (Curcio, 2005; Curcio et al., 1998).
4. Neutral lipids accumulate with age in BrM (Fig. 3)
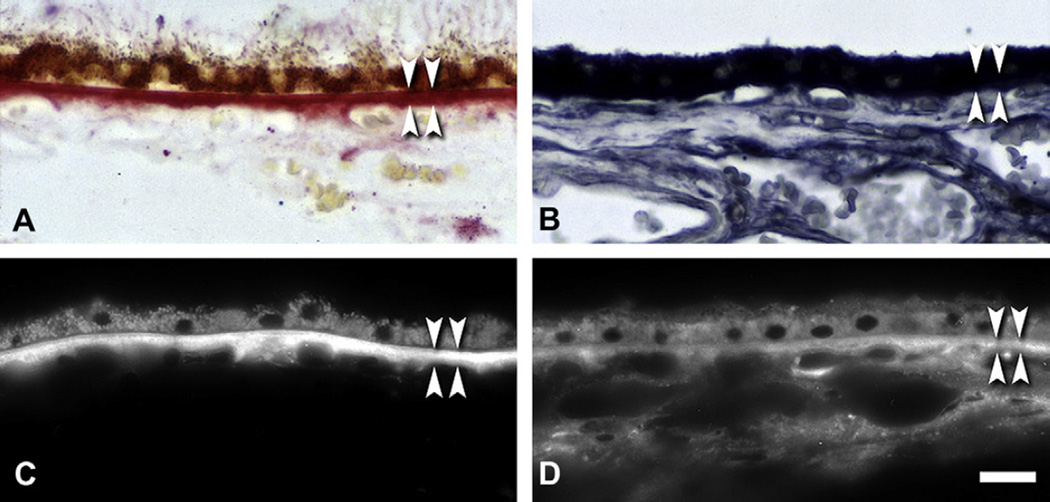
Fig. 3.
Lipid histochemistry of BrM and choroid. Normal macula of a 79 yr old donor. RPE is shown at the top, and choroid at the bottom, of each panel. BrM is bracketed by arrowheads. Bar in D, 20 µm. A. Oil red O stains BrM and lipofuscin. B. Sudan Bromine Black B stains BrM, RPE, and cells throughout choroid. C. Filipin for esterified cholesterol stains BrM. RPE lipofuscin autofluorescence is much less intense than filipin, and it is a different color under ultraviolet illumination. D. Filipin for unesterified cholesterol stains BrM, RPE, and choroidal cells.
Clinical observations on the natural history of serous (fluid-filled) RPE detachments in older adults led to the hypothesis by Bird and colleagues that a lipophilic barrier in BrM blocked the normal outwardly-directed fluid efflux from the RPE (Bird and Marshall, 1986). Impaired movement of fluid from RPE or from leaky vessels in choroidal neovascular membranes was thought to contribute to the formation of RPE detachments in ARMD patients (Marshall et al., 1998). Lipophilic material in BrM, if unevenly distributed, could explain the non-uniform distribution of water-soluble sodium fluorescein in clinical angiography of RPE detachments.
This hypothesis was tested in a seminal laboratory study by Pauliekhoff and colleagues (Pauleikhoff et al., 1990), who used three histochemical stains to identify lipids in cryosections of eyes from 30 donors (1–95 yr) with grossly normal maculas. Bromine Sudan Black B (BSBB) binds phospholipid (PL), triglyceride (TG), EC, UC, and FA. Bromine-Acetone-Sudan Black B (BASBB) binds only acetone-resistant phospholipid after acetone removes other lipids. Oil red O binds TG, EC, and FA (Adams and Bayliss, 1975; Luna, 1968), which are hereafter called collectively neutral lipids. Of these methods, oil red O-binding material localized to BrM, while the two other dyes additionally labeled cells throughout the choroid. All stains gave similar results with regard to age: no eyes from donors <30 yr exhibited staining, eyes from donors 31–60 yr exhibited variable staining, and eyes from donors ≥61 yr exhibited moderate to intense staining for lipids (Fig. 3A,B). These findings were repeated (44 eyes, 4–88 yr, (Pauleikhoff et al., 2000); 17 eyes, 32 wk to 92 yr (Haimovici et al., 2001)), with less intense staining in peripheral BrM (Haimovici et al., 2001).
This finding had little historical precedent. Verhoeff speculated that lipid deposition might precede basophilia and fragmentation due to “lime salts in the elastic layer” of BrM (Verhoeff and Sisson, 1926). Lauber (Lauber, 1924) (cited by Rones (Rones, 1937)) treated phthisical eyes with a Sudan stain, noted that deposits between the “laminavitrea” (BrM) and the RPE and granules within the RPE both appeared bright red, and inferred chemically similar substances in both locations and an RPE origin for the deposits. Years later the oil red O staining of retinoids was recognized within lipofuscin, a distinct age-pigment that accumulates markedly in the RPE (Eldred and Lasky, 1993; Pauleikhoff et al., 1990), without evidence that lipofuscin and BrM lipids are chemically related. An early histochemical description of drusen (Wolter and Falls, 1962) can be interpreted as the first evidence for neutral lipid in BrM. Wolter and Falls stated that in two patients 65 and 83 years of age “hyaline bodies {drusen} are in contact with and of similar staining characteristics of {BrM} … they stain reddish with … oil red O,” recognizing the common constituents of these two extracellular structures.
5. apoB, MTP, and atherosclerotic cardiovascular disease (Figs. 4,5)
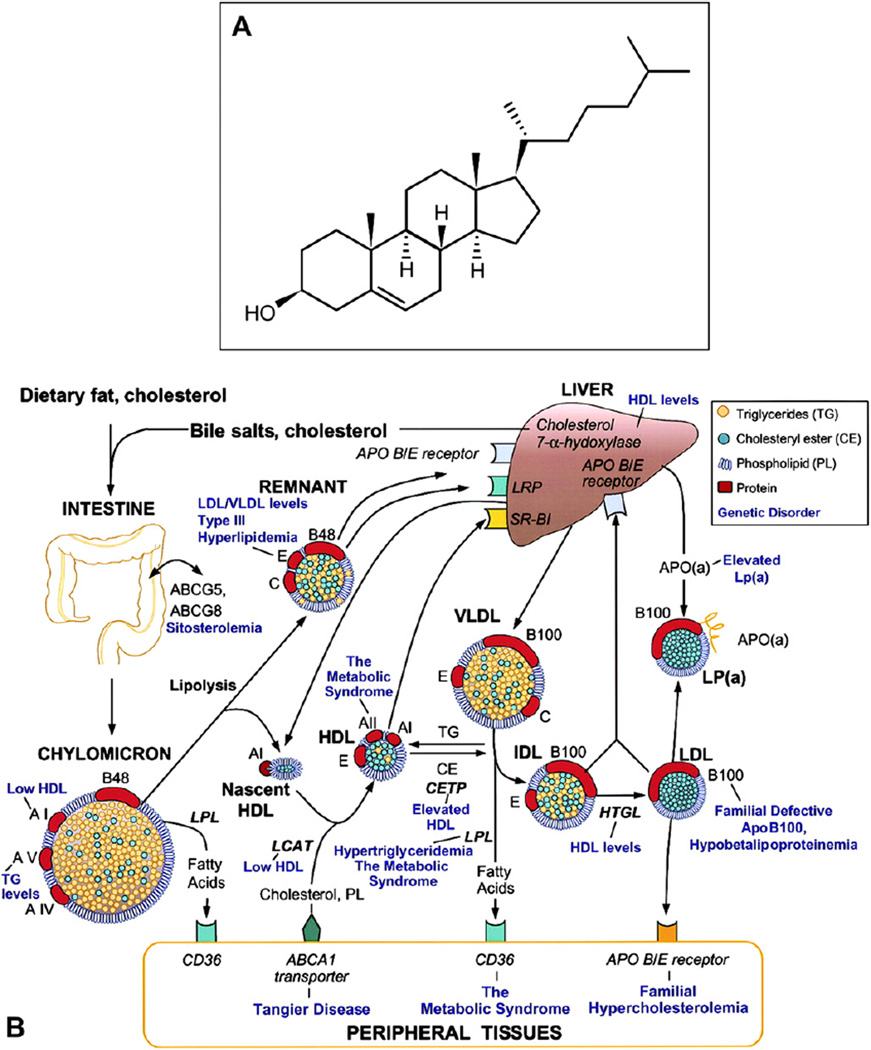
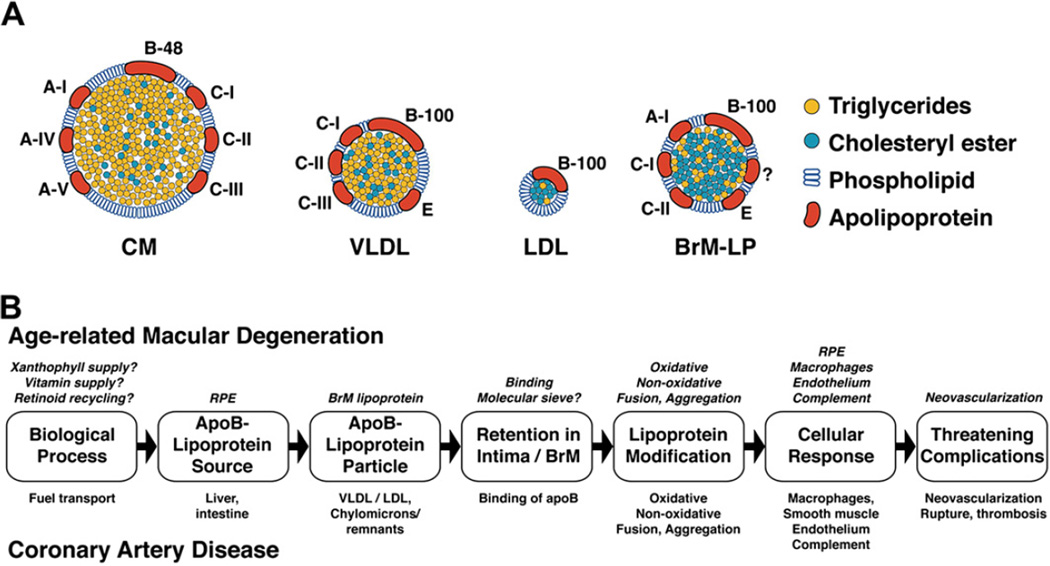
Fig. 4.
Cholesterol and lipoprotein basics. A. Cholesterol structure. Cholesterol is esterified to long chain (>16:0) fatty acids at the 3 β-hydroxy group. B. Lipoprotein homeostasis in plasma (with permission (Lusis et al., 2004)). See Section 5.3 for details.
Fig. 5.
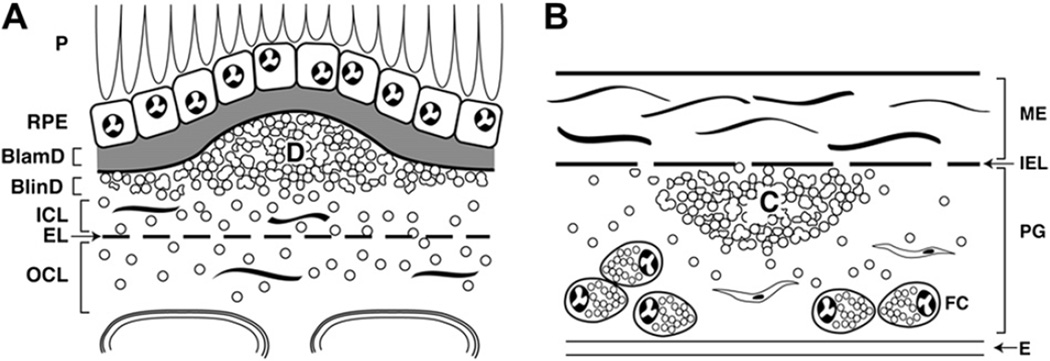
ARMD lesions vs plaque. Schematic cross-sections of BrM from an eye with ARMD (B) and atherosclerotic arterial intima (B). Endothelium and vascular lumens (choriocapillary, A; artery, B) are at the bottom. Drawings are not at scale. The thickness of normal BrM and intima is 4–6 µm and 100–300 µm, respectively. Small circles in BrM (A) and PG layer (B) indicate EC-rich lipoproteins, native and modified. A. P = photoreceptors, RPE = retinal pigment epithelium, R-BL = RPE basal lamina, BlamD = basal laminar deposit, BlinD = basal linear deposit, D = druse, ICL = inner collagenous layer EL = elastic layer, OCL = outer collagenous layer. B. ME = musculo-elastic layer, IEL = internal elastic layer, C = lipid-rich core, PG = proteoglycan layer, FC = foam cells. Modified from Malek et al., (2003), with permission from the American Society For Investigative Pathology.
5.1. Cholesterol and its forms (Fig. 4A)
A hydrophobic alcohol built up from condensations of isoprene units, cholesterol is an essential component of all animal cell membranes (Fliesler and Keller, 1997) (Fig. 4A). Cholesterol exists in two chemical forms and three physical forms. The two chemical forms are unesterified (UC) or bound by an ester linkage to a fatty acid at the 3-beta-hydroxy group to produce esterified cholesterol (EC). The three physical forms are differentiated by the relative proportions of UC, EC, and solubilizing PL – oily droplets (EC >UC > PL), membranes (UC and PL), and crystals (UC only) (Small and Shipley, 1974).
Cellular cholesterol levels represent a balance of endogenous synthesis and uptake of exogenous cholesterol delivered via lipoproteins, on one hand, and turnover via release, on the other. Multiple processes for cellular cholesterol release (Cavelier et al., 2006) include passive diffusion from membranes, release to circulating high density lipoprotein (HDL) via ABCA1 for clearance by the liver as bile salts (reverse cholesterol transport), conversion via cytochrome P450-dependent cholesterol oxidases to either 24S-hydroxycholesterol (by neurons) or 27-hydroxycholesterol (by macrophages) for secretion, and secretion with endogenous apoE (by macrophages and glia). EC, the most highly hydrophobic neutral lipid, is the storage and transport form of cholesterol. It can be passively released from dying cells containing lipid droplets (e.g., (Ball et al.,1995)), and it can be actively secreted from cells as part of apoB-containing lipoprotein particles (e.g., (Temel et al., 2007)).
5.2. Lipoprotein particles (Fig. 4B)
Sometimes called nature’s nanoparticles, lipoproteins are multimolecular complexes that solubilize a neutral lipid core (essentially an oil droplet) containing TG and EC, within an approximately 2 nm thick surface of protein, UC, and PL (Havel and Kane, 2001; Jonas, 2002) (Fig. 4B). The major classes of lipoproteins were initially described and named based on their flotation properties in density gradient ultracentrifugation. Diameters range from 7 nm for HDL to 600 nm for dietary chylomicrons (CM). Low density lipoprotein (LDL) and HDL have the most EC-rich cores and consist of 30–50% protein. LDL (22 nm), very low density lipoprotein (VLDL, 75 nm), and CM have on their surfaces a single molecule of apoB (Section 5.4). In addition to apoB, VLDL additionally has apolipoproteins E and C-III, and CM, apolipoproteins E, C-III, A-I, and A-II, on their respective surfaces. CM and VLDL have highly TG-rich cores and the least protein (1–10%).
5.3. Lipoprotein metabolism in plasma (Fig. 4B)
Cholesterol is transported through the circulation as complexes with various apolipoproteins that sequester the lipids and also act as cofactors for enzymes or ligands for uptake by cellular receptors (Crispin, 2002; Havel and Kane, 2001; Jonas, 2002) (Fig. 4B). Dietary lipids are absorbed in the intestine, packaged into CM, and then secreted into lymph. Upon entering the circulation, TG are hydrolyzed through the action of lipoprotein lipase and the resulting remnants taken up by interaction of apoE with the LDL receptor (LDL-R, also called apoB, E receptor) and the LDL-R related protein. During lipolysis, surface PLs and CM proteins slough off to give rise to HDL precursors. Hepatocytes package TG and EC into VLDL particles. Lipoprotein lipase acts on them, hydrolyzing TG to release fatty acids to produce intermediate-density lipoproteins (IDL), which can be taken up at the LDL-R, or further lipolyzed, partly through hepatic lipase action, to produce LDL. Lipids transfer between lipoprotein particles by the activity of cholesteryl ester transfer protein and PL transfer protein.
LDL, the major cholesterol-carrying particle in most individuals, is removed from circulation by the LDL-R. Mature, spherical HDLs are formed largely in the circulation from apoA-I and apoA-II secreted by liver and intestine and from the surface PLs of CM and VLDL during their lipolysis. HDL precursors take up cholesterol from various tissues through interaction with ABCA1 transporter, and this cholesterol is esterified by lecithin: cholesterol acyltransferase (LCAT). EC is selectively taken up from HDL particles without degradation of apoA-I protein through interaction with the scavenger receptor B–I (SRB-I) at the liver and elsewhere.
5.4. Intracellular production of apoB-lipoproteins
One of the largest plasma proteins, apoB is a secretory glycoprotein with 16 N-linked oligosaccharides related to the egg yolk protein vitellogenin (Hussain et al., 2003; Olofsson and Boren, 2005; Shoulders and Shelness, 2005). One gene encodes 2 forms of apoB by the process of post-transcriptional mRNA editing implemented by apoB editing complex 1 (apoBEC-1). This deoxycytidine deaminase enzyme, present in intestine of all mammals and in liver of rodents, creates a stop-codon at position 2153 that truncates the nascent polypeptide at 48% of its full length. In contrast, the full-length 4536 residue apoB-100, is 512 kDa when fully glycosylated and secreted by liver. The 5 domains in apoB-100 (3 α-helical, 2 β-strand) confer amphipathic properties that promote binding to lipid in the particle core while interacting with plasma at the particle surface. ApoB is insoluble when delipidated, and uniquely among apolipoproteins, cannot transfer from one lipoprotein particle to another in plasma.
Assembly of apoB-lipoproteins requires microsomal triglyceride transfer protein (MTP), a soluble heterodimer in the lumen of endoplasmic reticulum (Gordon and Jamil, 2000; Wetterau et al., 1997). MTP consists of a widely expressed 58 kDa protein disulfide isomerase and a unique 97 kDa protein that transfers neutral lipid, preferentially TG, to apoB while the apoB transcript is translated (Athar et al., 2004; Jamil et al., 1995). The MTP gene has two splice variants that both encode functional proteins (Dougan et al., 2007; Mohler et al., 2007). MTP-mediated lipid transfer allows apoB to fold correctly and evade intracellular degradation via the ubiquitin-proteosome system and other pathways (Fisher and Ginsberg, 2002; Yao et al., 1997). ApoB and MTP together create a pocket that accommodates an expanding lipid droplet during transit through the endoplasmic reticulum and Golgi (Segrest et al., 1999). Cells expressing apoB without MTP cannot secrete lipoproteins (Gordon et al., 1994; Leiper et al., 1994; Sellers and Shelness, 2001; Wetterau et al., 1992).
ApoB’s principal function is delivering exogenous (CM) or endogenous (VLDL) TG and cholesterol to peripheral tissues. CM also deliver lipophilic vitamins A, E, and K. ApoB’s role in physiology has been expanded by evidence that it is also expressed in heart, kidney, and placenta (Madsen et al., 2004; Nielsen et al., 1998). The heart secretes lipoproteins to regulate cardiomyocyte TG content and forestall lipotoxicity by releasing fatty acids unconsumed by mitochondrial fatty acid b-oxidation (Björkegren et al., 2001). MTP is even more widely expressed than apoB (cardiomyocytes, kidney, testis, ovary, pancreas, placenta, and adipocytes), highlighting a generalized role in PL transfer (Hussain et al., 2008; Shoulders and Shelness, 2005). In mice, absence of apoB is lethal in utero, and absent or reduced apoB is associated with neural tube defects (Farese et al., 1995; Raabe et al., 1998). Mutations of the MTP gene cause the rare autosomal recessive disorder abetalipoproteinemia (MIM 200100, Bassen-Kornzweig disease) (Berriot-Varoqueaux et al., 2000; Wetterau et al., 1992), and truncation-specifying mutations of the APOB gene cause hypobetalipoproteinemia (MIM 107730), a genetically heterogeneous autosomal trait (Schonfeld, 2003). Both disorders include pigmentary retinopathies historically attributed to reduced delivery of lipophilic vitamins from absent or low apoB lipoproteins in plasma (Grant and Berson, 2001).
5.5. Role of apoB lipoproteins in producing atherosclerotic plaques (Fig. 5)
It is instructive to compare BrM to the inner wall of large arteries, which are divided into 3 layers (from inside out): the intima, media, and adventitia (Fig. 5). The intima is located between two diffusion barriers, an endothelial cell layer at the lumen and a dense elastic layer (Stary et al., 1992). Throughout life the intima of atherosclerosis-prone large arteries thickens adaptively to the mechanical stresses of blood flow and wall tension (Malek et al., 1999). Collagen, elastin, and proteoglycans at sites of intimal thickening specifically interact and bind with lipoprotein particles from plasma (Camejo et al., 1998). The deep intima, near the elastic layer, is the site of extracellular lipid accumulation. Macrophages and smooth muscle cells filled with EC-rich oil droplets (foam cells) accumulate in a region lateral to this lipid-rich core (called the shoulder). Smooth muscle cells also contribute to an overlying fibrous cap.
A widely supported hypothesis of atherogenesis states that CAD is a “Response-to-Retention” of the arterial intima to apoB-containing lipoproteins entering from plasma. This hypothesis, first formulated by Williams and Tabas in 1995 (Tabas, 1999; Williams and Tabas, 1995, 1998, 2005), summarized 8 decades of research, beginning with Anitchkow’s cholesterol-fed rabbits in 1913 (Steinberg, 2005). This work established that the earliest and largest cholesterol component in the lipid-rich core of atherosclerotic plaques derives from plasma apoB-containing lipoproteins that directly infuse into the intima (Chao et al., 1990; Guyton and Klemp, 1989; Katz and Small, 1980; Nievelstein et al., 1991; Smith, 1974). These are largely LDL with some partly hydrolyzed CM remnants. Lipoprotein particles enter intima via endothelial cell trancytosis and bind to specific proteoglycans (biglycan, decorin, and versican) (Kovanen and Pentikainen,1999; O’Brien et al., 2004; Pentikainen et al., 1997). This binding can be enhanced via a link to lipoprotein lipase (Pentikainen et al., 2000, 2002).
Oxidative and non-oxidative processes modify retained lipoproteins and evoke a cascade of downstream events. These events include deposition of cholesterol, differentiation of foam cells from extravasated monocytes-macrophages, proliferation and differentiation of smooth muscle cells, endothelial cell injury, cytokine release, neovascularization, rupture, and hemorrhage. One specific role of the lipid-rich core of a plaque is structural instability (Davies, 1996). Abundant foam cells and thin overlying cap contribute to the vulnerability of a plaque to rupture (Shah, 2007; Varnava et al., 2002).
Evidence supporting the Response-to-Retention hypothesis included the demonstration of early LDL entry through intact endothelium (Schwenke and Carew, 1989a,b), chemical and morphological description of insudated lipoproteins and their successor particles (Chao et al.,1990; Guyton and Klemp,1989), and the abatement of atherosclerosis by reduced apoB binding to intimal proteoglycans (Skålen et al., 2002). Although recent research has highlighted inflammatory processes (e.g., C-reactive protein levels) as independent risk factors, these may be insufficient to initiate disease in the absence of dyslipidemia (Ridker et al., 2002; Zacho et al., 2008).
With this knowledge about apoB-lipoprotein-instigated disease in arterial intima, the RPE/BrM complex can be interrogated for evidence of physical and chemical forms of cholesterol, lipid-rich and structurally unstable lesions, and lipoprotein particles as a source of extracellular cholesterol. To understand the antecedents of disease in normal physiology, we should also seek a cellular source of lipoprotein particles and biological processes to drive lipoprotein production.
6. Evidence for an intra-ocular apoB-containing lipoprotein
6.1. Cholesterol in aged BrM – histochemical, physicochemical studies (Fig. 6)
Fig. 6.
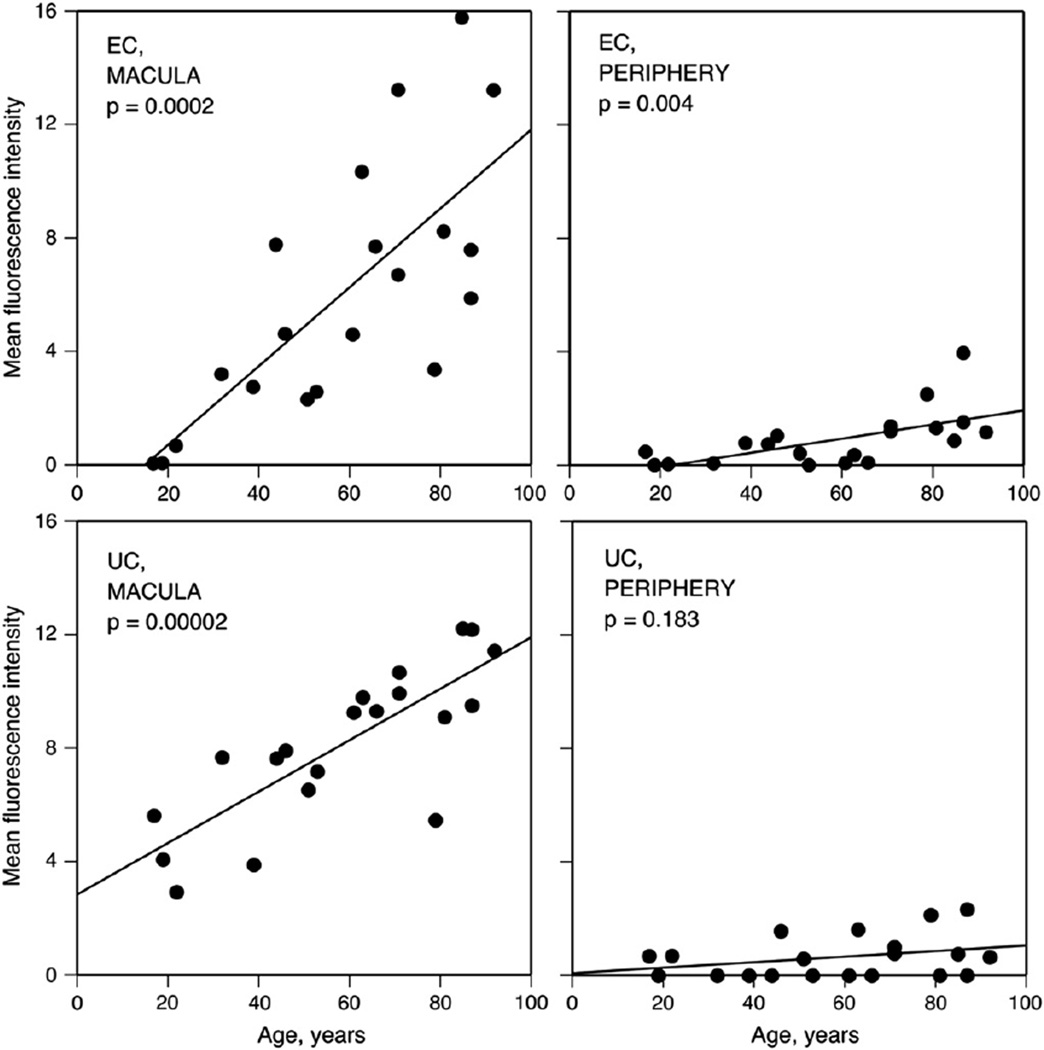
BrM cholesterol increases with age. Filipin fluorescence due to EC and UC increases with age in normal BrM. For EC in the macula (A) and periphery (B) and UC in the macula (C) and periphery (D), fluorescence intensity (x 10−6 arbitrary units) for each eye is corrected for background autofluorescence of BrM. Reprinted from (Curcio et al., 2001); copyright is held by Association for Research in Vision and Ophthalmology.
Lipids that bind the histochemical stain oil red O increase with age in normal human connective tissues, including the sclera (Broekhuyse, 1972), cornea (Gaynor et al., 1996), intima of large arteries (Smith, 1974), and BrM (Pauleikhoff et al., 1990). In the intima, the oil red O-positive material comprises small (60–200 nm) extracellular droplets highly enriched in EC relative to UC (69% EC, 22% UC, and 9% PL) (Bocan et al., 1986; Chao et al., 1990; Guyton and Klemp, 1988, 1989; Kruth, 1984a). The source of EC in sclera, cornea, and arterial intima is LDL translocated from plasma into connective tissues. EC-enriched particles are thought to arise from smaller LDL particles by extracellular matrix-mediated trapping of LDL, degradation of LDL protein and/or PL components, and fusion of the remaining lipid components (Kruth, 1997). It is thus important to determine whether lipid deposition in BrM is an ocular manifestation of this ongoing systemic process or a phenomenon unique to retina.
Curcio et al (Curcio et al., 2001) examined the EC and UC content of BrM using the fluorescent, polyene antibiotic filipin (Fig. 3C,D). This compound binds specifically to the 3-β-hydroxy group on cholesterol and other sterols, and it can be used to identify cholesterol in tissue sections (Kruth, 1984a,b) and in cultured cells (Lakkaraju et al., 2007). Importantly, EC can also be localized using filipin, by extracting native UC with ethanol, hydrolyzing EC with the enzyme cholesterol esterase, then binding the newly released UC with filipin. This method is an improvement over oil red O, because it is specific for one class of neutral lipids, and because the fluorescence, while labile, is readily quantifiable with digital microscopy. With this method, EC was localized in macula and temporal periphery of 20 normal eyes from age 17–92 years (Curcio et al., 2001) (Fig. 6). In the macula, EC was undetectable before 22 yr and then rose linearly throughout adulthood to reach high and variable levels in aged donors. EC was detectable in periphery at roughly 1/7 the level of macula, but still increased significantly with age. In the same eyes, UC in macular BrM also increased throughout adulthood, although not as steeply as EC, and it did not increase significantly with age in peripheral retina. Extending the original findings of lipids accumulating with age in BrM (Pauleikhoff et al., 1990), this study identified a specific class of molecules (EC) and quantified their increase with age.
Haimovici and colleagues (Haimovici et al., 2001) used hot stage polarizing microscopy, a physicochemical technique (Small, 1988; Waugh and Small, 1984), to examine the birefringence and melting characteristics of lipids in BrM and sclera. EC in tissue sections appears as liquid crystals (“Maltese crosses”) when examined through a polarizing filter. When sections are heated and cooled slowly, liquid crystals melt and reform at characteristic temperatures dictated by the saturation level of the major ester. BrM contained Maltese crosses that melted at a higher temperature than those in sclera. This higher melting temperature suggests that BrM EC contains less polyunsaturated fatty acid than scleral EC, which accumulates cholesteryl linoleate (18:2n6)1 from plasma lipoproteins with age (Smith, 1973). Few birefringent crystals signifying TG were found in either tissue.
6.2. BrM lipoprotein morphology and distribution (Fig. 7)
Fig. 7.
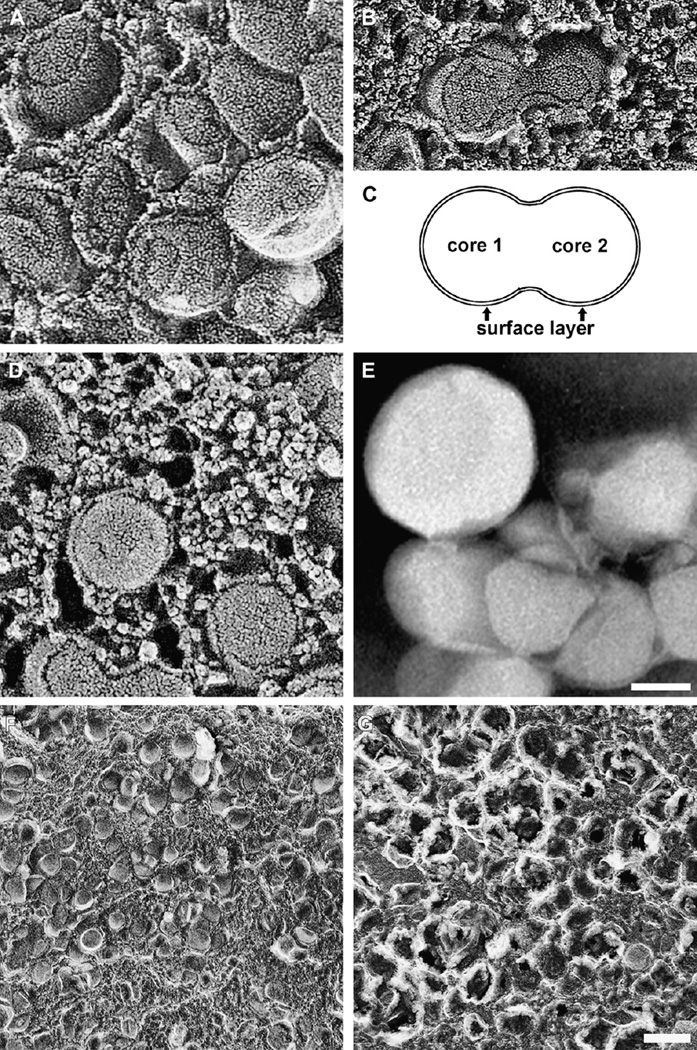
BrM lipoproteins, in situ and isolated. A–B, D, F–G. QFDE images of normal BrM (Huang, 2007). E. Negative stain electron microscopy (Li et al., 2005a). A. Core and surface of tightly packed lipoproteins. B. Fused lipoproteins. C. Interpretive schematic based on panel B. D. Small granular particles associated with lipoproteins. E. Isolated particles are large, spherical, and electron-lucent. F,G. Lipoproteins in untreated tissue (F) and in tissue treated with chloroform-methanol to remove lipid (G). Only some surface components, presumably apolipoproteins, remain after extraction. Bar in E = 50 nm, applies to panels A–E. Bar in G = 200 nm, applies to panels F and G.
Ultrastructural studies since the 1960’s have illustrated numerous small (<100 nm), round electron-lucent spaces in BrM of older eyes (Bairaiti and Orzalesi, 1963; Feeney-Burns and Ellersieck, 1985; Green and Enger, 1993; Hogan et al., 1971; Killing sworth, 1987; Nakaisumi et al., 1964; Pauleikhoff et al., 1990). The occasional presence of a single electron-dense line at the borders of these spaces presumably accounts for their frequent description as membranous or vesicular (liposomes with aqueous interiors). However, conventional methods of tissue preparation for thinsection transmission electron microscopy extract tissue lipids, resulting in sub-optimal visualization of fine structure. Tissue post-fixation with osmium-tannic acid-paraphenylenediamine (OTAP) preserves neutral lipid (Guyton and Klemp, 1988) and was used to show that BrM vesicles were actually solid and electron dense (Curcio et al., 2001, 2005b). Because biochemical evidence in Sections 6.3–6.4 firmly indicates that these particles are lipoproteins, this name will be used henceforth.
More dramatic views of the particles were made possible by quick-freeze/deep-etch (QFDE), an ultrastructural tissue preparation technique (Shotton and Severs, 1995) that reveals lipids and extracellular matrix with stunning detail. QFDE was used to demonstrate the accumulation of lipoproteins in the aortic wall at the earliest stages of atherosclerosis (Frank and Fogelman, 1989; Nievelstein et al., 1991; Tamminen et al., 1999). Briefly, tissue is rapidly frozen, fractured, and then “etched” by sublimating vitrified water at the fracture surface, leaving only frozen matrix behind. This matrix is then rotary-shadowed with a platinum/carbon mixture, creating a replica that is stabilized with more carbon. The tissue itself is then dissolved away, and the replica is viewed using a transmission electron microscope.
QFDE revealed that the vesicles accumulating in BrM with age were not only solid particles but also distinguished by a surface and core structure (Fig. 7A) (Huang et al., 2007b; Ruberti et al., 2003). Particles typically varied in size from 60 to 100 nm but could be as large as 300 nm. Occasionally particles appeared to coalesce with one another (Fig. 7B,C). Particle appearance in QFDE preparations was consistent with their designation as lipoproteins, in several ways (Huang et al., 2007b). 1) Particles appeared solid and did not etch significantly during the QFDE process, indicating that they contained little water. 2) Particle appearance was similar to that seen previously in QFDE studies of LDL particles accumulating in the aortic intima (Frank and Fogelman, 1989). 3) Particles were found in the same locations in BrM as the lipid-containing particles identified using OTAP (Curcio et al., 2001). 4) Particle cores could be extracted with the Folch reagent for lipid, leaving the surface largely intact (Huang et al., 2007a) (Fig. 7F,G). 5) The age-related accumulation of particles within BrM throughout adulthood (see Section 7.1 ) was consistent with light microscopic studies and biochemical assessment of lipid deposition in this tissue (Huang et al., 2007b, 2008a). Also seen by QFDE was the accumulation in BrM of small granules (<10 nm) of not yet determined identity that formed complexes with the lipoproteins (Fig. 7D) (Huang et al., 2007b).
6.3. Assays of BrM/choroid and isolated lipoproteins (Tables 1–3)
Table 1.
BrM lipid composition: studies using tissues.
| Reference | N | Age, yr {1} | D to P, hr {2} | Fresh/fixed | Region | Tissues | Prep | Assays |
|---|---|---|---|---|---|---|---|---|
| (Sheraidah et al., 1993) | 27 | 1.5, 97 | n.a. | Fixed 24 h | M | RPE/BrM/Ch, BrM/Ch | Cryosections | TLC; GC, FID |
| (Holz et al., 1994) | 32 | 3, 97 | 17.6 | Fresh | M, P | BrM/Ch | 7 mm | TLC |
| (Gülcan et al., 1993) | 12 pr | 4th, 9th decade |
<24 | Fixed 1 h | M, P | Ret; RPE; BrM/Ch | 4 mm | HPLC; saponification & HPLC for FA |
| (Pauleikhoff et al., 1994) | 27 | 1, 97 | n.a. | Fixed 24 h | M | RPE/BrM/Ch | Cryosections | TLC; GC, FID |
| (Spaide et al., 1999) | 13 | 40, 78 | <24 | Fresh | M | BrM/Ch | 7 mm; vessels removed; histo | Saponified; HPLC for FA |
| (Curcio et al., 2001) | 10 | 71, 87 | <4 | Fixed, various times |
M (10), P (3) |
Ret; BrM/Ch; Ch vessels |
8 mm; vessels removed; histo | Enzymatic fluorimetric assay * |
| (Li et al., 2005a,b) | 2 | >60 | <6 | Fresh | All | Ret; BrM | 8 mm; vessels removed; histo | ESI/MS |
| (Bretillon et al., 2008a,b) | 27 | 59, 95 | 34 | Fresh | All | Ret; RPE/Ch | Entire retina, entire RPE/Ch | TLC; GC, FID; saponification & HPLC for FA in retina PL |
| (Wang et al., 2009a,b) | 4 | 37, 86 | <8 | Fresh | All | BrM/Ch | Entire BrM, vessels removed | TLC; GC, FID |
Notes and abbreviations (see also table at beginning of article).
Age: Minimum and maximum age.
D to P: Death to preparation time.
Fresh/fixed: fixatives were formalin or 4% paraformaldehyde.
Tissues: Ch, choroid.
Region: M, macula; P, periphery.
Prep: Diameter of circular punch given; Histo, quality of dissection monitored by histology.
Assays: ESI/MS, electrospray ionization mass spectrometry; FID, flame ionization detection of fatty acids; GC, gas chromatography; HPLC, high performance liquid chromatography; TLC, thin layer chromatography.
n.a., not available.
EC mass was originally reported as nmol/g dry weight and should be (nmol × 1000)/g dry weight.
Table 3.
BrM lipoproteins vs plasma apoB-lipoproteins.
| Lipoprotein | Diameter, nm |
Apolipoproteins | EC/ (EC + UC) |
EC/ TG |
PC/ PE |
SPM/ PC |
|---|---|---|---|---|---|---|
| BrM | 66 | A-I, B-100, E, C-I, -II | 0.58 | 11.32 | 1.29 | 0.70 |
| Chylomicron | >70 | A-I, B-48, C-I, -II, -III | 0.59 | 0.11 | 6.09 | 0.28 |
| VLDL | 25–70 | B-100, C-I, -II, -II, E | 0.45 | 0.13 | 8.30 | 0.28 |
| LDL | 19–23 | B-100 | 0.69 | 8.52 | 14.90 | 0.37 |
EC: esterified cholesterol; TG: triglyceride; PC: phosphatidylcholine; PE: phosphatidylethanolamine; UC: unesterified cholesterol; SPM: sphingomyelin.
Values for BrM lipoproteins and plasma lipoprotein lipids from (Li et al., 2005a,b) and (Wang et al., 2009a,b).
Values for plasma lipoprotein diameters and apolipoproteins from (Burtis and Ashwood, 1999).
Values for plasma lipoprotein SPM/PC calculated from (Jonas, 2002).
Determining the composition of lipids accumulating in BrM is challenging due to the size and marked heterogeneity of the choroid. At 100–300 µm, the choroid is 1–2 orders of magnitude thicker than BrM itself (2–6 µm) (Ramrattan et al., 1994; Spraul et al., 1996). Moreover, the choroid contains numerous blood vessels (arteries, veins, and capillaries) with plasma lipoproteins and blood cells, as well as resident cells (e.g., vascular endothelium, fibroblasts, melanocytes, macrophages, and neurons). Thus it is important that direct assays be validated by results of histochemical studies utilizing tissue sections.
Surprisingly, the potential for contamination from plasma in such assays is probably small. Less than 6% of EC from extracts of partially stripped choroid could be accounted for by plasma lipoproteins retained in vessels, which drain of blood at death (Curcio et al., 1998, 2001). Further, in cryosections only a few choriocapillaris lumens contain immunoreactivity for apoB (presumably on plasma lipoproteins) (Malek et al., 2003). The greater source of contaminating lipids, then, is the extraneous choroidal vessels and stroma left on BrM, as PLs and UC both localize to all choroidal cells. Removal of choroidal tissue is highly operator-dependent and ideally should be monitored with histology.
Table 1 lists 7 studies that assayed BrM lipids from tissue extracts (as opposed to lipoproteins released from BrM, see below). Most studies used fresh tissue, some used formalin-preserved tissue to allow separation of layers or to allow correlative histo-chemical staining, and some used other parts from the same eyes as positive control or comparison tissues. Studies used vertically oriented cryosections or circular punches of choroid 4–8 mm in diameter, sometimes with major choroidal arteries and veins removed under histological monitoring. Extracted lipids were generally separated into classes by thin layer chromatography or liquid chromatography, followed by derivatization of the fatty acids for analysis by flame ionization detection. Other studies used thin layer chromatography and densitometry for all lipid classes, enzymatic fluorimetry for total and unesterified cholesterol, or electrospray ionization mass spectrometry for EC only. Data were reported as mass (mg or nmoles normalized by dry weight or nominal area of choroidal sample) or as composition (% of total lipids, or % of class).
From these 7 studies, a consensus description of BrM lipid composition (mole percent of the major classes and their fatty acids) was sought. Results obtained with comprehensive assays, results repeated with different assays and/or by different laboratories, and consistency with histochemical findings were given greater weight. There is now agreement on the composition of the neutral lipid that accumulates with age in BrM (Table 2): 1)RPE/BrM/choroid is much more enriched in neutral lipid/EC than neurosensory retina (Bretillon et al., 2008b; Curcio et al., 2001; Gülcan et al., 1993). 2) Within RPE/BrM/choroid, EC is the prominent neutral lipid, exceeding TG by 4–10-fold (Bretillon et al., 2008b; Li et al., 2005a; Wang et al., 2009b), despite an early report to the contrary (Holz et al., 1994). 3) EC represents 50–60% of the cholesterol detected (Bretillon et al., 2008b; Curcio et al., 2001; Wang et al., 2009b). 4) The predominant fatty acids in the neutral lipid/EC fraction are linoleate (18:2, 45.1%), oleate (18:1, 20.3%), palmitate (16:0, 13.9%), arachidonate (20:4n6, 6.8%), and stearate (18:0, 2.5%) (Bretillon et al., 2008b; Li et al., 2005a; Wang et al., 2009b). Together these compounds account for 88–89% of the EC detected. 5) The fatty acid docosahexaenoate (22:n6, 0.5%) is present in minute quantities (Bretillon et al., 2008b; Gülcan et al., 1993; Li et al., 2005a; Spaide et al., 1999; Wang et al., 2009b). 6) Relative to macula, peripheral BrM/choroid has neutral lipid of similar fatty acid composition and less EC relative to UC (Curcio et al., 2001; Gülcan et al., 1993). 7) Neutral lipids increase markedly with age relative to PLs, especially after age 60 yr (Holz et al., 1994; Sheraidah et al., 1993).
Table 2.
Lipid profile of BrM/choroid and isolated particles.
| EC | DG | FA | TG | CL | LYPC | PC | PE | PS | UC | SPM | RE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BrM/choroid | ||||||||||||
| mole%a | 29.8 | 0.7 | 3.6 | 3.0 | 1.5 | 0.6 | 15.4 | 12.6 | 6.0 | 26.7 | n.a | n.a. |
| Isolated particles | ||||||||||||
| mole%a | 32.4 | 1.7 | 6.3 | 3.3 | 3.2 | 1.8 | 14.2 | 9.5 | 4.6 | 22.9 | n.a | n.a. |
| nmol/eye | 270.6 | 11.7 | 44.5 | 23.6 | 21.4 | 11.4 | 109.7 | 79.4 | 35.2 | 184.2 | 79.5 | 0.117 |
EC: esterified cholesterol; DG: diglyceride; FA: non-esterified fatty acid; TG: triglyceride; CL: cardiolipin; LYPC: lysophosphatidylcholine; PC: phosphatidylcholine; PE: phosphatidylethanolamine; PS: phosphatidylserine; UC: unesterified cholesterol; SPM: sphingomyelin; RE: retinyl ester.
n.a., not available.
Calculated relative to the sum of all lipids except SPM and RE.
Understanding the role of lipoproteins in aging and ARMD has been furthered by direct evidence that particles with lipoprotein characteristics are isolable from BrM/Ch, as they are from arterial intima (Chao et al., 1990; Chung et al., 1994; Rapp et al., 1994). Following a double, high-salt buffer extraction from tissue homogenates (Li et al., 2005a; Wang et al., 2009b), particles released from BrM exhibit a density similar to plasma VLDL (0.95–1.006 g/ml), relative enrichment with EC (59% of total cholesterol), and spherical particle morphology indicative of a neutral lipid core (Fig. 7E). By negative stain electron microscopy, these highly electron-lucent particles have a mean diameter of 66 nm. Sub-fractionation produces an additional, higher density peak with less relative EC and a particle appearance consistent with loss of core neutral lipids (Li et al., 2005a). The fragility of larger lipoproteins is well recognized (Anderson et al., 1989; Forte and Nordhausen, 1986), and sub-fractionation is not recommended for future studies of BrM lipoproteins.
These two studies (Li et al., 2005a; Wang et al., 2009b) yielded salient characteristics of BrM lipoproteins that along with large size and enrichment with EC justify appellation of the particles as bona fide lipoproteins. The neutral lipid composition (Table 2) was very similar to that determined for native BrM/Ch, suggesting that appropriate lipoprotein isolation techniques can retain most particle properties. The following conclusions can be drawn.1) EC is the predominant neutral lipid. Most notable was the lack of TG, the major core lipid of plasma apoB lipoproteins in the same size class. EC was 11.6-fold more abundant than TG (moles), even higher than in the tissue studies. 2) The distribution of EC fatty acids as determined with two different methods is as follows: linoleate (18:2, 41.3%), oleate (18:1, 18.9%), palmitate (16:0, 17.4%), arachidonate (20:4n6, 6.5%), stearate (18:0, 3.0%), and docosahexaenoate (22:n6, 0.5%), highly similar to the tissue studies. 3) Phosphatidylcholine is more abundant than phosphatidylethanolamine by a factor of 1.29, and sphingomyelin, which binds tightly to cholesterol, is abundant relative to phosphatidylcholine (0.70). These ratios differ markedly from plasma lipoproteins. 4) Particles contain measurable retinyl ester, the transport form of dietary vitamin A. 5) Lipoprotein fractions contain apoB, apoA-I, and apoE, all of which have been identified in BrM; other apolipoproteins have not yet been sought. 6) The distribution of the major lipid classes in BrM lipoproteins differ importantly from apoB- containing plasma lipoproteins (Table 3), indicating that the particles in BrM are not simply a transudate from the circulation. 7) Within each lipid class, however, BrM lipoprotein fatty acids are overall remarkably similar to those plasma lipoproteins, with the principal exception of ∼20% lower linoleate (18:2n6) among all lipid classes. This finding raises the possibility that plasma lipoproteins are a source of individual lipid classes in BrM lipoproteins.
6.4. RPE lipid processing
Attempts to deduce the source of BrM lipids from compositional studies like those just described operate under the assumption that composition should reflect the source(s). Conclusions based on this reasoning have been hampered by limited data on composition and/or inadequate attention to whether the lipids discovered were localized only in BrM or throughout the choroid as well (Curcio et al., 2001; Holz et al., 1994; Sheraidah et al., 1993; Spaide et al., 1999). This review has summarized converging and repeatable evidence from light microscopic histochemistry, physical chemistry, ultrastructure, and lipid profiling of tissues and isolated lipoproteins (Sections 6.1–6.4) that establishes EC as the primary lipid accumulating with age in BrM. Further, of the major lipid classes, only EC is exclusively localized to BrM, i.e., not also distributed throughout the choroid.
What does high EC concentration within aged BrM signify about its source? Similar questions were raised decades ago regarding arterial intima, in that the earliest EC in atherosclerotic plaques was thought to consist of numerous oil droplets released from dying foam cells. Multiple lines of evidence indicated a composition consistent with a plasma lipoprotein transudate rather than cells (Chao et al., 1990; Guyton and Klemp, 1989; Kruth, 1997; Smith et al., 1967), a key conclusion that contributed to plasma lipid-lowering therapy via statins for treatment of atherosclerosis.
In the eye, various insults evoke engorgement of RPE cells with large oil red O-binding intracellular droplets (El Baba et al., 1986; Feeney-Burns et al., 1981; Fine and Kwapien, 1978; Majji et al., 2000). Such cells could conceivably contribute to BrM neutral lipid if they die and release their droplets. However, the intracellular droplets are much larger (1–2 µm) than BrM particles (66 nm), they apparently have little EC (Anderson et al., 2006) and abundant retinyl ester (Redmond et al.,1998), few RPE cells exhibit droplets in any one normal eye, lipoidal degeneration is not widely prevalent across eyes, and the rate of age-related RPE cell death (Del Priore et al., 2002) is probably too slow to account for the large and universal age-related accumulation of EC in BrM.
Excluding the possibility that lipids accumulating in BrM with age arise from dying RPE cells leaves the best-documented way to release EC from a healthy cell, namely, within the core of an apoB-containing lipoprotein. What could be the source of such a lipoprotein? Perhaps particles of hepatic or intestinal origin exit plasma in the choriocapillaris and adhere to extracellular matrix in BrM, analogous to events in arterial intima or even arcus (Section 5.5). However, isolated BrM lipoproteins differ from plasma lipoproteins in composition (Section 6.3), and indirect evidence from patients argues against a plasma origin (Section 9.2.2). While it is possible that BrM lipoproteins are modified plasma lipoproteins, available evidence indicates an important contribution of directly secreted lipoproteins from RPE
6.4.1. Expression of lipoprotein pathway genes of RPE
Determining the genes expressed by native human RPE is challenging even for the highly sensitive reverse transcriptase polymerase chain reaction (RT-PCR). Specific impediments to obtaining high quality RNA are the small amount of starting material (6–11 mg total RNA from 2 eyes), variable post-mortem delay to processing, potential contamination with closely associated blood, vasculature, and neurosensory retina, and the presence of melanin, an RT-PCR inhibitor (Eckhart et al., 2000; Giambernardi et al., 1998). Data interpretation is aided by the use of positive controls like liver and brain. Despite these difficulties, mRNA transcripts for both apoE and apoB were confirmed in human RPE and RPE/choroid preparations (Li et al., 2005b; Malek et al., 2003; Mullins et al., 2000), with apoE expression levels third behind brain and liver (Anderson et al., 2001). RPE contains mRNA transcripts for apos A-I, C-I, and C-II, but not C-III (Li et al., 2006; Malek et al., 2003; Tserentsoodol et al., 2006a).
Full-length apoB protein was localized to native RPE using a specific monoclonal antibody (Li et al., 2005b; Malek et al., 2003). Of significance to the apoB system, apoBEC-1 mRNA is not detectable in human RPE (Li et al., 2005b). However, it is present in rat RPE and the rat-derived RPE-J cell line (L. Wang, unpublished data), along with an apoB-48-like protein immunoprecipitable by anti-rat apoB (Wang et al., 2009b), suggesting that rat RPE expresses both apoB-100 and apoB-48 like liver in this species. Of major importance to the apoB system is the presence of both mRNA and protein for the MTP large subunit within native human RPE. The latter was localized with apoB itself to punctate intracellular bodies, presumably endoplasmic reticulum, within both the RPE and, surprisingly, ganglion cells of the neurosensory retina (Li et al., 2005b). In mouse RPE, both MTP isoforms are expressed (Fujihara et al., 2009). The dual expression of apoB and MTP signifies that RPE has the capability of secreting lipoprotein particles (Section 5.4). Finally, RPE expresses other genes encoding proteins important in cholesterol and lipoprotein metabolism, including ACAT-1,-2,2 LCAT, ABCA1, fatty acid binding protein 5, LDL-R, scavenger receptors B–I and -I, and CD-36 (Duncan et al., 2002; Li et al., 2005a,b; Ryeom et al., 1996; Tian et al., 2005; Tserentsoodol et al., 2006a; Yamada et al., 2008). Roles of these genes in retinal physiology are only beginning to be understood.
These RPE gene expression data provides a basis for redesignating the pigmentary retinopathies of abetalipoproteinemia and hypobetalipoproteinemia (Section 5.4) as intrinsic retinal degenerations. That the retinopathies associated with these mutations of MTP and APOB genes, respectively, are only partly alleviated by dietary supplementation with lipophilic vitamins carried on plasma apoB-lipoproteins (Chowers et al., 2001) is consistent with this view.
6.4.2. RPE lipid composition and apolipoprotein secretion
Lipid droplets are ultrastructurally detectable in basal cytoplasm of mammalian RPE cells (Hirosawa and Yamada, 1976; Robison and Kuwabara, 1977), including humans (Bairaiti and Orzalesi, 1963), and they increase in number and retinyl ester content after dietary or genetic manipulation of retinoid processing (Hirosawa and Yamada, 1976; Redmond et al., 1998; Robison and Kuwabara, 1977). Lipofuscin, which accumulates with age in RPE cells, contains oil red O-binding lipid in proportions different from outer segments, i.e., less PL, more nonesterified palmitate (16:0), arachidonate (20:4n6), and oleate (18:1n9), and less docosahexaenoate (22:6n3) (Bazan et al., 1990). The lipids in lipofuscin are also distinct from those accumulating with age in BrM (Section 6.3). The major fluorophor of RPE lipofuscin, the retinoid derivative A2E, at concentrations similar to those in vivo, can interfere with cholesterol processing in cultured RPE cells, leading to accumulation of EC, UC, and Nieman-Pick transporter in lysosomes, without affecting other lysosomal functions (Finnemann et al., 2002; Lakkaraju et al., 2007). Thus A2E, prominent in aging eyes, could influence the overall amount of cellular cholesterol available for export from RPE by lipoprotein secretion or other pathways (Section 5.1).
Neutral lipids have also been directly assayed in RPE. Frog RPE contains measurable TG and EC (Chen and Anderson, 1992), and outer segment docosahexaenoate is transiently stored as newly synthesized, rapidly hydrolysable TG before being recycled to retina (Bazan et al., 1992; Rodriguez de Turco et al., 1999). Native human RPE and ARPE-19 cells contain UC and EC in lower quantities than the in vitro lipoprotein secretor HepG2 (hepatoma). The EC fatty acid distribution in RPE resembles that of the particles accumulating with age in (i.e., rich in linoleate and poor in docosahex-aenoate (Li et al., 2005a,b)).
Recent evidence indicates that the RPE releases apolipoproteins and cholesterol using pathways previously well characterized in other cell types (Sections 5.3–5.4)3. Cultured RPE constitutively secrete 37 kDa apoE into high-density fractions (d = 1.18– 1.35 g/ml); lower density fractions were not examined (Ishida et al., 2004). These investigators also demonstrated a transfer of radio-labeled docosahexaenoate from outer segment membranes to HDL or lipid-free apoA-I in the medium, presumably by ABCA1-medi-ated mechanisms (Ishida et al., 2006). ARPE-19 cells (from human) and RPE-J cells (from rat) secrete EC into a lipoprotein-containing fraction following a standard fatty acid supplementation paradigm (oleate for ARPE-19, palmitate for RPE-J) (Li et al., 2005b; Wang et al., 2009b). Similar in size (mean, 56 nm) to the particles found in native BrM (Section 6.3), they contain little TG. Importantly, RPE-J cells and medium also contain immunoprecipitable [35S]-methionine-labeled apoB, in a full-length (512 kDa) form and a lower molecular weight band that may be apoB-48 (Wang et al., 2009b). This assay for protein synthesis and secretion, considered definitive, was also used to show apoB secretion from ARPE-19 cells (Fijalkowski et al., 2009).
The apoB-containing lipoproteins secreted by cultured RPE cell lines are unusual in several respects compared to other such lipoproteins: 1) Particles are EC-rich, despite being as large as TG-rich VLDL. 2) Particles are EC-rich when newly secreted, unlike LDL and HDL, whose composition is achieved by enzymatic remodeling in plasma (Section 5.3). 3) Fusion of smaller particles to create larger ones, as postulated for LDL in arterial intima (Kruth, 1997), is not required to achieve the size distribution seen in aged BrM (Huang et al., 2008a).
6.4.3. Source of lipids found in RPE lipoproteins (Table 4)
Table 4.
Fatty acids in lipoproteins and outer segments (mole%).
| Common name | Molecular name | EC in BrM lipoproteins |
EC in plasma lipoproteins* |
Phospholipids in outer segments ** |
|||
|---|---|---|---|---|---|---|---|
| HDL | LDL | VLDL | CM | ||||
| Palmitic acid | 16:0 | 15.6 | 13.2 | 12.0 | 11.3 | 12.9 | 16.3 |
| Stearic acid | 18:0 | 3.7 | 1.4 | 0.7 | 2.3 | 3.9 | 23.9 |
| Oleic acid | 18:1n9 | 18.4 | 21.1 | 16.0 | 19.2 | 20.4 | 9.1 |
| Linoleic acid | 18:2n6 | 41.6 | 54.4 | 56.7 | 50.8 | 47.0 | 0.9 |
| Arachidonic acid | 20:4n6 | 7.8 | 6.7 | 6.9 | 6.2 | 5.8 | 5.6 |
| Docosahexaenoic acid | 22:6n3 | 0.5 | 0.6 | 0.6 | 0.5 | 33.5 | |
HDL, high density lipoprotein; CM, chylomicron.
(Iglesias et al., 1996) for HDL (20 normolipemic subjects). (Wang et al., 2009a,b) for LDL, VLDL, CM (4 normolipemic subjects).
(Rapp et al., 2000), for outer segment phospholipids in human donor eyes.
There has been speculation in older literature that the age-related accumulation of BrM neutral lipid is related to photoreceptor phagocytosis, either by direct deposition of debris (Grindle and Marshall, 1978) or by stress-related basolateral secretion subsequent to engorgement of RPE with lipofuscin (Young, 1987). The purpose of the BrM lipoprotein is likely similar to lipoproteins elsewhere, i.e., secretion of excess lipid that cells cannot use or process. An initially attractive hypothesis that an apoB-lipoprotein from the RPE could eliminate fatty acids released by lysosomal phospholipases after outer segment phagocytosis (Li et al., 2005b) is now considered unlikely, for several reasons. 1) Neither the neutral lipids nor the PLs of BrM lipoproteins have a fatty acid composition that resembles that of outer segments (Table 4) particularly with regard to docosahexaenoate (22:6n3)(Fliesler and Anderson, 1983; Rapp et al., 2000), which is abundant in outer segments and sparse in BrM lipoproteins (Table 4). 2) BrM lipoproteins and drusen are highly enriched in EC (Sections 6.3, 8.1), which is essentially absent from outer segments (Fliesler and Anderson, 1983). 3) BrM lipoproteins and drusen are more highly enriched in UC (Sections 6.3, 8.1) than outer segments or isolated disk membranes (Boesze-Battaglia et al., 1990; Fliesler and Anderson, 1983). 4) Phagocytosis is not required for secretion of neutral lipids or apolipoproteins in RPE cell lines (Ishida et al., 2004; Li et al., 2005b; Wang et al., 2009b).
Several explanations for the dissimilarity between BrM lipoproteins and outer segments are possible. 1) Outer segment lipids may be catabolized by RPE (Bazan et al., 1990) prior to re-synthesis as particle components. 2) Docosah exaenoate (22:6n3) is efficiently recycled back to the retina (Bazan et al., 1992), leaving little for export. 3) Outer segment lipids may be present in a form (e.g., oxidized (Suzuki et al., 2007)) not recognizable by the assays utilized. 4) Some lipids within BrM lipoproteins may be selectively and extensively hydrolyzed in the extracellular compartment (e.g., by choroid-resident (Casaroli-Marano et al., 1996) or RPE-secreted lipases), an option made less likely by concordant results from whole tissues, isolated particles, and cultured cells. 5) BrM lipoproteins are dominated by other sources, specifically plasma lipoproteins, which they closely resemble with regard to fatty acid composition (Table 4), although BrM lipoproteins differ from plasma in other important respects (Section 6.3). This last explanation is the simplest.
Plasma lipoproteins LDL and HDL are both taken up by RPE, which expresses functional receptors for both LDL (LDL-R) and HDL (scavenger receptor B–I, SRB-I) (Duncan et al., 2002; During et al., 2008; Elner, 2002; Hayes et al.,1989; Tserentsoodol et al.,2006a). This system is likely in place for re-supply of essential lipophilic nutrients. LDL-derived cholesterol partitions with remarkable speed (within hours) into membranes of the neurosensory retina (Gordiyenko et al., 2004; Tserentsoodol et al., 2006a). The xanthophylls lutein and zeaxanthin, major components of macular pigment, are carried equally on LDL and HDL in humans (Loane et al., 2008). ARPE-19 cells preferentially take up xanthophylls at SRB-I (During et al., 2008), and avian retina uniquely depends on HDL-mediated xanthophyll delivery to maintain high tissue levels in vivo (Connor et al., 2007). Vitamin E, essential for outer retinal health (Friedrichson et al.,1995; Yokota et al., 2001), is also delivered by lipoproteins via LDL-R- and SRB-I-dependent pathways (Hacquebard and Carpentier, 2005). Thus, delivery of cholesterol, xanthophylls, and vitamin E are the most plausible reasons for a major plasma lipoprotein input to RPE. With regard to its nutrition, then, retina differs markedly from brain, which relies almost exclusively on endogenous cholesterol synthesis (Björkhem and Meaney, 2004) and contains little lutein or zeaxanthin (Connor et al., 2007).
A less likely reason for lipoprotein-mediated delivery to the RPE is the re-supply of retinal docosahexaenoate, a mechanism postulated but not directly demonstrated two decades ago (Scott and Bazan, 1989). A recent comprehensive analysis of retina, RPE/ choroid, and orbital fat lipid composition (Bretillon et al., 2008b) concluded that only a small portion of retinal docosahexaenoate is derived from diet, with intra-retinal biosynthesis and recycling the most likely source, but left open the possibility that RPE/choroid is influenced by diet. It is also possible that retinal docosahexaenoate will prove to be also delivered by albumin, the major plasma carrier of non-esterified fatty acids to body tissues (Goldberg et al., 2009).
Given that the lipids in BrM lipoproteins are not likely to originate in outer segments and that the fatty acid composition of these lipoproteins is markedly similar to that of plasma LDL and HDL, it is tempting to conclude that the BrM lipoprotein is a mechanism to eliminate plasma lipoprotein residues from RPE after specific nutrients (e.g., xanthophylls, cholesterol, vitamin E) are extracted for use by photoreceptors. According to this model, EC-rich lipoproteins from plasma are taken up by RPE, stripped of nutrients, and repackaged for secretion in BrM as large, EC-rich apoB-lipoproteins. Rather than representing a system to dispose of outer segments, then, the BrM lipoprotein may be a by-product of an immense and poorly understood system to deliver specific lipophilic nutrients to retina. Within the polarized RPE, the apically-directed recycling of docosahexaenoate to the retina (Bazan et al., 1992) may be accompanied by an independent, basally-directed recycling of plasma lipoproteins repackaged for egress to the systemic circulation across BrM.
7. BrM, lipids, and transport
7.1. EC-rich barrier in aged BrM; Lipid Wall (Figs. 8–10)
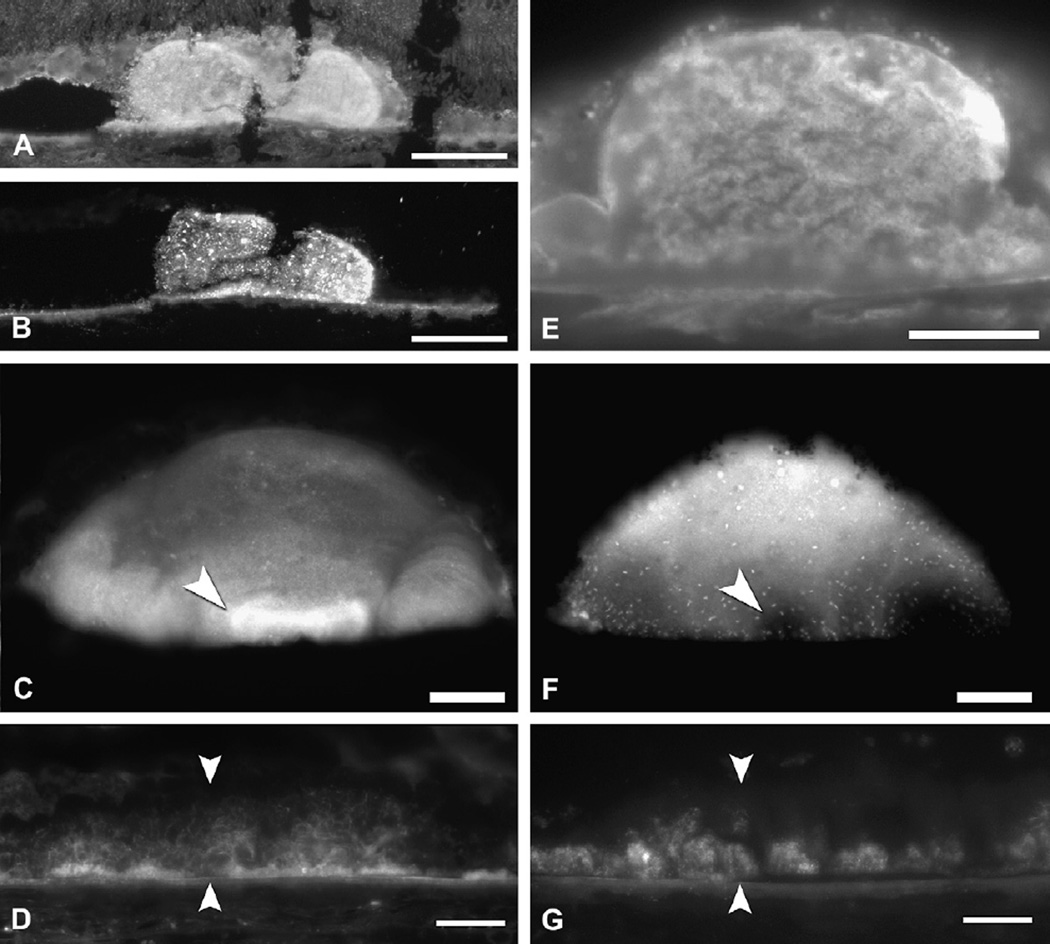
Fig. 8.
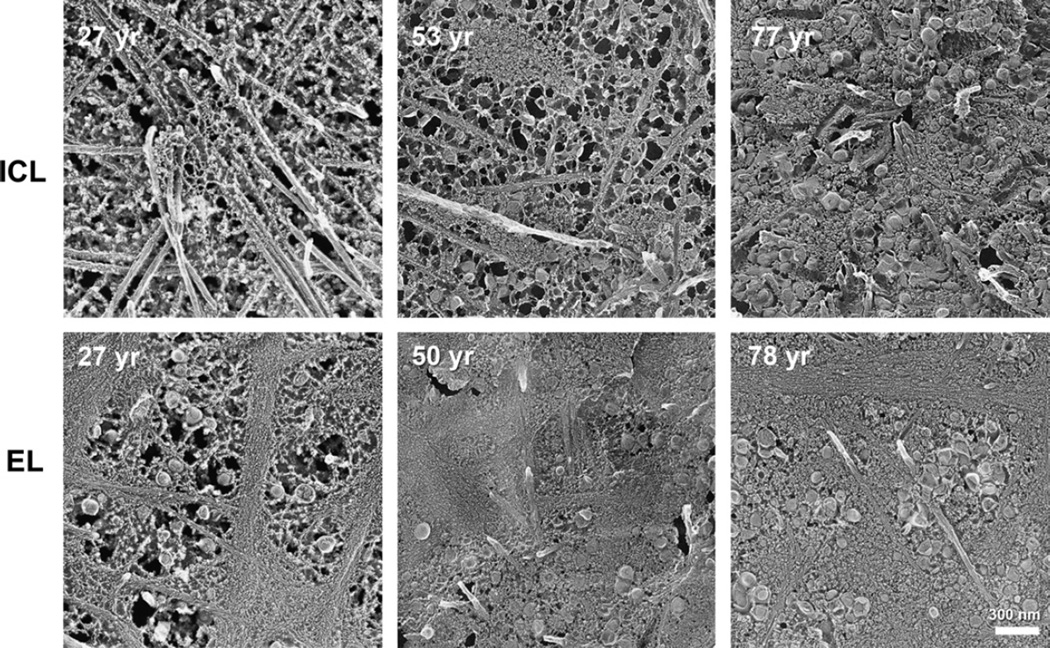
Lipoprotein deposition in BrM layers throughout adulthood. Apparent lipoprotein particle accumulation appears at earlier ages in elastic layer (EL) than inner collagenous layer (ICL). Oblique views of BrM were obtained from normal maculas prepared using QFDE. Bar is 300 nm. Reprinted from (Huang et al., 2007b) with permission from Elsevier.
Fig. 10.
Lipid Wall. In this oblique view of BrM, lipo proteins are densely packed in the Lipid Wall (lower right corner). Normal macula of an 82-year-old donor, prepared by QFDE. Taken from (Huang et al., 2007b) BI, basal infolding; BL-RPE, basal lamina of the RPE.
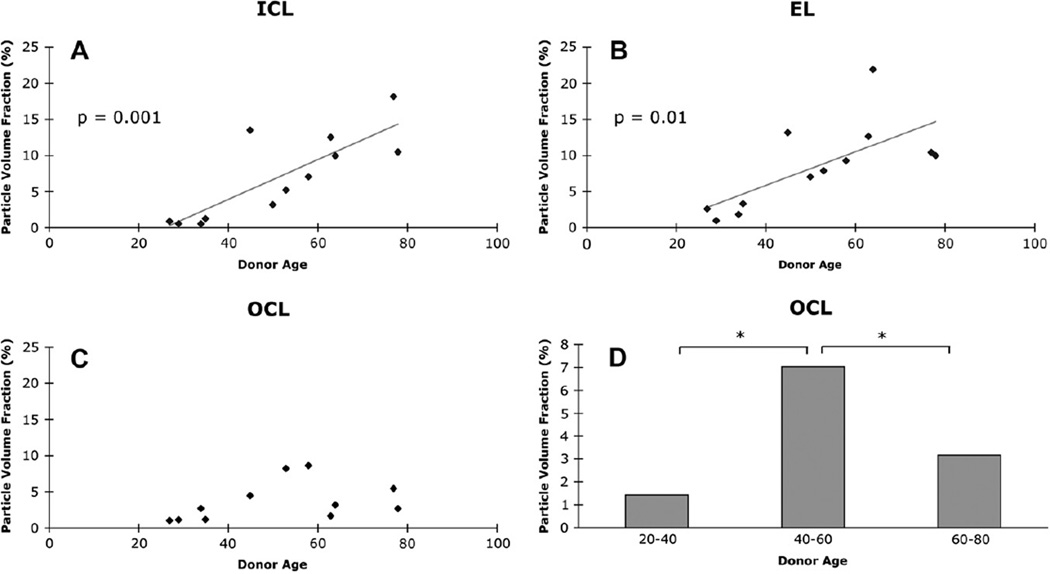
As revealed by QFDE analysis of normal eyes of different ages (Huang et al., 2007b, 2008a; Ruberti et al., 2003), significant lipoprotein accumulation begins during the third or fourth decade of life in or near the elastic layer of macular BrM. This process is reminiscent of the preferential deposition of lipoprotein-derived EC near elastin in arterial intima (Guyton et al., 1985). With advancing age, following the elastic layer becoming filled with these particles and other debris, accumulation was also seen in the inner collagenous layer (Fig. 8), as if particles were “backing up” into this region. The volume fractions occupied by lipoprotein particles in these tissues increased in roughly a linear fashion (Fig. 9) (Huang et al., 2008a). The outer collagenous layer showed a more complicated pattern (Fig. 9), with an early accumulation that decreases later in life, as if their source had diminished. During this time the small (10 nm) granular particles (Section 6.2) also accumulate throughout the layers.
Fig. 9.
Volume occupied by lipoproteins in BrM layers. Lipoproteins occupy steadily greater volume with age in inner collagenous and elastic layers (ICL, EL) but increase then decrease in outer collagenous layer (OCL). Asterisk indicates significant difference. Reprinted from (Huang et al., 2008a); copyright is held by Association for Research in Vision and Ophthalmology.
In eyes over 60 years of age, accumulated particles fill most of the inter-fibrillar space in the inner collagenous layer, and groups of particles appear between this layer and the RPE basal lamina. In eyes over 70 years of age, this process culminates in the formation of a new layer in BrM that we have termed the Lipid Wall (Ruberti et al., 2003). This dense band 3–4 particles thick external to the RPE basal lamina was previously illustrated by others without comment (Fig. 10). This tightly packed layer of space-filling particles, too dense for volume fraction measurements, displaces the structural collagen fibers at the same location in younger eyes that help bind BrM to the RPE basal lamina. Interestingly, particle accumulation and Lipid Wall formation occurs not only in macular BrM, but also in the periphery, although less prominent in the latter region, and in somewhat older eyes (Johnson et al., 2007). Taken together, these observations are consistent with an interpretation that lipoprotein accumulation starts in the elastic layer, backs up into the inner collagenous layer, and eventually forms the Lipid Wall. This blocks the source of lipoproteins to the outer collagenous layer and explains why the concentration of particles in this layer drops in eyes >60 years of age.
The striking spatial correspondence of the Lipid Wall in aged eyes and BlinD in eyes with ARMD makes the Lipid Wall a likely direct antecedent to BlinD (Section 8.2).
7.2. The barrier hypothesis: lipid accumulation and transport through BrM (Figs. 11,12)
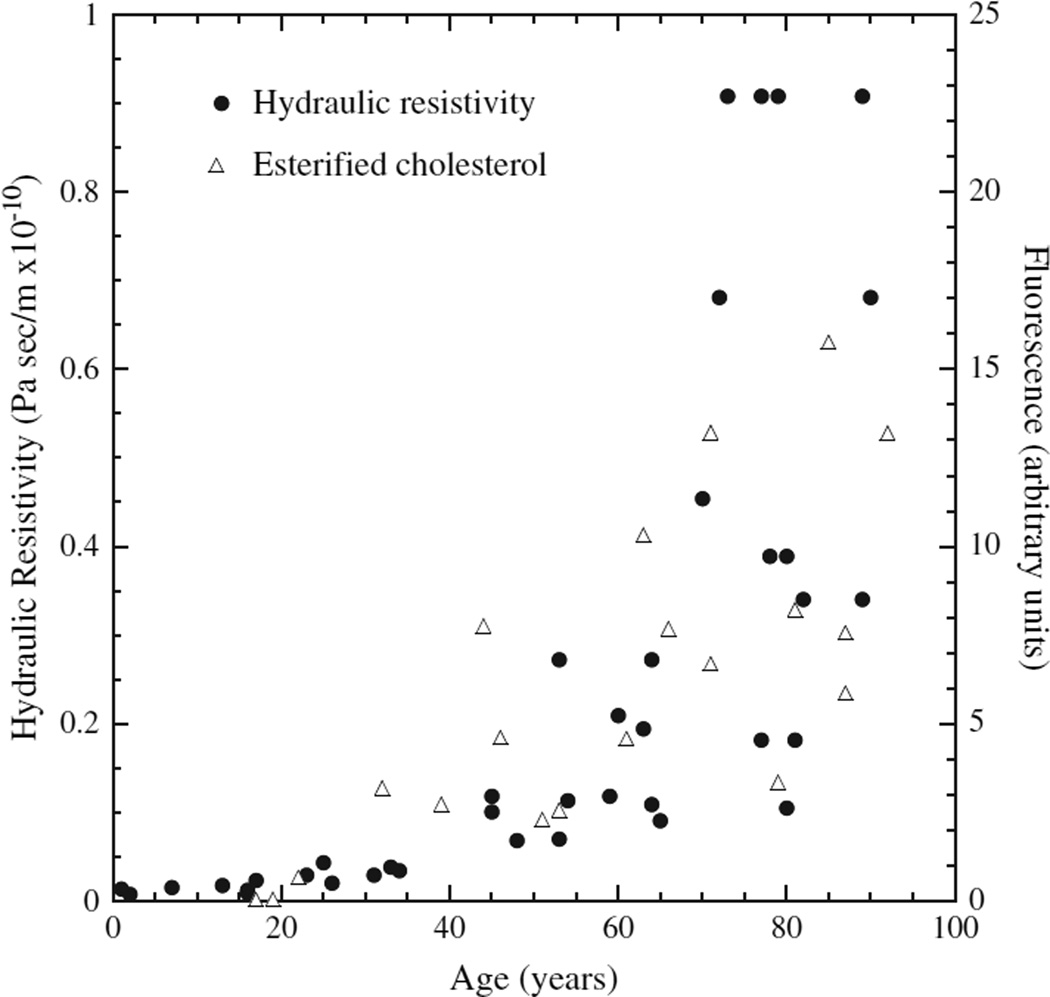
Fig. 11.
Hydraulic resistivity and BrM EC in aging. Hydraulic resistivity (Marshall et al., 1998) of excised BrM/choroid (closed symbols) and fluorescence due to histochemically detected esterified cholesterol in sections of normal BrM (Curcio et al., 2001) (open symbols) as a function of age. From (Ethier et al., 2004) Reprinted, with permission, from the Annual Review of Biomedical Engineering, Volume 6 (c)2004 by Annual Reviews www.annualreviews.org.
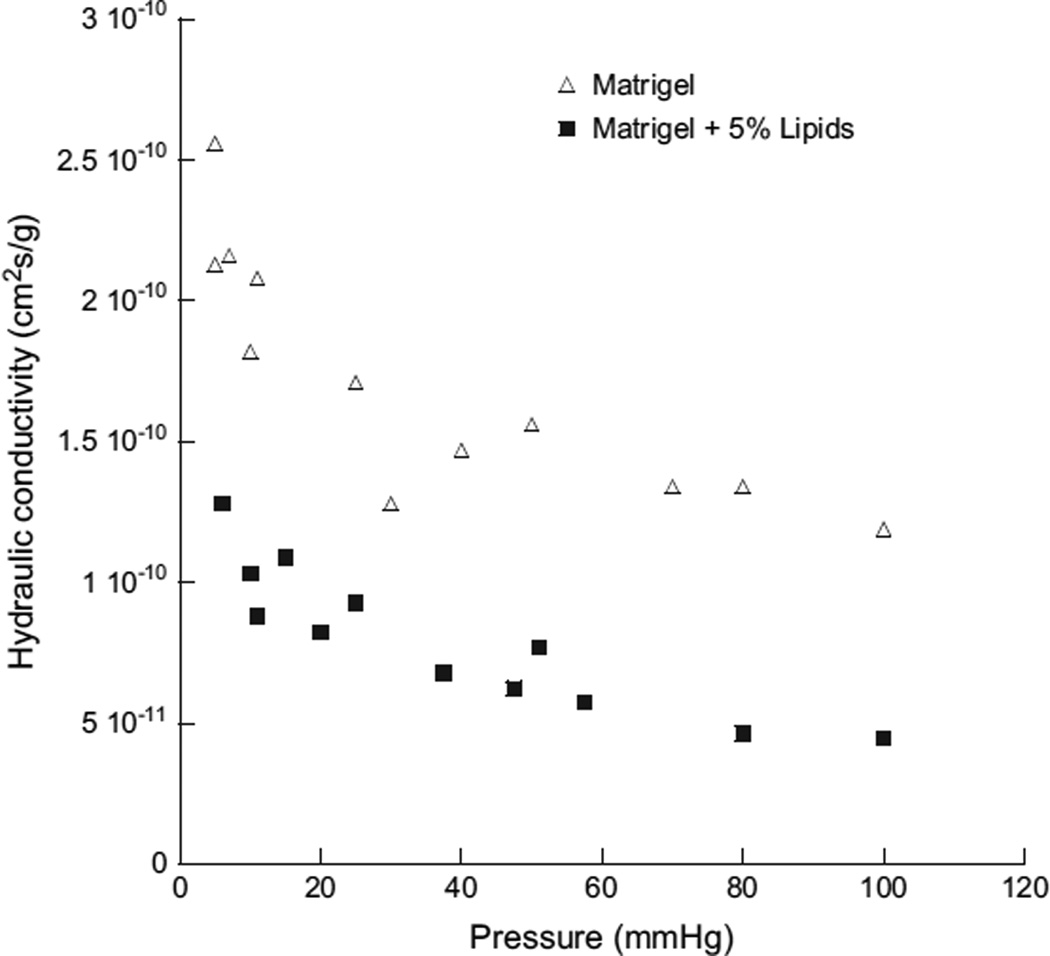
Fig. 12.
Lipoprotein-derived lipids reduce fluid transport through an artificial matrix. The hydraulicconductivity,asafunctionofperfusionpressure,of1%Matrigel (opentriangle) or 1% Matrigel with 5% LDL added (closed squares). Modified from (McCarty et al., 2008).
Deposition of lipoproteins, and especially EC within their cores, may render BrM increasingly hydrophobic with age and impede transport of hydrophilic moieties between the RPE and choroidal vessels. Over twenty years ago, Bird and Marshall first introduced the hypothesis of a physical barrier in this tissue (Bird and Marshall, 1986), distinct from the physiological blood-retina barrier formed by retinal capillary endothelium and RPE junctional complexes (Kaur et al., 2008). A BrM transport barrier could pre-dispose older individuals to multiple retinal conditions (Bird and Marshall, 1986; Chuang and Bird, 1988; Kuntz et al., 1996). Marshall and colleagues provided substantial support for a BrM transport barrier, demonstrating reduced hydraulic conductivity and permeability to solutes and macromolecules in BrM of aged and ARM eyes (Hussain et al., 2002; Moore and Clover, 2001; Moore et al., 1995; Starita et al., 1996). The role of lipids in this barrier was tested by examining the correlation between the accumulation of BrM lipids and increased BrM hydraulic resistivity, as measured in BrM/choroid explants from eyes of different ages (Moore et al., 1995; Starita et al., 1996, 1997). Marshall’s group concluded that since lipid accumulation occurs mostly after age 40, and the majority of decrease in hydraulic conductivity of BrM was observed before this age, these events are unrelated (Marshall et al., 1998).
However, the hydraulic resistivity (inverse of hydraulic conductivity) of BrM actually correlates strongly with its lipid content (Marshall et al., 1998). Indeed, the age-related increase in hydraulic resistivity of BrM exactly mirrors that of the age-related increase of histochemically detected EC in BrM (Ethier et al., 2004) (Fig. 11). Hydraulic resistances add when flow-limiting regions are in series, while hydraulic conductances add when they are in parallel. Lipid accumulation in BrM adds a new hydraulic resistance in series with an existing resistance. It cannot be bypassed, as the flow must pass through this layer. It is therefore not surprising that hydraulic resistivity shows a better correlation with lipid accumulation than does hydraulic conductivity, which would have an inverse relationship with lipid accumulation in any case.
Recent evidence (McCarty et al., 2008) indicates that lipid deposition can significantly decrease the hydraulic conductivity of a model extracellular matrix, Matrigel™. In this experimental system, the hydraulic conductivity of Matrigel™ was modified by the addition of LDL-derived lipids4. Addition of 5% LDL-derived lipids (by weight) to Matrigel™ lowered its hydraulic conductivity by greater than 50% (Fig. 12). This effect was surprisingly large, much larger than if 5% latex spheres were added to the Matrigel™, and larger than predicted by the Debye–Brinkman equation used to model interstitial fluid movement (McCarty et al., 2008).
8. Cholesterol and apolipoproteins in sub-RPE lesions
Here we consider the cholesterol and apolipoproteins in the extracellular sub-RPE lesions associated with aging and ARMD (Section 3.2 for definitions and descriptions). Lesion composition is best known for drusen, which are amenable both for histochemistry in tissue sections and for isolation and direct assay. The relationship between normal aging and an ARMD-specific lesion may be best appreciated for the Lipid Wall and BlinD. The latter may be more correctly described as containing lipoprotein-derived debris rather than membranous debris, as occurs in many descriptions of this lesion. BlamD lipid content is also discussed.
8.1. Druse histochemistry and immunohistochemistry (Fig. 13)
Fig. 13.
Cholesterol and apolipoproteins in drusen and deposits. Filipin fluorescence in A–D and F–G. Immunofluorescence in E. Bars in A, B, E 50 µm. Bars in C,D,F,G 20 µm. A. Druse and surrounding chorioretinal tissue contain UC. B. Druse (same as A) and BrM contain EC. Speckles represent lakes of EC. C. A bright UC-rich core (arrowhead) at the base of an isolated, extra-macular druse. D. A thick BlamD (between arrowheads) has bright, delicate fluorescence for UC. E. ApoB immunofluorescence in a druse. F. The core at the base of the same druse as panel C is EC-poor core (arrowhead). This druse also contains EC-rich lakes (speckles). G. A thick BlamD (same as D, between arrowheads) has fluorescence for EC confined to its outer half. Adapted from (Curcio et al., 2005a; Li et al., 2007; Malek et al., 2003).
Studies using sudanophilic dyes or polarizing microscopy identified both neutral lipids and polar lipids in age-related drusen (Haimovici et al., 2001; Malek et al., 2003; Pauleikhoff et al., 1992; Wolter and Falls, 1962). In macular drusen of ARMD eyes and extra-macular drusen of non-ARMD aged eyes (Curcio et al., 2001, 2005a; Li et al., 2007; Malek et al., 2003) (Fig. 13A – D), virtually every druse, whether hard or soft, has abundant EC and UC as revealed by filipin. Cholesterol in drusen assumes different morphologies reflecting different chemical composition and formative processes. Soft druse contents contain distinctive, loosely packed UC-rich whorls (Fig. 10D). Lakes of EC (Fig. 13A) occur in macular and extra-macular drusen, with larger lakes in the macula. Druse cores are ∼15 µm diameter basally located regions (Mullins and Hageman, 1999) that rich in UC (Fig. 13C,D) and in their very centers, poor in EC (Li et al., 2007). This disposition of cholesterol in cores may reflect the activity of invading cellular processes during druse biogenesis (Hageman et al., 2001), with the greater size of UC-rich cores relative to EC-poor cores reflecting a declining gradient of enzymatic activity with increased radial distance from the putative invaders. Finally, extra-macula drusen also exhibited highly fluorescent EC-rich shells subjacent to its cap of RPE (Anderson et al., 2004; Malek et al., 2003). No drusen containing cholesterol crystals have been observed. Nor have there been reports of the distinctive clefts that signify crystals in tissue sections, a distinct difference from atherosclerotic intima or lipid keratopathy (Crispin, 2002; Guyton and Klemp, 1993).
Multiple apolipoproteins are found in drusen, making a lipoprotein particle containing them (Section 6.3) an efficient mechanism to explain the presence of all of them (as speculated (Anderson et al., 2001)). ApoE appears in macular and extra-macular drusen, within a tri-laminar distribution in BrM, and it does not localize to lipofuscin within RPE (Anderson et al., 2001; Klaver et al., 1998). Other studies (Li et al., 2006; Malek et al., 2003) found apoE and apoB in 80–100% of peripheral drusen in ARMD and normal eyes and in fewer (55–60%) macular drusen of ARMD eyes, attributed to apolipoprotein dilution by other druse constituents. ApoE but not apoB was found in the drusen proteomics study (Crabb et al., 2002), perhaps due to sample preparation with a chloroform-methanol extraction that de-lipidates and precipitates apoB. ApoA-I immunoreactivity is present in 62% of peripheral drusen (Li et al., 2005a, 2006). Of interest is the relationship between apoCs in plasma and drusen, such that apoC-III, which is abundant in normolipemic plasma, is present in fewer drusen (16.6%) than apoC-I (93.1%), which is sparse. This result suggests either a specific retention mechanism for plasma-derived apolipoproteins with drusen, or a local source.
Immunoreactivity for all apolipoproteins is present throughout each individual druse. This pattern resembles that of abundant druse components like TIMP-3, vitronectin, and CFH (Hageman et al., 1999, 2005; Ruiz et al., 1996) and contrast with non-abundant druse components like amyloid or zinc that preferentially localize to small sub-regions (Johnson et al., 2002; Lengyel et al., 2007; Luibl et al., 2006). Abundant drusen constituents that are also present in normal aged BrM are good candidates for pathways involved at the earliest phases of disease (e.g, EC, UC, apoE, TIMP-3, AGE). Molecules enriched in inner BrM by light microscopy (e.g., apoE, apoB) are likely present in the Lipid Wall and BlinD (Anderson et al., 2001; Malek et al., 2003).
8.2. BlinD – Membranous debris vs lipoprotein-derived debris, and its evolution from the Lipid Wall (Fig. 14)
Fig. 14.
Ultrastructure of cholesterol-containing components of drusen and deposits. Tissue post-fixed with OTAP, from (Curcio et al., 2005b). A. RPE migrating anteriorly and thick BlamD containing cellular processes, bracketed by large arrowheads. B. BlamD containing basal mounds of membranous debris (arrowhead). Bar, 200 nm C. Membranous debris with contents, interior of a large druse. Bar, 200 nm. D. Individual profiles in the basal mound of panel B are solid and surrounded by an electron-dense band. E,F. Bar is 500 nm. E. Linear tracks of partially extracted material resembling membranous debris (double arrows) in BlamD. F. Linear tracks of solid particles (double arrows) in BlamD of the same eye as E, post-fixed with OTAP.
The morphology of extracellular cholesterol is best described for the lipid-rich core of atherosclerotic plaque in arterial intima. In that location, morphologically distinct components differ in their relative proportions of UC, EC, and PL and the disease stage at which they appear (Curcio et al., 2005b; Guyton and Klemp, 1989, 1996). From incipient to advanced disease, these forms include EC-rich LDL particles (22 nm), small EC-rich droplets (100 nm), multi-lamellar liposomes of UC and PL (40–200 nm), irregular lakes of EC delimited by a PL monolayer (6–20 µm), large EC-rich droplets (1–4 µm) shed from dying foam cells, and crystals of pure UC (4 µm), a sign of plaque maturity.
In ARMD, the principal lipid-containing lesion component has been called membranous debris by Shirley and John Sarks (Sarks et al., 1988, 2007; Sarks, 1980). As viewed by transmission electron microscopy following osmium tetroxide post-fixation, membranous debris appears as variably sized, contiguous coils or whorls of uncoated membranes consisting of uni- or multilamellar electrondense lines surrounding an electron-lucent center. This material dominates four extracellular lesions – soft drusen, BlinD, basal mounds between the RPE and its basal lamina, and aggregations within the sub-retinal space – and in one intracellular location – intracellular vacuoles within the RPE. By light microscopy, these locations contain histochemically detectable PL and UC, with quantitative evidence indicating much higher UC enrichment than surrounding cells (Curcio et al., 2005a; Pauleikhoff et al., 1992). Accordingly, membranous debris fine structure does not resemble nearby RPE plasma membranes (Curcio et al., 2005a), since it is thick, occasionally multilamellar, and highly electron dense (Fig. 14C,D).
Although membranous debris is frequently interpreted as vesicles with aqueous interiors, recently evidence indicates that it is not vesicular but rather, aggregations of solid particles that could account for the abundant EC in drusen and deposits more plausibly than membranes containing UC and PL (Curcio et al., 2000, 2005a; Malek et al., 2003; Pauleikhoff et al., 1992). The ultrastructural correlate of neutral lipid in lesions is better revealed in tissue post-fixed by the OTAP method, since these lipids are extracted using routine tissue preparation for transmission electron microscopy (Section 6.2). Linear tracks of solid particles cross BlamD in the same configuration as tracks of membranous debris (Fig. 14E,F). Soft drusen and basal mounds contain ovoid-to-spherical bodies with homogeneous, moderately electron-dense interiors and highly electron-dense exteriors, and pools of neutral lipid (Fig. 14C,D). These images were interpreted to indicate that membranous debris represents the UC-rich exteriors of fused lipoprotein particles whose neutral lipid interiors are not uniformly well preserved in typical preparations of post-mortem tissue (Section 6.2). Lipid-preserving methods can minimize this loss. This interpretation is supported by evidence that when isolated BrM lipoprotein particles lose their neutral lipid cores during extensive sub-fractionation, only UC-enriched surfaces of individual or fused lipoproteins remain (Li et al., 2005a). From this perspective, then, we propose that the major lipid-containing component of ARMD-specific lesions should be called “lipoprotein-derived debris” rather than “membranous debris.”
Although lipoprotein-derived debris could explain EC, UC, and PL within lesions, actual membranes containing UC, not derived from lipoproteins, are also likely present in these locations. Early and influential micrographs showing membrane-bounded cytoplasmic packets shed from surrounding RPE (Ishibashi et al., 1986a,b) suggested that at least some UC could arrive in extracellular lesions by this route. Recently, proteins of known RPE origin have been detected in BrM and drusen, including RGR (Lin et al., 2007; Nickle and Robinson, 2007) and exosome markers CD63, CD81, and LAMP2 (Lakkaraju and Rodriguez-Boulan, 2008; Wang et al., 2009a). Because these proteins are normally membranebound, their presence in lesions is consistent with direct shedding or blebbing of plasma or intracellular membranes by RPE, perhaps secondary to oxidant injury (Strunnikova et al., 2001).
What is the relationship of age-related deposits found in BrM to BlinD in ARMD? The Lipid Wall, located between the inner collagenous layer and RPE basal lamina, is at the same site of BlinD, suggesting that the Lipid Wall might be the precursor of BlinD. Support for this hypothesis comes from ultrastructural examination of eyes at different ARMD stages (Curcio and Millican, 1999; Sarks et al., 2007). We have identified transitional forms between the Lipid Wall and BlinD supporting evolution of BlinD from the Lipid Wall (Messinger et al., 2009). In addition, their correspondence in physical location and similarity in structure support this conclusion. Whereas the Lipid Wall consists of packed lipoproteins of similar size, BlinD in its earliest forms consisted of material resembling fused lipoproteins of irregular size. These findings suggest that the Lipid Wall is the likely immediate precursor of BlinD, of significance because Lipid Wall composition can be inferred in part from that of the BrM lipoprotein particles (Section 6.4).
Since the Lipid Wall is found in all aged eyes, while BlinD is specifically associated with ARMD, this suggests that the “Response-to-Retention” of lipoproteins in BrM that is key step in the pathogenesis of ARMD lesions. It also suggests that formation of the Lipid Wall is a critical step in this disease process.
Sections 6 and 7 described a model wherein RPE production of apoB lipoproteins for normal physiological transport to the choriocapillaris is gradually blocked by age-related accumulation of these particles in BrM eventually filling this tissue, resulting in a new layer caused by the backwards accumulation of these particles. Once this Lipid Wall begins to form between the inner collagenous layer and the RPE basal lamina, more lipids preferentially fill this potential space, leading to the formation of BlinD, which is linear because of the geometry of the space containing it. Formation of BlinD may damage RPE cells via decreased transport of nutrients and waste products. Their response to this insult may include production of excessive basal lamina materials. The presence of BlinD and remaining Lipid Wall prevents removal of these materials, contributing to local accumulation of BlamD, which may have already begun due to other RPE stresses. BlamD would have to be internal to BlinD based on this argument.
8.3. BlamD
BlamD, internal to the RPE basal lamina, consists largely of basal lamina-like proteins and glycosaminoglycans (Kliffen et al., 1996; Lommatzsch et al., 2008; Marshall et al., 1992) but also contains lipoprotein-related components. In light microscopic using cryosections and oil red O or filipin, BlamD is easily discernable, and it is stained lightly near the RPE and heavily near BrM (Curcio et al., 2005a; Lommatzsch et al., 2008; Malek et al., 2003; Pauleikhoff et al., 1992). A widespread, thick BlamD associated with mutations of the C1QTNF5 (CTRP5) short-chain collagen gene (Hayward et al., 2003) have abundant EC and UC, apoB immunoreactivity, and linear tracks of contiguous electron-dense solid particles (Milam et al., 2000). These tracks suggest an organized mechanism for tethering and transporting lipoproteins particles to the RPE basal lamina, which they cross in order to reach BrM. These observations are consistent with the idea that the importance of BlamD lies in indicating RPE stress and detaining components en route to drusen and BlinD where they will have greater pathogenic significance (Sarks et al., 2007).
9. Response-to-retention of an intra-ocular apoB lipoprotein
Our view of the role of BlinD and soft drusen in ARMD pathogenesis and progression centers around three concepts: 1) a barrier that prevents aqueous phase nutrients in plasma (Pauleikhoff et al., 1990) or other compounds with potential for clearance (e.g., HDL) from gaining access to RPE, thus obstruction the elimination of waste products; 2) a plane that sequesters angiogenic compounds, cytokines, modified lipids, or proteins (Crabb et al., 2002; Spaide et al., 1999; Yamada et al., 2008) that collectively evoke a local inflammatory response; and 3) a cleavage plane for lateral dissection by opportunistic choriocapillary endothelial cells that have breached BrM (Sarks et al., 1980). The latter is strengthened by observations of pooled neutral lipid in BlinD with little surrounding proteinaceous material to provide cohesion (Curcio et al., 2005b), the detachment of the RPE basal lamina from collagen fibrils of the inner collagenous layer by the Lipid Wall (Section 7.1 ), and the physical fragility of macular soft drusen (Malek et al., 2003; Rudolf et al., 2008a).
9.1. Statement of model (Fig. 15A,B)
Fig. 15.
Response-to-retention: ARMD, CAD. A. BrM lipoprotein (BrM-LP) compared to other apoB-containing lipoproteins well characterized in plasma. Particle diameters are to scale. Not all the proteins on the BrM lipoprotein are known. B. The hypothesized progression of ARMD has many parallels to the Response-to-Retention hypothesis of atherosclerotic coronary artery disease (Tabas et al., 2007), beginning with apoB-lipoprotein deposition in a vessel wall.
Collectively, the data reviewed in Sections 6 and 7 imply that the RPE constitutively secretes an apoB-containing lipoprotein particle from its basolateral aspect into BrM for eventual clearance into plasma. We envision a large lipoprotein (Fig. 15A) in the VLDL size and density class that contains apoB-100, apoA-I, apoE, apoC-I, apoC-II, and possibly other proteins. It is secreted with an EC-rich neutral lipid core. Plasma lipoproteins taken up by the RPE (Tserentsoodol et al., 2006b) can deliver UC, lipophilic vitamins, and/or xanthophylls for ultimate use by the photoreceptors. However, this process might raise intracellular UC to toxic levels within RPE. Lipoprotein secretion by RPE cells would allow disposal of excess UC in concert with recycling EC and retinoids back to plasma (Qtaishat et al., 2003). This model does not preclude other mechanisms of cholesterol release from RPE (Sections 5.1 and 10.1.1). Although it is possible that smaller lipoproteins secreted by the RPE accumulate in BrM and fuse together to form large particles, the size distribution of particles in aged BrM is inconsistent with a continuously evolving population (Huang et al., 2008a).
We propose that in younger eyes, lipoprotein particles cross BrM for egress to plasma. With advanced age, transit time across BrM increases due to changes in extracellular matrix, particle character, and/or clearance mechanisms, resulting in the accumulation of either native or modified particles, especially at the site of the Lipid Wall. In conjunction with other cellular processes, lipoprotein accumulation and modification eventually causes the formation of BlinD and drusen and contributes to ongoing RPE stress and formation of BLamD. Our model does not indicate that deposit formation alone initiates ARMD. Instead, it is likely that the “Response-to-Retention” of lipoproteins (Tabas et al., 2007) in BrM gives rise to, and may even be required for, other key events including complement activation, inflammation, neovascularization, and RPE cell death.
This model allows us to compare ARMD and CAD at many steps (Fig. 15B), importantly, near the beginning where tissue-specific processes are most likely involved. Lipoproteins retained in arterial intima undergo numerous modifications, including oxidation, aggregation, fusion, glycation, immune complex formation, proteoglycan complex formation, and conversion to cholesterol-rich liposomes (Tabas, 1999). Potentially all these processes may also occur in the RPE/choroid complex (Handa, 2007), as oxygen levels are high (Wangsa-Wirawan and Linsenmeier, 2003), high levels of irradiation could promote lipid peroxide formation (Zarbin, 2004), BrM contains proteoglycans and AGE are found to accumulate with age (Handa et al., 1999; Hewitt et al.,1989; Kliffen et al., 1996; Newsome et al., 1987), and the immediately adjacent RPE is a source of secreted enzymes (e.g., sphingomyelinase (Lakkaraju et al., 2007)) that can act on lipoproteins.
The lipid components of BrM lipoproteins are potentially subject to oxidative modification that leads to further deleterious consequences. Antibodies vs copper sulfate-oxidized LDL reveal immunoreactivity in BrM and basal deposits of eyes with ARMD (Yamada et al., 2008). Immunoreactivity for oxidized phosphatidylcholine is present in photoreceptor outer segments, some RPE cells, scattered drusen in eyes with ARMD, and in surgically excised neovascular membranes (Kamei et al., 2007; Suzuki et al., 2007). ARPE-19 cells exposed to oxidized LDL undergo numerous phenotypic shifts, including altered gene expression (Yamada et al., 2008). Oxysterols are metabolites of UC generated in vivo primarily by mitochondrial enzymes like CYP27A1 and CYP46A1 (Javitt,2008), which are present in retina and RPE (Bretillon et al., 2007; Lee et al., 2006). Oxysterols are also studied as in vitro as toxic byproducts of LDL oxidation that can contribute to macrophage differentiation into foam cells and induce cell death in RPE cell lines (Joffre et al.,2007; Moreira et al., 2009; Ong et al., 2003; Rodriguez et al., 2004). 7-ketocholesterol is present near BrM in adult monkey eyes and is thought to originate from BrM lipoprotein deposits that oxidized in the high-oxygen choroidal environment (Moreira et al., 2009). These oxidation products can contribute to disease progression, as intravitreal injections of linoleate hydroperoxide can elicit choroidal neovascularization and chorioretinal atrophy (Tamai et al., 2002), and 7-ketocholesterol can up-regulate VEGF expression (Moreira et al., 2009).
The hypothesis that an RPE-derived apoB-lipoprotein retained in a vascular intima evokes downstream consequences (Fig. 15) also provides a new context for the recently recognized roles of inflammatory proteins and regulators in ARMD ((Hageman et al., 2001; Nussenblatt and Ferris, 2007; Richards et al., 2007)). The alternative complement pathway has been implicated by findings of complement components CFH, C5b-9, and others within drusen, and increased risk for ARMD associated with variants in CFH, FB, and C3 genes (Gold et al., 2006; Hageman et al., 2005; Johnson et al., 2000; Yates et al., 2007). For comparison, atherosclerotic plaques contain many of these same molecules, and plaque progression is affected in mice deficient in these proteins (Oksjoki et al., 2007). Within the deep intima, the membrane attack complex C5b-9 co-localizes with modified LDL, appearing shortly after experimental diet-induced hypercholesterolemia (Seifert et al., 1989; Torzewski et al., 1998). Sub-endothelial LDL is thus considered an important activator of complement early in plaque development. That C5b-9 appears in normal BrM and drusen (Johnson et al., 2000; Seth et al., 2008) along with apoB-containing lipoproteins indicates that complement activation could occur by a similar route early in ARMD pathogenesis.
9.2. ARMD ≠ CAD
Like atherosclerotic plaques, drusen and basal deposits feature cholesterol and apoB deposition, and BrM, like arterial intima and other connective tissues, accumulates lipoprotein-derived EC with advanced age. Although knowledge about CAD can provide a powerful conceptual framework for developing new hypotheses about ARMD pathobiology, RPE physiology, and new therapeutic approaches (Curcio et al., 2001), these two complex multi-factorial diseases differ in important and ultimately informative ways, both at the level of the vessel wall and in patient populations.
9.2.1. At the vessel wall – similarities and differences
BrM and arterial intima have notable similarities (Sivaprasad et al., 2005): 1) similar extracellular matrix components (collagen, elastin, glycosaminoglycans including dermatan sulfate); 2) approximately 2–3 fold thickening with age; 3) decreased collagen solubility with age; 4) involvement of MMP-2 and MMP-9 in new vessel growth; 5) presence of TIMP-3, a regulator of matrix turnover; 6) significant age-related build-up of cellular debris and altered extracellular matrix; 7) age-related accumulation of apoB-containing lipoproteins rich in cholesteryl linoleate; and 8) common proteins accumulating in deposits including C5b-9, C3, C5, clusterin, AGE, apoB, and apoE.
But BrM and arterial intima also have notable differences: 1) BrM and intima likely have different major sources of neutral lipid-bearing lipoproteins (RPE vs liver and intestine via plasma); 2) absolute differences in thickness (2–4 µm for BrM, 100–300 µm for intima); 3) hemodynamics of a capillary bed (choriocapillaris vs muscular artery); 4) BrM, located between an endothelium and epithelium, is influenced by both cell types, whereas intima supports an endothelium only; 5) foam cells congregate distinctly in plaques but macrophages are associated primarily with neovascularization in ARMD; 6) smooth muscle cells, which make the fibrous cap of plaques, are scattered throughout the choroid and have an undefined role in ARMD; 7) fibrous collagens (types I and III) and elastin are major components of incipient lipid-rich plaque core but are absent from drusen and BlinD in ARMD; and 9) cholesterol crystals do not occur in BrM or sub-RPE lesions.
9.2.2. In patients – opposite effects (Table 5)
Table 5.
Age-related Macular Degeneration vs Coronary Artery Disease.
| Factor | Age-related maculopathy |
Coronary artery disease |
|---|---|---|
| ApoE4 genotype | ▼Risk | ▲Risk |
| Elevated plasma cholesterol | – | ▲Risk |
| Elevated plasma HDL | – | ▼Risk |
| Diabetes (Type 2) | – | ▲Risk |
| Statin therapy | – | Benefit |
| Anti-oxidant therapy | Benefit | – |
No effect.
If the deposition of neutral lipids and the formation of cholesterol-rich lesions in BrM were an ocular manifestation of a systemic process, then elevated plasma cholesterol should be a defining risk factor for ARMD, as it is for CAD (2001b; Verschuren et al., 1995). Yet since 1963, 26 studies measuring plasma cholesterol in ARMD patients provided largely inconclusive results (Dashti et al., 2006). Similar arguments can be made for HDL, considered protective against cardiovascular disease. Plasma apoB and apoA-I concentrations in ARMD patients, a measure now recognized as advantageous for cardiovascular risk analysis (Contois et al., 2009), are unrelated to ARMD stage (Dashti et al., 2006). Consistent with this view are the mixed outcomes of plasma lipid-lowering treatments (e.g., statins) on ARMD (Chuo et al., 2007) and the weak association of ARMD with Type 2 diabetes (Adiels et al., 2008; Klein et al., 2004).
The apoE4 genotype is well established as a modest but consistent risk factor for CAD, conferring decreased longevity and increased mortality (Smith, 2002), yet surprisingly protects against ARMD, reducing risk by 40% (Klaver et al., 1998; Thakkinstian et al., 2006). ApoE in human populations exhibits E2, E3, and E4 isoforms, of which E3 is the most common (Hatters et al., 2006). Isoforms differ from each other by Cys to Arg substitutions at positions 112 and 158, so that E2 has a neutral, and E4 a positive, charge. ApoE4 forms partially unfolded structures more readily than apoE3 or apoE2, allowing it to bind lipid particles like lipoproteins or lysosomal membranes with higher affinities or rates and rendering it more sensitive to proteolysis and degradation. Literature too vast to enumerate here relates effects of apoE isoforms on plasma LDL clearance, hepatic apoB production, and cholesterol efflux from intimal macrophages. The opposite polarity of apoE influence in ARMD and CAD could occur at any or all of these sites within RPE, which shares lipoprotein production and clearance pathways with hepatocytes and macrophages. The following scenario suggests one way in which apoE might have different effects in the two tissues. Cholesterol efflux into the intima prevents macrophages from differentiating into foam cells, and apoE4 hinders this efflux, thus exacerbating atherosclerosis (Cullen et al., 1998). In contrast, cholesterol exported from RPE within lipoproteins accumulates in BrM and promotes lesion formation and neovascularization. Perhaps the opposing effect of cellular cholesterol export in BrM and intima helps explain the opposite direction of apoE4’s influence in ARMD and CAD.
10. Future directions
10.1. Directions for laboratory research
The central role of RPE and BrM in ARMD pathogenesis has been recognized for almost 4 decades (Hogan, 1972). The studies reviewed herein have major implications for research in RPE cell biology, as information about the homeostasis of cholesterol and neutral lipid in this polarized epithelium and its supporting basement membrane is surprisingly sparse. While a connection between age-related lipid accumulation in BrM and ARMD was postulated 2 decades ago, many questions remain. Why does lipid start to accumulate in BrM and where does it come from? What is the mechanism of this accumulation? How does this accumulation initiate secondary processes that lead to ARMD? How are waste products from the RPE normally transported away? How can this lipid accumulation and/or its consequences be prevented?
10.1.1. RPE cell biology
The goal of new therapeutic approaches for ARMD may be best served by a comprehensive under standing of the RPE as a polarized, constitutive lipoprotein secretor. Here, ample work on hepatocytes, enterocytes, macrophages, cell lines, and mouse models can provide guidance to the critical questions about the apoB system. Recently developed high fidelity, polarized RPE cultures systems (Maminishkis et al., 2006) and tools permitting tissue-specific modulation of RPE gene expression in vivo (Le et al., 2008; Mori et al., 2002) will be essential.
Some outstanding issues about an RPE apoB system include: 1) Identifying the full range of input lipids to an RPE lipoprotein. Current evidence implicates plasma lipoproteins (Section 6.4.3) but which one(s), how it (they) gain(s) access to RPE, which are quantitatively dominant, and to what purpose, remain to be learned. This knowledge, in turn, would stimulate development of new methods that are both reliable and biologically justified for lipid-loading cultured RPE to elicit secretion. 2) Identifying the full range of RPE lipoprotein outputs. Further study may prove that RPE releases cholesterol by an apoB-dependent mechanism involving a large lipoprotein, apoB-independent mechanisms including ABCA1-mediated release to circulating HDL, and production of its own apoE-containing HDL (Iqbal et al., 2003; Zhang et al., 1996). 3) How an apoB-lipoprotein of RPE origin compares to those from other well-characterized apoB secretors. Specific points of interest are whether B-100 and B-48 is synthesized in RPE of various species, whether other apolipoproteins or proteins are present on secreted particles, what if any tissue-specific promoters drive expression in RPE, how assembly and secretion are regulated (e.g., via nuclear receptors or hormonal influences), how this pathway interacts with retinoid processing (Qtaishat et al., 2003), and how lipoprotein secretion forestalls lipotoxicity. 4) Defining the relationship of basolaterally directed apoB secretion to the apically-directed lipid transport systems to the neurosensory retina (Bazan et al., 1992; Gonzalez-Fernandez and Ghosh, 2008; Tserentsoodol et al., 2006a).
10.1.2. Animal models
The macula is a highly specialized area unique to humans and other primates, and thus far, other than humans, only macaque monkeys have been shown to accumulate constitutively neutral lipid in BrM (Anderson et al., 2006) and apoE in drusen (Umeda et al., 2005). A short-lived species that also displays these characteristics and permits in vivo tests of disease initiation remains a high priority research goal. Because ARMD and atherosclerosis share several key mechanisms, it is not surprising that eyes of established mouse and rabbit models of atherosclerosis have already been examined in pursuit of that goal.
Several general comments can be made about studies using mice. 1) Mice are naturally resistant to atherosclerosis and differ from humans in key aspects of lipoprotein metabolism. Rodents carry plasma cholesterol principally in HDL rather than LDL, they lack the cholesterol ester transfer protein, and they synthesize apoB-48 in hepatocytes as well as enterocytes (Sparks et al., 1981; Zak et al., 2002). Commonly used models are generated by genetically manipulating apolipoprotein and cholesterol processing pathways (Veniant et al., 2008). 2) The best described models are based on C57BL/6 mice and include knock outs of apoE or LDL-R (Dithmar et al., 2000; Ong et al., 2001; Rudolf et al., 2005; Schmidt-Erfurth et al., 2008) or transgenes of human apoB-100, or apoE (Bretillon et al., 2008a; Kliffen et al., 2000; Malek et al., 2005; Sallo et al., 2009). 3) Interpretation of studies using transgenic mice expressing lipoprotein-related genes are complicated by RPE expression of the same genes (Section 6.4.1), preventing definitive attribution of effects to plasma lipoproteins of hepatic/intestinal origin or to lipoproteins of RPE origin. The ocular changes seen in the animal models described below occur in the setting of hyperlipidemia, which is not associated with ARMD pathogenesis (Section 9.2.2). Future animal models that independently manipulate RPE- and plasma-derived lipoproteins, e.g., by using Cre-loxP technology to effect tissue-specific gene deletion (Le et al., 2008; Mori et al., 2002), will thus be highly informative. 4) Murine BrM is only 250 to ∼600 nm thick, presenting a challenge to studies of its morphology and composition. Transmission electron microscopy is often used, using tissue preparation steps that extract lipids. As a result lipids are commonly demonstrated indirectly as empty and round electron-lucent vesicles scattered through BrM (Dithmar et al., 2000; Rudolf et al., 2005; Sallo et al., 2009). One study has used the OTAP technique to neutral lipid-containing particles in inner BrM (Fujihara et al., 2009). Unresolved is whether partially extracted electron-dense material in BlamD is sub-optimally preserved neutral lipid or disintegrating basal RPE infoldings. 5) Histochemical detection of lipids in mouse BrM is also difficult because BrM is extremely thin and can be obscured by surrounding pigmentation, but it is possible to detect neutral lipid with oil red O or filipin (Bretillon et al., 2008a; Malek et al., 2005; Rudolf et al., 2005).
Studies of established atherosclerotic mouse models with hyperlipidemia have reported BrM changes comparable to alterations found in humans. These changes include thickening, loss of laminar architecture, and accumulation of apparent lipid vesicles (Dithmar et al., 2000; Fujihara et al., 2009; Kliffen et al., 2000; Rudolf et al., 2005; Schmidt-Erfurth et al., 2008). In apoE−/− and LDL-R−/− mice (Dithmar et al., 2000; Ong et al., 2001; Rudolf et al., 2005; Schmidt-Erfurth et al., 2008) or mice expressing human apoB-100 or apoE transgenes (Bretillon et al., 2008a; Fujihara et al., 2009; Kliffen et al., 2000; Malek et al., 2005; Sallo et al., 2009), main factors promoting BrM lipid deposition were advanced age and, in contrast to humans, elevated levels of plasma lipids. High-fat or high cholesterol diets have been used to provoke the desired lesions consistently (Dithmar et al., 2000; Edwards and Malek, 2007; Miceli et al., 2000; Ong et al., 2001; Rudolf et al., 2005). Mice expressing variants of the apoE gene exhibit among other things varying degrees of basal deposits (Dithmar et al., 2000; Kliffen et al., 2000; Malek et al., 2005). Blue light-exposed mice have increased BlamD-like material in the sub-RPE space (Cousins et al., 2002; Espinosa-Heidmann et al., 2004).
A hint as to why lipids get trapped in BrM may come from an apoB-transgenic mouse that also over-expresses biglycans (Sallo et al., 2009). Biglycan expression causes BrM thickening and is suspected of promoting BrM lipid accumulation by interacting with lipoprotein-associated proteins. Older mice expressing human apoE2, E3, or E4 isoforms and consuming a high-fat diet accumulate oil red O-binding material in the sub-RPE space (Malek et al., 2005). Surprisingly, ApoE4, which is protective in humans (Section 9.2.2.), produced the most severe effect of any ApoE allele, showing even spontaneous choroidal neovascularization (Malek et al., 2005).
Not only BrM is affected in these hypercholesterolemic mice, but also the tissues surrounding it. The RPE demonstrates vacuolization, loss of intracellular compartments, reduced cell height, and fewer apical microvilli (Miceli et al., 2000; Rudolf et al., 2005 Schmidt-Erfurth et al., 2008). Further, photoreceptors and the choriocapillary endothelium are compromised as well (Bretillon et al., 2008a; Schmidt-Erfurth et al., 2008). Degenerative ultra-structural changes in aged mice are associated with local oxidative stress measurable by increased presence of nitric oxide metabolites or lipid peroxidation (Sadaba et al., 2008). In these mice, such phenomena can lead to increased expression of ARMD-relevant stress-related growth factors like VEGF, the major stimulus for choroidal neovascularization (Rudolf et al., 2005; Schmidt-Erfurth et al., 2008).
Rabbits are also commonly used in atherosclerosis research, as they demonstrate hypercholesterolemia-inducible aortic lesions resembling human fatty streaks (Moghadasian, 2002). In this merangiotic species, only one vascular system (choroidal) serves the retina, unlike humans. Watanabe heritable hyperlipidemic and New Zealand rabbits after diet-induced hypercholesterolemia exhibit high levels of LDL cholesterol and lesions in the ocular posterior pole (Kouchi et al., 2006; Ramírez et al., 2006; Salazar et al., 2007; Triviño et al., 2006; Walton and Dunkerley, 1974). Principal changes appear in the choroid, with diffuse thickening and accumulation of lipid-rich macrophages resembling foam cells. Of note, the inner collagenous layer of BrM thickens, with electron dense and electron-lucent particles and BlamD, and the RPE acquires lipid inclusions. Changes in choroid, BrM, and RPE were partly reversible after plasma cholesterol levels are normalized (Ramírez et al., 2006; Salazar et al., 2007).
10.1.3. Model systems
Unlike age-related accumulation of cholesterol in other connective tissues, that which occurs in BrM is apparently unique in its potential impact on fluid and nutrient exchange, because of importance of this tissue to transport to and from the RPE. The new biochemical model for BrM lipid accumulation may facilitate the construction of and informed experimentation with in vitro model systems to study transport across natural and artificial matrices. Such systems could use readily available LDL as an acceptable surrogate because of its high EC content or VLDL because of its size similarity to the lipoproteins seen to accumulate in BrM. Use of such systems have already demonstrated that LDL can pass through bovine BrM (Huang et al., 2008b), although slowed in transit, and that LDL deposition significantly decreases the hydraulic conductivity of an extracellular matrix (McCarty et al., 2008). Future studies should focus on determining the mechanism by which lipid accumulates in BrM and impede transport.
There are several changes that might explain why lipoproteins begin to accumulate in BrM. These steps include changes in the extracellular matrix of BrM (e.g. non-enzymatic glycation (Cerami et al., 1986) or increased proteoglycans (Sallo et al., 2009)) can promote lipid accumulation, changes in the amount or character of lipoproteins that transit through BrM (e.g., oxidation (Chang et al., 2001; Wang et al., 2001) such that they are more likely to accumulate, or changes in the mechanism of lipoprotein clearance. Use of natural and artificial matrices would allow investigation of such possibilities (Huang et al., 2008b, 2009; McCarty et al., 2008).
Once trapped or restricted by the extracellular matrix, the process by which lipids continue to accumulate and lead to formation of a transport barrier is also unknown. It is unlikely that the lipid particles simply begin to accumulate in BrM, and this accumulation directly leads to the formation of a transport barrier like the Lipid Wall. Such a process would be expected to be nonlinear, with the initial accumulation being slow and latter stages accelerated as those lipids already trapped impede further transport of lipids. The age-related lipid accumulation rate in BrM is at best only slightly non-linear (Fig. 9A), inconsistent with this two-stage model. This suggests multiple processes (including clearance) that might be best studied by perfusing natural or artificial matrices with lipids and then using imaging techniques (e.g. QFDE, OTAP) to characterize this accumulation process.
10.2. Directions for clinical research
10.2.1. Dietary manipulation
The model presented herein provides a basis for designing dietary enhancement of RPE function via LDL or HDL uptake (Elner, 2002; Tserentsoodol et al., 2006a,b). Such knowledge will be critical for interpreting results emanating from the Age-related Eye Disease Study (http://www.nei.nih.gov/AREDS2), which will test the effects of dietary lutein and docosahexanoate supplementation on ARMD incidence and progression.
10.2.2. Refurbishing BrM for RPE cell replacement
RPE re-populates BrM poorly following surgical extirpation of choroidal neovascular membranes in older patients, unlike younger patients (Tezel et al., 2004). The RPE’s difficulty in attaching, growing, and servicing photoreceptors on aged BrM (Sun et al., 2007) complicates the prospects of RPE transplants in ARMD patients, leading to intensive efforts to optimize the substrate for RPE growth by refurbishing aged BrM (Del Priore and Tezel, 1998; Gullapalli et al., 2008; Tezel et al., 2004). The deleterious effect of lipid accumulation on transport across BrM (Section 7. 2 ) is another factor to consider in addition to the adhesive properties of the RPE basal lamina (inner sub-layer of BrM) (Afshari and Fawcett, 2009). Without more information about inputs to and purpose of an RPE apoB-lipoprotein, interfering with its production by RPE (e.g., by MTP inhibitors (Shoulders and Shelness, 2005) or apoB anti-sense (Soutschek et al., 2004) may be premature. However, agents that remove EC or LDL-like particles from BrM should certainly be sought and tested. In particular BrM can serve as a surgical bed for autologous grafts or stem cells to replace damaged RPE and photoreceptors (Binder et al., 2007), and thus refurbishing should include lipid sequestering or solubilizing using detergents (Tezel et al., 2004), neutral pH cholesterol esterases (Holm and Osterlund, 1999), reconstituted HDL (Chung et al., 2005), apolipoprotein mimetics (White et al., 2009) and bioremediation (Rittmann and Schloendorn, 2007).
10.2.3. Imaging ARMD in patients
Subtle features in druse structure seen in living patients, whether by biomicroscopy or dye angiography, have been attributed to differences in the composition of druse interiors and exteriors (Bird and Marshall, 1986; Pauleikhoff et al., 1992). Neutral lipids identified in drusen (Wolter and Falls, 1962) (Section 8.1) were postulated to account for the variable staining during clinical angiography by the hydrophilic dye fluorescein (Pauleikhoff et al., 1992). Spectral domain optical coherence tomography is fast approaching the ability to reveal druse contents (Khanifar et al., 2008). The ubiquity and abundance of cholesterol in drusen suggests that methods capable of revealing cholesterol forms or lipoproteins in atherosclerotic plaques (e.g., magnetic resonance spectroscopy) (Ruberg et al., 2006; Yuan et al.,1997)) may someday disclose details of druse evolution and provide a biochemical basis for the increased risk indicated by these structures. Finally, the distinctly different compositions of BlinD and BlamD should enable their eventual visualization in living patients.
10.3. Conclusion
We have presented a body of work strongly implicating the RPE as constitutive secretor of EC-rich apoB-lipoproteins which when retained and accumulated in BrM contributes importantly to impeding RPE and photoreceptor function and to forming the specific lesions of ARMD. The conceptual framework, borrowed heavily from decades of atherosclerosis research, provides a wide knowledge base and sophisticated clinical armamentarium that can be readily exploited for the benefit of ARMD patients.
Acknowledgments
We are grateful for support from NIH grants EY06109 and EY014662, International Retinal Research Foundation, American Health Assistance Foundation, EyeSight Foundation of Alabama, Research to Prevent Blindness, Inc., Macula Vision Research Foundation, Roger Johnson Prize in Macular Degeneration Research, and Deutsche Forschungsgemeinschaft. CAC thanks especially the Alabama Eye Bank, members of Lipid Breakfast (Atherosclerosis Research Unit, J.P. Segrest, MD, PhD, Director, Department of Medicine, UAB), Lanning B. Kline, MD, Peter A. Dudley, PhD, John Guyton, MD, Howard Kruth, MD, and Clyde Guidry, PhD. David Fisher provided graphical design.
Abbreviations
- ARMD
age-related macular degeneration
- apo
apolipoprotein
- BlamD
basal laminar deposit
- BlinD
basal linear deposit
- BrM
Bruch’s membrane
- CAD
coronary artery disease
- CM
chylomicron
- EC
esterified cholesterol
- LDL
low density lipoprotein
- MTP
microsomal triglyceride transfer protein
- PL
phospholipid
- RPE
retinal pigment epithelium
- TG
triglyceride
- UC
unesterified cholesterol
- VLDL
very low density lipoprotein
Footnotes
We use these terms for fatty acids. Chemical notation: CXX:Yn-Z, where XX is carbon chain length, Y is the number of double bonds along the carbon chain, and Z is the position of the first double bond, counting from the methyl carbon. Saturated, no double bonds. Unsaturated, one or more double bonds.
Acyl coenzyme A cholesterol acyl transferase 1, 2 is also called sterol-O-acyl-transferase 1, 2 (SOAT).
We omit from this section clusterin, a widely expressed, multifunctional 70 kDa glycoprotein whose strong association with plasma HDL earned it the name of apolipoprotein J (de Silva et al., 1990; Vaisar et al., 2007). Although secreted by RPE and present in drusen, it is not universally considered a true apo (Alaupovic, 2003), so it is not discussed further here.
Hydraulic conductivity is reported rather than resistivity for consistency with previous literature.
References
- A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. AREDS Report No. 8. Arch. Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Adams CWM, Bayliss OB. Lipid histochemistry. In: Glick D, Rosenbaum RM, editors. Techniques of Biochemical and Biophysical Morphology. New York: John Wiley and Sons; 1975. pp. 100–156. [Google Scholar]
- Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- Afshari FT, Fawcett JW. Improving RPE adhesion to Bruch’s membrane. Eye. 2009 doi: 10.1038/eye.2008.411. [DOI] [PubMed] [Google Scholar]
- Alaupovic P. The concept of apolipoprotein-defined lipoprotein families and its clinical significance. Curr. Atheroscler. Rep. 2003;5:459–467. doi: 10.1007/s11883-003-0036-8. [DOI] [PubMed] [Google Scholar]
- Anderson LJ, Boyles JK, Hussain MM. A rapid method for staining large chylomicrons. J. Lipid Res. 1989;30:1819–1824. [PubMed] [Google Scholar]
- Anderson DH, Ozaki S, Nealon M, Neitz J, Mullins RF, Hageman GS, Johnson LV. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am. J. Ophthalmol. 2001;131:767–781. doi: 10.1016/s0002-9394(00)00961-2. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp. Eye Res. 2004;78:243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Anderson MD, Dawson WW, Martinez-Gonzalez J, Curcio CA. Drusenoid lesions and lipid-filled retinal pigment epithelium cells in a rhesus macula. Vet. Ophthalmol. 2006;9:201–207. doi: 10.1111/j.1463-5224.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- Athar H, Iqbal J, Jiang XC, Hussain MM. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 2004;45:764–772. doi: 10.1194/jlr.D300026-JLR200. Epub 2004 Feb 2001. [DOI] [PubMed] [Google Scholar]
- Bairaiti A, Orzalesi N. The ultrastructure of the pigment epithelium and of the photoreceptor-pigment epithelium interface. J. Ultrastruct. Res. 1963;9:484–496. doi: 10.1016/s0022-5320(63)80080-5. [DOI] [PubMed] [Google Scholar]
- Ball RY, Stowers EC, Burton JH, Cary NR, Skepper JN, Mitchinson MJ. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-s. [DOI] [PubMed] [Google Scholar]
- Bazan HEP, Bazan NG, Feeney-Burns L, Berman ER. Lipids in human lipofuscin-enriched subcellular fractions of two age population. Invest. Ophthalmol. Vis. Sci. 1990;31:1433–1443. [PubMed] [Google Scholar]
- Bazan NG, Gordon WC, Rodriguez de Turco EB. Docosahexaenoic acid uptake and metabolism in photoreceptors: retinal conservation by an efficient retinal pigment epithelial cell-mediated recycling process. Adv. Exp. Med. Biol. 1992;318:295–306. doi: 10.1007/978-1-4615-3426-6_26. [DOI] [PubMed] [Google Scholar]
- Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglyceride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 2000;20:663–697. doi: 10.1146/annurev.nutr.20.1.663. [DOI] [PubMed] [Google Scholar]
- Binder S, Stanzel BV, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog. Retin. Eye Res. 2007;26:516–554. doi: 10.1016/j.preteyeres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Bird AC, Marshall J. Retinal pigment epithelial detachments in the elderly. Trans. Ophthalmol. Soc. UK. 1986;105:674–682. [PubMed] [Google Scholar]
- Björkegren J, Veniant M, Kim SK, Withycombe SK, Wood PA, Hellerstein MK, Neese RA, Young SG. Lipoprotein secretion and triglyceride stores in the heart. J. Biol. Chem. 2001;276:38511–38517. doi: 10.1074/jbc.M106839200. [DOI] [PubMed] [Google Scholar]
- Björkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb. Vasc. Biol. 2004;5:5. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- Bocan TM, Schifani TA, Guyton JR. Ultrastructure of the human aortic fibrolipid lesion. Formation of the atherosclerotic lipid-rich core. Am. J. Pathol. 1986;123:413–424. [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Fliesler SJ, Albert AD. Relationship of cholesterol content to spatial distribution and age of disk membranes in retinal rod outer segments. J. Biol. Chem. 1990;265:18867–18870. [PMC free article] [PubMed] [Google Scholar]
- Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR. Clinicopathological correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994;14:130–142. [PubMed] [Google Scholar]
- Bretillon L, Diczfalusy U, Bjorkhem I, Maire MA, Martine L, Joffre C, Acar N, Bron A, Creuzot-Garcher C. Cholesterol-24S-hydroxylase (CYP46A1) is specifically expressed in neurons of the neural retina. Curr. Eye Res. 2007;32:361–366. doi: 10.1080/02713680701231857. [DOI] [PubMed] [Google Scholar]
- Bretillon L, Acar N, Seeliger MW, Santos M, Maire MA, Juaneda P, Martine L, Gregoire S, Joffre C, Bron AM, Creuzot-Garcher C. ApoB100, LDLR−/−mice exhibit reduced electroretinographic response and cholesteryl esters deposits in the retina. Invest. Ophthalmol. Vis. Sci. 2008a;49:1307–1314. doi: 10.1167/iovs.07-0808. [DOI] [PubMed] [Google Scholar]
- Bretillon L, Thuret G, Gregoire S, Acar N, Joffre C, Bron AM, Gain P, Creuzot-Garcher CP. Lipid and fatty acid profile of the retina, retinal pigment epithelium/choroid, and the lacrimal gland, and associations with adipose tissue fatty acids in human subjects. Exp. Eye Res. 2008b;87:521–528. doi: 10.1016/j.exer.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Broekhuyse RM. Lipids in tissues of the eye. VII. Changes in concentration and composition of sphingomyelins, cholesterol esters and other lipids in aging sclera. Biochim. Biophys. Acta. 1972;280:637–645. [PubMed] [Google Scholar]
- Burtis CA, Ashwood ER. Philadelphia: W.B. Saunders; 1999. Tietz Textbook of Clinical Chemistry. [Google Scholar]
- Camejo G, Hurt-Camejo E, Wiklund O, Bondjers G. Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis. 1998;139:205–222. doi: 10.1016/s0021-9150(98)00107-5. [DOI] [PubMed] [Google Scholar]
- Canter JA, Olson LM, Spencer K, Schnetz-Boutaud N, Anderson B, Hauser MA, Schmidt S, Postel EA, Agarwal A, Pericak-Vance MA, et al. Mito-chondrial DNA polymorphism A4917G is independently associated with age-related macular degeneration. PLoS One. 2008;3:e2091. doi: 10.1371/journal.pone.0002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaroli-Marano RP, Peinado-Onsurbe J, Reina M, Staels B, Auwerx J, Vilaro S. Lipoprotein lipase in highly vascularized structures of the eye. J. Lipid Res. 1996;37:1037–1044. [PubMed] [Google Scholar]
- Cavelier C, Lorenzi I, Rohrer L, von Eckardstein A. Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim. Biophys. Acta. 2006;1761:655–666. doi: 10.1016/j.bbalip.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Cerami A, Vlassara H, Brownlee M. Role of nonenzymatic glycosylation in atherogenesis. J. Cell Biochem. 1986;30:111–120. doi: 10.1002/jcb.240300203. [DOI] [PubMed] [Google Scholar]
- Chang MY, Potter-Perigo S, Wight TN, Chait A. Oxidized LDL bind to nonproteoglycan components of smooth muscle extracellular matrices. J. Lipid Res. 2001;42:824–833. [PubMed] [Google Scholar]
- Chao FF, Blanchette-Mackie E, Chen Y-J, Dickens BF, Berlin E, Amende LM, Skarlatos SI, Gamble W, Resau JH, Mergner WT, Kruth HS. Characterization of two unique cholesterol-rich lipid particles isolated from human atherosclerotic lesions. Am. J. Pathol. 1990;136:169–179. [PMC free article] [PubMed] [Google Scholar]
- Chen H, Anderson RE. Lipids of frog retinal pigment epithelium: comparison with rod outer segments, retina, plasma and red blood cells. Curr. Eye Res. 1992;11:793–800. doi: 10.3109/02713689209000752. [DOI] [PubMed] [Google Scholar]
- Chowers I, Banin E, Merin S, Cooper M, Granot E. Long-term assessment of combined vitamin A and E treatment for the prevention of retinal degeneration in abetalipoproteinaemia and hypobetalipoproteinaemia patients. Eye. 2001;15:525–530. doi: 10.1038/eye.2001.167. [DOI] [PubMed] [Google Scholar]
- Chuang EL, Bird AC. Bilaterality of tears of the retinal pigment epithelium. Br. J. Ophthalmol. 1988;72:918–920. doi: 10.1136/bjo.72.12.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung BH, Tallis G, Yalamoori V, Anantharamaiah GM, Segrest JP. Liposome-like particles isolated from human atherosclerotic plaques are structurally and compositionally similar to surface remnants of triglyceride-rich lipoproteins. Arterioscler Thromb. 1994;14:622–635. doi: 10.1161/01.atv.14.4.622. [DOI] [PubMed] [Google Scholar]
- Chung BH, Mishra V, Franklin F, Liang P, Doran S, Curcio CA. Phosphatidylcholine-rich acceptors, but not native HDL or its apolipoproteins, mobilize cholesterol from cholesterol-rich insoluble components of human atherosclerotic plaques. Biochem. Biophys. Acta Mol. Cell Biol. Lipids. 2005;1733:76–89. doi: 10.1016/j.bbalip.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Chuo JY, Wiens M, Etminan M, Maberley DA. Use of lipid-lowering agents for the prevention of age-related macular degeneration: a meta-analysis of observational studies. Ophthalmic Epidemiol. 2007;14:367–374. doi: 10.1080/09286580701421684. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Rosenfeld PJ. Antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. Curr. Opin. Ophthalmol. 2009;20:158–165. doi: 10.1097/ICU.0b013e32832d25b3. [DOI] [PubMed] [Google Scholar]
- Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Connor WE, Duell PB, Kean R, Wang Y. The prime role of HDL to transport lutein into the retina: evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Invest. Ophthalmol. Vis. Sci. 2007;48:4226–4231. doi: 10.1167/iovs.06-1275. [DOI] [PubMed] [Google Scholar]
- Contois JH, McConnell JP, Sethi AA, Csako G, Devaraj S, Hoefner DM, Warnick GR. Apolipoprotein B and cardiovascular disease risk: position statement from the AACC lipoproteins and vascular diseases Division working group on best Practices. Clin. Chem. 2009;55:407–419. doi: 10.1373/clinchem.2008.118356. [DOI] [PubMed] [Google Scholar]
- Cousins SW, Espinosa-Heidmann DG, Alexandridou A, Sall J, Dubovy S, Csaky K. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp. Eye Res. 2002;75:543–553. doi: 10.1006/exer.2002.2047. [DOI] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispin S. Ocular lipid deposition and hyperlipoproteinaemia. Prog. Retin. Eye Res. 2002;21:169–224. doi: 10.1016/s1350-9462(02)00004-6. [DOI] [PubMed] [Google Scholar]
- Cullen P, Cignarella A, Brennhausen B, Mohr S, Assman G, von Eckardstein A. Phenotype-dependent differences in apolipoprotein E metabolism and cholesterol homeostasis in human monocyte-derived macrophages. J. Clin. Invest. 1998;101:1670–1677. doi: 10.1172/JCI119887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA. Imaging maculopathy in the post-mortem human retina. Vis. Res. 2005;45:3496–3503. doi: 10.1016/j.visres.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Medeiros NE, Millican CL. The Alabama age-related macular degeneration grading system for donor eyes. Invest. Ophthalmol. Vis. Sci. 1998;39:1085–1096. [PubMed] [Google Scholar]
- Curcio CA, Saunders PL, Younger PW, Malek G. Peripapillary chorioretinal atrophy: bruch’s membrane changes and photoreceptor loss. Ophthalmology. 2000;107:334–343. doi: 10.1016/s0161-6420(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch’s membrane. Invest. Ophthalmol. Vis. Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- Curcio CA, Presley JB, Medeiros NE, Malek G, Avery DV, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp. Eye Res. 2005a;81(6):731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Presley JB, Millican CL, Medeiros NE. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp. Eye Res. 2005b;80:761–775. doi: 10.1016/j.exer.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch. Ophthalmol. 1999;117:329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- Dashti N, McGwin G, Jr, Owsley C, Curcio CA. Plasma apolipoproteins and risk for age-related maculopathy. Br. J. Ophthalmol. 2006;90:1028–1033. doi: 10.1136/bjo.2006.093856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation. 1996;94:2013–2020. doi: 10.1161/01.cir.94.8.2013. [DOI] [PubMed] [Google Scholar]
- Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, Ferris FL, Bressler SB, Milton RC. The age-related eye disease study severity scale for age-related macular degeneration: AREDS report No. 17. Arch. Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Priore LV, Kuo Y-H, Tezel TH. Age-related changes in human RPE cell density and apoptosis proportion in situ. Invest. Ophthalmol. Vis. Sci. 2002;43:3312–3318. [PubMed] [Google Scholar]
- Del Priore LV, Tezel TH. Reattachment rate of human retinal pigment epithelium to layers of human Bruch’s membrane. Arch. Ophthalmol. 1998;116:335–341. doi: 10.1001/archopht.116.3.335. [DOI] [PubMed] [Google Scholar]
- Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- Dithmar S, Curcio C, Le N-A, Brown S, Grossniklaus H. Ultrastructural changes in Bruch’s membrane of apolipoprotein E-deficient mice. Invest. Ophthalmol. Vis. Sci. 2000;41:2035–2042. [PubMed] [Google Scholar]
- Dougan SK, Rava P, Hussain MM, Blumberg RS. MTP regulated by an alternate promoter is essential for NKT cell development. J. Exp. Med. 2007;204:533–545. doi: 10.1084/jem.20062006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KG, Bailey KR, Kane JP, Schwartz DM. Human retinal pigment epithelial cells express scavenger receptors BI and BII. Biochem. Biophys. Res. Commun. 2002;292:1017–1022. doi: 10.1006/bbrc.2002.6756. [DOI] [PubMed] [Google Scholar]
- During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared to beta -carotene by retinal cells via a scavenger receptor BI-dependent mechanism. J. Lipid Res. 2008;49:1715–1724. doi: 10.1194/jlr.M700580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart L, Bach J, Ban J, Tschachler E. Melanin binds reversibly to thermostable DNA polymerase and inhibits its activity. Biochem. Biophys. Res. Commun. 2000;271:726–730. doi: 10.1006/bbrc.2000.2716. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Malek G. Molecular genetics of AMD and current animal models. Angiogenesis. 2007;10:119–132. doi: 10.1007/s10456-007-9064-2. [DOI] [PubMed] [Google Scholar]
- El Baba F, Green WR, Fleischmann J, Finkelstein D, de la Cruz ZC. Clin-icopathologic correlation of lipidization and detachment of the retinal pigment epithelium. Am. J. Ophthalmol. 1986;101:576–583. doi: 10.1016/0002-9394(86)90948-7. [DOI] [PubMed] [Google Scholar]
- Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- Elner VM. Retinal pigment epithelial acid lipase activity and lipoprotein receptors: effects of dietary omega-3 fatty acids. Trans. Am. Ophthalmol. Soc. 2002;100:301–338. [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Sall J, Hernandez EP, Cousins SW. Basal laminar deposit formation in APO B100 transgenic mice: complex interactions between dietary fat, blue light, and vitamin E. Invest. Ophthalmol. Vis. Sci. 2004;45:260–266. doi: 10.1167/iovs.03-0910. [DOI] [PubMed] [Google Scholar]
- Ethier CR, Johnson M, Ruberti J. Ocular biomechanics and biotransport. Annu. Rev. Biomed. Eng. 2004;6:249–273. doi: 10.1146/annurev.bioeng.6.040803.140055. [DOI] [PubMed] [Google Scholar]
- Farese RV, Jr, Ruland SL, Flynn LM, Stokowski RP, Young SG. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc. Natl. Acad. Sci. U S A. 1995;92:1774–1778. doi: 10.1073/pnas.92.5.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariss RN, Apte SS, Olsen BR, Iwata K, Milam AH. Tissue inhibitor of metalloproteinases-3 is a component of Bruch’s membrane of the eye. Am. J. Pathol. 1997;150:323–328. [PMC free article] [PubMed] [Google Scholar]
- Feeney-Burns L, Ellersieck MR. Age-related changes in the ultrastructure of Bruch’s membrane. Am. J. Ophthalmol. 1985;100:686–697. doi: 10.1016/0002-9394(85)90625-7. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L, Malinow R, Klein ML, Neuringer M. Maculopathy in cynomologus monkeys. A correlated fluorescein angiographic and ultrastructural study. Arch. Ophthalmol. 1981;99:664–672. doi: 10.1001/archopht.1981.03930010664013. [DOI] [PubMed] [Google Scholar]
- Fijalkowski N, Fujihara M, Nagai N, Wu T, Tian J, Bartels E, Nielsen LB, Handa JT. Transgenic mice that express human apolipoprotein B100 (ApoB100) in the retinal pigmented epithelium (RPE) and liver Develop phenotypic features of early age-related macular degeneration (AMD) Invest. Ophthalmol. Vis. Sci. 2009;50:e774. [Google Scholar]
- Fine BS, Kwapien RP. Pigment epithelial windows and drusen: an animal model. Invest. Ophthalmol. Vis. Sci. 1978;17:1059–1068. [PubMed] [Google Scholar]
- Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc. Natl. Acad. Sci. U S A. 2002;99:3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Progr Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Keller RK. Isoprenoid metabolism in the vertebrate retina. Int. J. Biochem. Cell Biol. 1997;29:877–894. doi: 10.1016/s1357-2725(97)00018-6. [DOI] [PubMed] [Google Scholar]
- Forte TM, Nordhausen RW. Electron microscopy of negatively stained lipoproteins. Methods Enzymol. 1986;128:442–457. doi: 10.1016/0076-6879(86)28086-6. [DOI] [PubMed] [Google Scholar]
- Frank JS, Fogelman AM. Ultrastructure of the intima in WHHL and cholesterol-fed rabbit aortas prepared by ultra-rapid freezing and freezeetching. J. Lipid Res. 1989;30:967–978. [PubMed] [Google Scholar]
- Friedman E. Update of the vascular model of AMD. Br. J. Ophthalmol. 2004;88:161–163. doi: 10.1136/bjo.2003.036277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichson T, Kalbach HL, Buck P, van Kuijk FJGM. Vitamin E in macular and peripheral tissues of the human eye. Curr. Eye Res. 1995;14:693–701. doi: 10.3109/02713689508998497. [DOI] [PubMed] [Google Scholar]
- Fujihara M, Bartels ED, Nielsen LB, Handa JT. A human apoB100 transgenic mouse expresses human apoB100 in the RPE and develops features of early AMD. Exp. Eye Res. 2009;88:1115–1123. doi: 10.1016/j.exer.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor PM, Zhang WY, Salehizadeh B, Pettiford B, Kruth HS. Cholesterol accumulation in human cornea: evidence that extracellular cholesteryl ester-rich lipid particles deposit independently of foam cells. J. Lipid Res. 1996;37:1849–1861. [PubMed] [Google Scholar]
- Giambernardi TA, Rodeck U, Klebe RJ. Bovine serum albumin reverses inhibition of RT-PCR by melanin. Biotechniques. 1998;25:564–566. doi: 10.2144/98254bm03. [DOI] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 2009 doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez F, Ghosh D. Focus on Molecules: interphotoreceptor retinoid-binding protein (IRBP) Exp. Eye Res. 2008;86:169–170. doi: 10.1016/j.exer.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Gordiyenko N, Campos M, Lee JW, Fariss RN, Sztein J, Rodriguez IR. RPE cells internalize low-density lipoprotein (LDL) and oxidized LDL (oxLDL) in large quantities in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 2004;45:2822–2829. doi: 10.1167/iovs.04-0074. [DOI] [PubMed] [Google Scholar]
- Gordon DA, Jamil H, Sharp D, Mullaney D, Yao Z, Gregg RE, Wetterau J. Secretion of apolipoprotein B-containing lipoproteins from HeLa cells is dependent on expression of the microsomal triglyceride transfer protein and is regulated by lipid availability. Proc. Natl. Acad. Sci. U S A. 1994;91:7628–7632. doi: 10.1073/pnas.91.16.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DA, Jamil H. Progress towards understanding the role of microsomal triglyceride transfer protein in apolipoprotein-B lipoprotein assembly. Biochim. Biophys. Acta. 2000;1486:72–83. doi: 10.1016/s1388-1981(00)00049-4. [DOI] [PubMed] [Google Scholar]
- Grant CA, Berson EL. Treatable forms of retinitis pigmentosa associated with systemic neurological disorders. Int. Ophthalmol. Clin. 2001;41:103–110. doi: 10.1097/00004397-200101000-00010. [DOI] [PubMed] [Google Scholar]
- Green WR, Enger C. Age-related macular degeneration histopathologic studies: the 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Grindle CFJ, Marshall J. Ageing changes in Bruch’s membrane and their functional implications. Trans. Ophthalmol. Soc. U K. 1978;98:172–175. [PubMed] [Google Scholar]
- Grisanti S, Tatar O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog. Retin. Eye Res. 2008;27:372–390. doi: 10.1016/j.preteyeres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Green WR. Choroidal neovascularization. Am. J. Ophthalmol. 2004;137:496–503. doi: 10.1016/j.ajo.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Gülcan H, Alvarez R, Maude M, Anderson R. Lipids of human retina, retinal pigment epithelium, and Bruch’s membrane/choroid: comparison of macular and peripheral regions. Invest. Ophthalmol. Vis. Sci. 1993;34:3187–3193. [PubMed] [Google Scholar]
- Gullapalli VK, Sugino IK, Zarbin MA. Culture-induced increase in alpha integrin subunit expression in retinal pigment epithelium is important for improved resurfacing of aged human Bruch’s membrane. Exp. Eye Res. 2008;86:189–200. doi: 10.1016/j.exer.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Guyton JR, Bocan TMA, Schifani TA. Quantitative ultrastructural analysis of perifibrous lipid and its association with elastin in nonatherosclerotic human aorta. Arteriosclerosis. 1985;5:644–652. doi: 10.1161/01.atv.5.6.644. [DOI] [PubMed] [Google Scholar]
- Guyton JR, Klemp KF. Ultrastructural discrimination of lipid droplets and vesicles in atherosclerosis: value of osmium-thiocarbohydrazide-osmium and tannic acid-paraphenylenediamine techniques. J. Histochem. Cytochem. 1988;36:1319–1328. doi: 10.1177/36.10.2458408. [DOI] [PubMed] [Google Scholar]
- Guyton JR, Klemp KF. The lipid-rich core region of human atherosclerotic fibrous plaques. Prevalence of small lipid droplets and vesicles by electron microscopy. Am. J. Pathol. 1989;134:705–717. [PMC free article] [PubMed] [Google Scholar]
- Guyton JR, Klemp KF. Transitional features in human atherosclerosis. Intimal thickening, cholesterol clefts, and cell loss in human aortic fatty streaks. Am. J. Pathol. 1993;143:1444–1457. [PMC free article] [PubMed] [Google Scholar]
- Guyton JR, Klemp KR. Development of the lipid-rich core in human atherosclerosis. Arterioscler Thromb. 1996;16:4–11. doi: 10.1161/01.atv.16.1.4. [DOI] [PubMed] [Google Scholar]
- Hacquebard M, Carpentier YA. Vitamin E: absorption, plasma transport and cell uptake. Curr. Opin. Clin. Nutr. Metab. Care. 2005;8:133–138. doi: 10.1097/00075197-200503000-00005. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Mullins RG, Russell SR, Johnson LV, Anderson DH. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999;13:477–484. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, Chong NHC, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Progr Ret Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovici R, Gantz DL, Rumelt S, Freddo TF, Small DM. The lipid composition of drusen, Bruch’s membrane, and sclera by hot stage polarizing microscopy. Invest. Ophthalmol. Vis. Sci. 2001;42:1592–1599. [PubMed] [Google Scholar]
- Handa JT. New molecular histopathologic insights into the pathogenesis of age-related macular degeneration. Int. Ophthalmol. Clin. 2007;47:15–50. doi: 10.1097/IIO.0b013e31802bd546. [DOI] [PubMed] [Google Scholar]
- Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, Miyata T, Hjelmeland LM. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Invest. Ophthalmol. Vis. Sci. 1999;40:775–779. [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem. Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Havel RJ, Kane JP. Introduction: structure and metabolism of plasma lipoproteins. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill; 2001. pp. 2707–2716. [Google Scholar]
- Hayes KC, Lindsey S, Stephan ZF, Brecker D. Retinal pigment epithelium possesses both LDL and scavenger receptor activity. Invest. Ophthalmol. Vis. Sci. 1989;30:225–232. [PubMed] [Google Scholar]
- Hayward C, Shu X, Cideciyan AV, Lennon A, Barran P, Zareparsi S, Sawyer L, Hendry G, Dhillon B, Milam AH, et al. Mutation in a short-chain collagen gene, CTRP5, results in extracellular deposit formation in late-onset retinal degeneration: a genetic model for age-related macular degeneration. Hum. Mol. Genet. 2003;12:2657–2667. doi: 10.1093/hmg/ddg289. [DOI] [PubMed] [Google Scholar]
- Hewitt TA, Nakasawa K, Newsome DA. Analysis of newly synthesized Bruch’s membrane proteoglycans. Invest. Ophthalmol. Vis. Sci. 1989;30:478–486. [PubMed] [Google Scholar]
- Hillenkamp J, Hussain AA, Jackson TL, Cunningham JR, Marshall J. Taurine uptake by human retinal pigment epithelium: implications for the transport of small solutes between the choroid and the outer retina. Invest. Ophthalmol. Vis. Sci. 2004;45:4529–4534. doi: 10.1167/iovs.04-0919. [DOI] [PubMed] [Google Scholar]
- Hirosawa K, Yamada E. Localization of vitamin A in the mouse retina as revealed by autoradiography; Paper presented at: The structure of the Eye, Third Symposium (Tokyo, Japanese Journal of Ophthalmology).1976. [Google Scholar]
- Hogan MJ. Role of the retinal pigment epithelium in macular disease. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1972;76:64–80. [PubMed] [Google Scholar]
- Hogan MJ, Alvarado JA, Weddell JE. An Atlas and Textbook. Philadelphia: W.B. Saunders; 1971. Histology of the Human Eye. [Google Scholar]
- Holm C, Osterlund T. Hormone-sensitive lipase and neutral cholesteryl ester lipase. Methods Mol. Biol. 1999;109:109–121. doi: 10.1385/1-59259-581-2:109. [DOI] [PubMed] [Google Scholar]
- Holz FG, Sheraidah G, Pauleikhoff D, Bird AC. Analysis of lipid deposits extracted from human macular and peripheral Bruch’s membrane. Arch. Ophthalmol. 1994;112:402–406. doi: 10.1001/archopht.1994.01090150132035. [DOI] [PubMed] [Google Scholar]
- Huang J-D. Aging Bruch’s Membrane. Evanston, Illinois: Northwestern University; 2007. Lipid accumulation. [Google Scholar]
- Huang J-D, Chimento MF, Presley JB, Siddik Z, Curcio CA, Johnson M. Lipoprotein-like particles represent a major portion of lipids found in human macular Bruch’s membrane (BrM) Invest. Ophthalmol. Vis. Sci. E-Abstract. 2185 [Google Scholar]
- Huang J-D, Presley JB, Chimento MF, Curcio CA, Johnson M. Age-related changes in human macular Bruch’s membrane as seen by quick-freeze/ deep-etch. Exp. Eye Res. 2007b;85:202–218. doi: 10.1016/j.exer.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J-D, Curcio CA, Johnson M. Morphometric analysis of lipoprotein-like particle accumulation in aging human macular Bruch’s membrane. Invest. Ophthalmol. Vis. Sci. 2008a;49:2721–2727. doi: 10.1167/iovs.07-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J-D, Kruth HS, Johnson M. Perfusion of low-density lipoprotein through Bruch’s membrane. Invest. Ophthalmol. Vis. Sci. 2008b;49 E-1752. [Google Scholar]
- Huang J-D, Kruth HS, Johnson M. Transport of low-density lipoproteins through Bruch’s membrane and choroid. under review. 2009 doi: 10.1016/j.exer.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain AA, Rowe L, Marshall J. Age-related alterations in the diffusional transport of amino acids across the human Bruch’s-choroid complex. J. Opt. Soc. Am. A. 2002;19:166–172. doi: 10.1364/josaa.19.000166. [DOI] [PubMed] [Google Scholar]
- Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- Hussain MM, Rava P, Pan X, Dai K, Dougan SK, Iqbal J, Lazare F, Khatun I. Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr. Opin. Lipidol. 2008;19:277–284. doi: 10.1097/MOL.0b013e3282feea85. [DOI] [PubMed] [Google Scholar]
- Iglesias A, Arranz M, Alvarez JJ, Perales J, Villar J, Herrera E, Lasuncion MA. Cholesteryl ester transfer activity in liver disease and cholestasis, and its relation with fatty acid composition of lipoprotein lipids. Clin. Chim. Acta. 1996;248:157–174. doi: 10.1016/0009-8981(95)06251-3. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Anwar K, Hussain MM. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J. Biol. Chem. 2003;278:31610–31620. doi: 10.1074/jbc.M301177200. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Patterson R, Ohnishi Y, Inomata H, Ryan SJ. Formation of drusen in the human eye. Am. J. Ophthalmol. 1986a;101:342–353. doi: 10.1016/0002-9394(86)90830-5. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Sorgente N, Patterson R, Ryan SJ. Pathogenesis of drusen in the primate. Invest. Ophthalm Vis. Sci. 1986b;27:184–193. [PubMed] [Google Scholar]
- Ishida BY, Bailey KR, Duncan KG, Chalkley RJ, Burlingame AL, Kane JP, Schwartz DM. Regulated expression of apolipoprotein E by human retinal pigment epithelial cells. J. Lipid Res. 2004;45:263–271. doi: 10.1194/jlr.M300306-JLR200. [DOI] [PubMed] [Google Scholar]
- Ishida BY, Duncan KG, Bailey KR, Kane JP, Schwartz DM. High density lipoprotein mediated lipid efflux from retinal pigment epithelial cells in culture. Br. J. Ophthalmol. 2006;90:616–620. doi: 10.1136/bjo.2005.085076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GR, Curcio CA, Sloan KR, Owsley C. Photoreceptor degeneration in aging and age-related maculopathy. In: Penfold PL, Provis JM, editors. Macular Degeneration. Berlin: Springer-Verlag; 2005. pp. 45–62. [Google Scholar]
- Jamil H, Dickson JK, Jr, Chu CH, Lago MW, Rinehart JK, Biller SA, Gregg RE, Wetterau JR. Microsomal triglyceride transfer protein. Specificity of lipid binding and transport. J. Biol. Chem. 1995;270:6549–6554. doi: 10.1074/jbc.270.12.6549. [DOI] [PubMed] [Google Scholar]
- Javitt NB. Oxysterols: novel biologic roles for the 21st century. Steroids. 2008;73:149–157. doi: 10.1016/j.steroids.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Joffre C, Leclere L, Buteau B, Martine L, Cabaret S, Malvitte L, Acar N, Lizard G, Bron A, Creuzot-Garcher C, Bretillon L. Oxysterols induced inflammation and oxidation in primary porcine retinal pigment epithelial cells. Curr. Eye Res. 2007;32:271–280. doi: 10.1080/02713680601187951. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Exp. Eye Res. 2000;70:441–449. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in drusen formation and age related macular degeneration. Exp. Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer’s A{beta}-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc. Natl. Acad. Sci. U S A. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Huang J-D, Presley JB, Chimento MF, Curcio CA. Comparison of morphology of human macular and peripheral Bruch’s membrane in older eyes. Curr. Eye Res. 2007;32:791–799. doi: 10.1080/02713680701550660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas A. Lipoprotein structure. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. Amsterdam: Elsevier; 2002. pp. 483–504. [Google Scholar]
- Kamei M, Yoneda K, Kume N, Suzuki M, Itabe H, Matsuda K, Shimaoka T, Minami M, Yonehara S, Kita T, Kinoshita S. Scavenger receptors for oxidized lipoprotein in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2007;48:1801–1807. doi: 10.1167/iovs.06-0699. [DOI] [PubMed] [Google Scholar]
- Kamei M, Hollyfield JG. TIMP-3 in Bruch’s membrane: changes during aging and in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 1999;40:2367–2375. [PubMed] [Google Scholar]
- Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc. Natl. Acad. Sci. U S A. 2007;104:16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwatowski WSS, Jeffried TE, Duance VC, Albon J, Bailey AJ, Easty DL. Preparation of Bruch’s membrane and analysis of the age-related changes in the structural collagens. Br. J. Ophthalmol. 1995;79:944–952. doi: 10.1136/bjo.79.10.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz SS, Small DM. Isolation and partial characterization of the lipid phases of human atherosclerotic plaques. J. Biol. Chem. 1980;255:9753. [PubMed] [Google Scholar]
- Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog. Retin. Eye Res. 2008;27:622–647. doi: 10.1016/j.preteyeres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Khanifar AA, Koreishi AF, Izatt JA, Toth CA. Drusen ultrastructure imaging with spectral domain optical coherence tomography in age-related macular degeneration. Ophthalmology. 2008;115:1883–1890. doi: 10.1016/j.ophtha.2008.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth MC. Age-related components of Bruch’s membrane. Graefes Arch. Clin. Exp. Ophthalmol. 1987;225:406–412. doi: 10.1007/BF02334166. [DOI] [PubMed] [Google Scholar]
- Klaver CC, Kliffen M, van Duijn CM, Hofman A, Cruts M, Grobbee DE, van Broeckhoven C, de Jong PT. Genetic association of apolipoprotein E with age-related macular degeneration. Am. J. Hum. Genet. 1998;63:200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Davis MD, Magli YL, Segal P, Klein BEK, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmol. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am. J. Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Kliffen M, Mooy CM, Luider TM, Huijmans JGM, Kerkvliet S, de Jong PTVM. Identification of glycosaminoglycans in age-related macular deposits. Arch. Ophthalmol. 1996;114:1009–1014. doi: 10.1001/archopht.1996.01100140217021. [DOI] [PubMed] [Google Scholar]
- Kliffen M, Lutgens E, Daemen MJAP, de Muinck ED, Mooy CM, de Jong PTVM. The APO*E3-Leiden mouse as an animal model for basal laminar deposit. Br. J. Ophthalmol. 2000;84:1415–1419. doi: 10.1136/bjo.84.12.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knupp C, Munro PM, Luther PK, Ezra E, Squire JM. Structure of abnormal molecular assemblies (collagen VI) associated with human full thickness macular holes. J. Struct. Biol. 2000;129:38–47. doi: 10.1006/jsbi.1999.4202. [DOI] [PubMed] [Google Scholar]
- Kouchi M, Ueda Y, Horie H, Tanaka K. Ocular lesions in Watanabe heritable hyperlipidemic rabbits. Vet. Ophthalmol. 2006;9:145–148. doi: 10.1111/j.1463-5224.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- Kovanen PT, Pentikainen MO. Decorin links low-density lipoproteins (LDL) to collagen: a novel mechanism for retention of LDL in the atherosclerotic plaque. Trends Cardiovasc. Med. 1999;9:86–91. doi: 10.1016/s1050-1738(99)00013-4. [DOI] [PubMed] [Google Scholar]
- Kruth HS. Histochemical detection of esterified cholesterol within human atherosclerotic lesions using the fluorescent probe filipin. Atherosclerosis. 1984a;51:281–292. doi: 10.1016/0021-9150(84)90175-8. [DOI] [PubMed] [Google Scholar]
- Kruth HS. Localization of unesterified cholesterol in human atherosclerotic lesions. Demonstration of filipin-positive, oil red O-negative particles. Am. J. Pathol. 1984b;114:201–208. [PMC free article] [PubMed] [Google Scholar]
- Kruth HS. The fate of lipoprotein cholesterol entering the arterial wall. Curr. Opin. Lipidol. 1997;8:246–252. doi: 10.1097/00041433-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Kuntz CA, Jacobson SG, Cideciyan AV, Li Z-Y, Stone EM, Possin D, Milam AH. Sub-retinal pigment epithelial deposits in a dominant lateonset retinal degeneration. Invest. Ophthalmol. Vis. Sci. 1996;37:1772–1782. [PubMed] [Google Scholar]
- Lakkaraju A, Finnemann SC, Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc. Natl. Acad. Sci. U S A. 2007;104:11026–11031. doi: 10.1073/pnas.0702504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18:199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber The origin of hyaline formations within the eye. Ber Ü D Deutsch Ophth Gesellsch. 1924;44:216. [Google Scholar]
- Le YZ, Zheng W, Rao PC, Zheng L, Anderson RE, Esumi N, Zack DJ, Zhu M. Inducible expression of cre recombinase in the retinal pigmented epithelium. Invest. Ophthalmol. Vis. Sci. 2008;49:1248–1253. doi: 10.1167/iovs.07-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Fuda H, Javitt NB, Strott CA, Rodriguez IR. Expression and localization of sterol 27-hydroxylase (CYP27A1) in monkey retina. Exp. Eye Res. 2006;83:465–469. doi: 10.1016/j.exer.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiper JM, Bayliss JD, Pease RJ, Brett DJ, Scott J, Shoulders CC. Microsomal triglyceride transfer protein, the abetalipoproteinemia gene product, mediates the secretion of apolipoprotein B-containing lipoproteins from heterologous cells. J. Biol. Chem. 1994;269:21951–21954. [PubMed] [Google Scholar]
- Lengyel I, Flinn JM, Peto T, Linkous DH, Cano K, Bird AC, Lanzirotti A, Frederickson CJ, van Kuijk F. High concentration of zinc in sub-retinal pigment epithelial deposits. Exp. Eye Res. 2007;84:772–780. doi: 10.1016/j.exer.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Li C-M, Chung BH, Presley JB, Malek G, Zhang X, Dashti N, Li L, Chen J, Bradley K, Kruth HS, Curcio CA. Lipoprotein-like particles and cholesteryl esters in human Bruch’s membrane: initial characterization. Invest. Ophthalmol. Vis. Sci. 2005a;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- Li C-M, Presley JB, Zhang X, Dashti N, Chung BH, Medeiros NE, Guidry C, Curcio CA. Retina expresses microsomal triglyceride transfer protein: implications for age-related maculopathy. J. Lipid Res. 2005b;46:628–640. doi: 10.1194/jlr.M400428-JLR200. [DOI] [PubMed] [Google Scholar]
- Li C-M, Clark ME, Chimento MF, Curcio CA. Apolipoprotein localization in isolated drusen and retinal apolipoprotein gene expression. Invest. Ophthalmol. Vis. Sci. 2006;47:3119–3128. doi: 10.1167/iovs.05-1446. [DOI] [PubMed] [Google Scholar]
- Li C-M, Clark M, Rudolf M, Curcio CA. Distribution and composition of esterified and unesterified cholesterol in extra-macular drusen. Exp. Eye Res. 2007;85:192–201. doi: 10.1016/j.exer.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Lin MY, Kochounian H, Moore RE, Lee TD, Rao N, Fong HK. Deposition of exon-skipping splice isoform of human retinal G protein-coupled receptor from retinal pigment epithelium into Bruch’s membrane. Mol. Vis. 2007;13:1203–1214. [PubMed] [Google Scholar]
- Loane E, Nolan JM, O’Donovan O, Bhosale P, Bernstein PS, Beatty S. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Surv. Ophthalmol. 2008;53:68–81. doi: 10.1016/j.survophthal.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Löffler KU, Lee WR. Basal linear deposit in the human macula. Graefes Arch. Clin. Exp. Ophthalmol. 1986;224:493–501. doi: 10.1007/BF02154735. [DOI] [PubMed] [Google Scholar]
- Lommatzsch A, Hermans P, Muller KD, Bornfeld N, Bird AC, Pauleikhoff D. Are low inflammatory reactions involved in exudative age-related macular degeneration? Morphological and immunhistochemical analysis of AMD associated with basal deposits. Graefes Arch. Clin. Exp. Ophthalmol. 2008;246:803–810. doi: 10.1007/s00417-007-0749-4. [DOI] [PubMed] [Google Scholar]
- Luibl V, Isas JM, Kayed R, Glabe CG, Langen R, Chen J. Drusen deposits associated with aging and age-related macular degeneration contain non-fibrillar amyloid oligomers. J. Clin. Invest. 2006;116:378–385. doi: 10.1172/JCI25843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna LG. Manual of the Histologic Staining Methods of the Armed Forces Institute of Pathology. New York: McGraw-Hill; 1968. [Google Scholar]
- Lusis AJ, Fogelman AM, Fonarow GC. Genetic basis of atherosclerosis: part I: new genes and pathways. Circulation. 2004;110:1868–1873. doi: 10.1161/01.CIR.0000143041.58692.CC. [DOI] [PubMed] [Google Scholar]
- Madsen EM, Lindegaard ML, Andersen CB, Damm P, Nielsen LB. Human placenta secretes apolipoprotein B-100-containing lipoproteins. J. Biol. Chem. 2004;279:55271–55276. doi: 10.1074/jbc.M411404200. Epub 52004 Oct 55225. [DOI] [PubMed] [Google Scholar]
- Majji AB, Cao J, Chang KY, Hayashi A, Aggarwal S, Grebe RR, de Juan E., Jr Age-related retinal pigment epithelium and Bruch’s membrane degeneration in senescence-accelerated mouse. Invest. Ophthalmol. Vis. Sci. 2000;41:3936–3942. [PubMed] [Google Scholar]
- Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Jama. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- Malek G, Li C-M, Guidry C, Medeiros NE, Curcio CA. Apolipoprotein B in cholesterol-containing drusen and basal deposits in eyes with age-related maculopathy. Am. J. Pathol. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek G, Johnson LV, Mace BE, Saloupis P, Schmechel DE, Rickman DW, Toth CA, Sullivan PM, Rickman CB. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc. Natl. Acad. Sci. U S A. 2005;102:11900–11905. doi: 10.1073/pnas.0503015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest. Ophthalmol. Vis. Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GE, Konstas AGP, Reid GG, Edwards JG, Lee WR. Type IV collagen and laminin in Bruch’s membrane and basal linear deposit in the human macula. Br. J. Ophthalmol. 1992;76:607–614. doi: 10.1136/bjo.76.10.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, Hussain AA, Starita C, Moore DJ, Patmore AL. Aging and Bruch’s membrane. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium: Function and Disease. New York: Oxford University Press; 1998. pp. 669–692. [Google Scholar]
- McCarty WJ, Chimento MF, Curcio CA, Johnson M. Effects of particulates and lipids on the hydraulic conductivity of Matrigel. J. Appl. Physiol. 2008;105:621–628. doi: 10.1152/japplphysiol.01245.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger JD, Johnson M, Medeiros NE, Curcio CA. Transition from Lipid Wall to basal linear deposit In age-related maculopathy (ARM) Invest. Ophthalmol. Vis. Sci. E-abstract. 2009:4933. [Google Scholar]
- Miceli MV, Newsome DA, Tate DJ, Jr, Sarphie TG. Pathologic changes in the retinal pigment epithelium and Bruch’s membrane of fat-fed atherogenic mice. Curr. Eye Res. 2000;20:8–16. [PubMed] [Google Scholar]
- Milam AH, Curcio CA, Cideciyan AV, Saxena S, John SK, Kruth HS, Malek G, Heckenlively JR, Weleber RG, Jacobson SG. Dominant late-onset retinal degeneration with regional variation of sub-RPE deposits, retinal function, and photoreceptor degeneration. Ophthalmology. 2000;107:2256–2266. doi: 10.1016/s0161-6420(00)00419-x. [DOI] [PubMed] [Google Scholar]
- Moghadasian MH. Experimental atherosclerosis: a historical overview. Life Sci. 2002;70:855–865. doi: 10.1016/s0024-3205(01)01479-5. [DOI] [PubMed] [Google Scholar]
- Mohler PJ, Zhu MY, Blade AM, Ham AJ, Shelness GS, Swift LL. Identification of a novel isoform of microsomal triglyceride transfer protein. J. Biol. Chem. 2007;282:26981–26988. doi: 10.1074/jbc.M700500200. [DOI] [PubMed] [Google Scholar]
- Montezuma SR, Sobrin L, Seddon JM. Review of genetics in age related macular degeneration. Semin. Ophthalmol. 2007;22:229–240. doi: 10.1080/08820530701745140. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Clover GM. The effect of age on the macromolecular permeability of human Bruch’s membrane. Invest. Ophthalmol. Vis. Sci. 2001;42:2970–2975. [PubMed] [Google Scholar]
- Moore DJ, Hussain AA, Marshall J. Age-related variation in the hydraulic conductivity of Bruch’s membrane. Invest. Ophthalmol. Vis. Sci. 1995;36:1290–1297. [PubMed] [Google Scholar]
- Moreira EF, Larrayoz IM, Lee JW, Rodriguez IR. 7-Ketocholesterol is present in lipid deposits in the primate retina: potential implication in the induction of VEGF and CNV formation. Invest. Ophthalmol. Vis. Sci. 2009;50:523–532. doi: 10.1167/iovs.08-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Metzger D, Garnier JM, Chambon P, Mark M. Site-specific somatic mutagenesis in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2002;43:1384–1388. [PubMed] [Google Scholar]
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- Mullins RF, Hageman GS. Human ocular drusen possess novel core domains with a distinct carbohydrate composition. J. Histochem. Cytochem. 1999;47:1533–1539. doi: 10.1177/002215549904701205. [DOI] [PubMed] [Google Scholar]
- Nakaisumi Y, Hogan MJ, Feeney L. The ultrastructure of Bruch’s membrane. III. The macular area of the human eye. Arch. Ophthalmol. 1964;72:395–400. doi: 10.1001/archopht.1964.00970020395018. [DOI] [PubMed] [Google Scholar]
- Newsome DA, Hewitt AT, Huh W, Robey PG, Hassell JR. Detection of specific extracellular matrix molecules in drusen, Bruch’s membrane, and ciliary body. Am. J. Ophthalmol. 1987;104:373–381. doi: 10.1016/0002-9394(87)90227-3. [DOI] [PubMed] [Google Scholar]
- Nickle B, Robinson PR. The opsins of the vertebrate retina: insights from structural, biochemical, and evolutionary studies. Cell Mol. Life Sci. 2007;64:2917–2932. doi: 10.1007/s00018-007-7253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen LB, Veniant M, Borén J, Raabe M, Wong JS, Tam C, Flynn L, Vanni-Reyes T, Gunn MD, Goldberg IJ, et al. Genes for apolipoprotein B and microsomal triglyceride transfer protein are expressed in the heart: evidence that the heart has the capacity to synthesize and secrete lipoproteins. Circulation. 1998;98:13–16. doi: 10.1161/01.cir.98.1.13. [DOI] [PubMed] [Google Scholar]
- Nievelstein PF, Fogelman AM, Mottino G, Frank JS. Lipid accumulation in rabbit aortic intima 2 hours after bolus infusion of low density lipoprotein. A deep-etch and immunolocalization study of ultrarapidly frozen tissue. Arte-rioscler Thromb. 1991;11:1795–1805. doi: 10.1161/01.atv.11.6.1795. [DOI] [PubMed] [Google Scholar]
- Nussenblatt RB, Ferris F., 3rd Age-related macular degeneration and the immune response: implications for therapy. Am. J. Ophthalmol. 2007;144:618–626. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KD, Lewis K, Fischer JW, Johnson P, Hwang JY, Knopp EA, Kinsella MG, Barrett PH, Chait A, Wight TN. Smooth muscle cell biglycan overexpression results in increased lipoprotein retention on extracellular matrix: implications for the retention of lipoproteins in atherosclerosis. Atherosclerosis. 2004;177:29–35. doi: 10.1016/j.atherosclerosis.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Oksjoki R, Kovanen PT, Meri S, Pentikainen MO. Function and regulation of the complement system in cardiovascular diseases. Front. Biosci. 2007;12:4696–4708. doi: 10.2741/2419. [DOI] [PubMed] [Google Scholar]
- Olofsson SO, Boren J. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 2005;258:395–410. doi: 10.1111/j.1365-2796.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Ong JM, Zorapapel NC, Rich KA, Wagstaff RE, Lambert RW, Rosenberg SE, Moghaddas F, Pirouzmanesh A, Aoki AM, Kenney MC. Effects of cholesterol and apolipoprotein E on retinal abnormalities in apoE-deficient mice. Invest. Ophthalmol. Vis. Sci. 2001;42:1891–1900. [PubMed] [Google Scholar]
- Ong JM, Aoki AM, Seigel GM, Sacerio I, Castellon R, Nesburn AB, Kenney MC. Oxysterol-induced toxicity in R28 and ARPE-19 cells. Neurochem. Res. 2003;28:883–891. doi: 10.1023/a:1023223409798. [DOI] [PubMed] [Google Scholar]
- Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch’s membrane: a histochemical and morphological study. Ophthalmology. 1990;97:171–178. [PubMed] [Google Scholar]
- Pauleikhoff D, Zuels S, Sheraidah GS, Marshall J, Wessing A, Bird AC. Correlation between biochemical composition and fluorescein binding of deposits in Bruch’s membrane. Ophthalmol. 1992;99:1548–1553. doi: 10.1016/s0161-6420(92)31768-3. [DOI] [PubMed] [Google Scholar]
- Pauleikhoff D, Sheraidah G, Marshall J, Bird AC, Wessing A. Biochemical and histochemical analysis of age related lipid deposits in Bruch’s membrane. Ophthalmologe. 1994;91:730–734. [PubMed] [Google Scholar]
- Pauleikhoff D, Wojteki S, Muller D, Bornfeld N, Heiligenhaus A. Adhesive properties of basal membranes of Bruch’s membrane. Immunohistochemical studies of age-dependent changes in adhesive molecules and lipid deposits. Ophthalmologe. 2000;97:243–250. doi: 10.1007/s003470050520. [DOI] [PubMed] [Google Scholar]
- Pentikainen MO, Lehtonen EM, Oorni K, Lusa S, Somerharju P, Jauhiainen M, Kovanen PT. Human arterial proteoglycans increase the rate of proteolytic fusion of low density lipoprotein particles. J. Biol. Chem. 1997;272:25283–25288. doi: 10.1074/jbc.272.40.25283. [DOI] [PubMed] [Google Scholar]
- Pentikainen MO, Oorni K, Kovanen PT. Lipoprotein lipase (LPL) strongly links native and oxidized low density lipoprotein particles to decorin-coated collagen. Roles for both dimeric and monomeric forms of LPL. J. Biol. Chem. 2000;275:5694–5701. doi: 10.1074/jbc.275.8.5694. [DOI] [PubMed] [Google Scholar]
- Pentikainen MO, Oksjoki R, Oorni K, Kovanen PT. Lipoprotein lipase in the arterial wall - Linking LDL to the arterial extracellular matrix and much more. Arteriosclerosis Thromb. Vasc. Biol. 2002;22:211–217. doi: 10.1161/hq0102.101551. [DOI] [PubMed] [Google Scholar]
- Qtaishat NM, Redmond TM, Pepperberg DR. Acute radiolabeling of retinoids in eye tissues of normal and rpe65-deficient mice. Invest. Ophthalmol. Vis. Sci. 2003;44:1435–1446. doi: 10.1167/iovs.02-0679. [DOI] [PubMed] [Google Scholar]
- Raabe M, Flynn LM, Zlot CH, Wong JS, Veniant MM, Hamilton RL, Young SG. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. U S A. 1998;95:8686–8691. doi: 10.1073/pnas.95.15.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez AI, Salazar JJ, De Hoz R, Rojas B, Ruiz E, Tejerina T, Ramírez JM, Triviño A. Macroglial and retinal changes in hypercholesterolemic rabbits after normalization of cholesterol levels. Exp. Eye Res. 2006;83:1423–1438. doi: 10.1016/j.exer.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PGH, de Jong PTVM. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest. Ophthalmol. Vis. Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- Raoul W, Feumi C, Keller N, Lavalette S, Houssier M, Behar-Cohen F, Combadiere C, Sennlaub F. Lipid-bloated subretinal microglial cells are at the origin of drusen appearance in CX3CR1-deficient mice. Ophthalmic Res. 2008;40:115–119. doi: 10.1159/000119860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp JH, Lespine A, Hamilton RL, Colyvas N, Chaumeton AH, Tweedie-Hardman J, Kotite L, Kunitake ST, Havel RJ, Kane JP. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb. 1994;14:1767–1774. doi: 10.1161/01.atv.14.11.1767. [DOI] [PubMed] [Google Scholar]
- Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest. Ophthalmol. Vis. Sci. 2000;41:1200–1209. [PubMed] [Google Scholar]
- Reale E, Groos S, Eckardt U, Eckardt C, Luciano L. New components of ‘basal laminar deposits’ in age-related macular degeneration. Cells Tissues Organs. 2008 doi: 10.1159/000187632. [DOI] [PubMed] [Google Scholar]
- Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma J-X, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cisvitamin A in the retinal visual cycle. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- Richards A, Kavanagh D, Atkinson JP. Inherited complement regulatory protein deficiency predisposes to human disease in acute injury and chronic inflammatory statesthe examples of vascular damage in atypical hemolytic uremic syndrome and debris accumulation in age-related macular degeneration. Adv. Immunol. 2007;96:141–177. doi: 10.1016/S0065-2776(07)96004-6. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- Rittmann BE, Schloendorn J. Engineering away lysosomal junk: medical bioremediation. Rejuvenation Res. 2007;10:359–365. doi: 10.1089/rej.2007.0594. [DOI] [PubMed] [Google Scholar]
- Robison WG, Jr, Kuwabara T. Vitamin A storage and peroxisomes in retinal pigment epithelium and liver. Invest. Ophthalmol. Vis. Sci. 1977;16:1110–1117. [PubMed] [Google Scholar]
- Rodriguez IR, Alam S, Lee JW. Cytotoxicity of oxidized low-density lipoprotein in cultured RPE cells is dependent on the formation of 7-ketocholesterol. Invest. Ophthalmol. Vis. Sci. 2004;45:2830–2837. doi: 10.1167/iovs.04-0075. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Parkins N, Ershov AV, Bazan NG. Selective retinal pigment epithelial cell lipid metabolism and remodeling conserves photoreceptor docosahexaenoic acid following phagocytosis. J. Neurosci. Res. 1999;57:479–486. [PubMed] [Google Scholar]
- Rones B. Formation of drusen of the lamina vitrea. Arch. Ophthalmol. 1937;18:288–402. [Google Scholar]
- Ruberg FL, Viereck J, Phinikaridou A, Qiao Y, Loscalzo J, Hamilton JA. Identification of cholesteryl esters in human carotid atherosclerosis by ex vivo image-guided proton MRS. J. Lipid Res. 2006;47:310–317. doi: 10.1194/jlr.M500431-JLR200. [DOI] [PubMed] [Google Scholar]
- Ruberti JW, Curcio CA, Millican CL, Menco BP, Huang JD, Johnson M. Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch’s membrane. Invest. Ophthalmol. Vis. Sci. 2003;44:1753–1759. doi: 10.1167/iovs.02-0496. [DOI] [PubMed] [Google Scholar]
- Rudolf M, Winkler B, Aherrahou Z, Doehring LC, Kaczmarek P, Schmidt-Erfurth U. Increased expression of vascular endothelial growth factor associated with accumulation of lipids in Bruch’s membrane of LDL receptor knockout mice. Br. J. Ophthalmol. 2005;89:1627–1630. doi: 10.1136/bjo.2005.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, Clark ME, Chimento M, Li C-M, Medeiros NE, Curcio CA. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest. Ophthalmol. Vis. Sci. 2008a;49:1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, Malek G, Messinger JD, Wang L, Clark ME, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp. Eye Res. 2008b;87:402–408. doi: 10.1016/j.exer.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Brett P, Bok D. TIMP-3 is expressed in the human retinal pigment epithelium. Biochem. Biophys. Res. Commun. 1996;226:467–474. doi: 10.1006/bbrc.1996.1379. [DOI] [PubMed] [Google Scholar]
- Ryeom SW, Silverstein RL, Scotto A, Sparrow JR. Binding of anionic phospholipids to retinal pigment epithelium may be mediated by the scavenger receptor CD36. J. Biol. Chem. 1996;271:20536–20539. doi: 10.1074/jbc.271.34.20536. [DOI] [PubMed] [Google Scholar]
- Sadaba LM, Fernandez-Robredo P, Rodriguez JA, Garcia-Layana A. Anti-oxidant effects of vitamins C and E, multivitamin-mineral complex and flavonoids in a model of retinal oxidative stress: the ApoE-deficient mouse. Exp. Eye Res. 2008;86:470–479. doi: 10.1016/j.exer.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Salazar JJ, Ramírez AI, de Hoz R, Rojas B, Ruiz E, Tejerina T, Triviño A, Ramírez JM. Alterations in the choroid in hypercholesterolemic rabbits: reversibility after normalization of cholesterol levels. Exp. Eye Res. 2007;84:412–422. doi: 10.1016/j.exer.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Sallo FB, Bereczki E, Csont T, Luthert PJ, Munro P, Ferdinandy P, Santha M, Lengyel I. Bruch’s membrane changes in transgenic mice overexpressing the human biglycan and apolipoprotein b-100 genes. Exp. Eye Res. 2009 doi: 10.1016/j.exer.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br. J. Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarks SH. Council Lecture: drusen and their relationship to senile macular degeneration. Aust. J. Ophthalmol. 1980;8:117–130. doi: 10.1111/j.1442-9071.1980.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Sarks SH, van Driel D, Maxwell L, Killingsworth M. Softening of drusen and subretinal neovascularization. Trans. Ophthalmol. Soc. U K. 1980;100:414–422. [PubMed] [Google Scholar]
- Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- Sarks SH, Arnold JJ, Killingsworth MC, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age-related maculopathy: a clinicopathological study. Br. J. Ophthalmol. 1999;83:358–368. doi: 10.1136/bjo.83.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2007;48:968–977. doi: 10.1167/iovs.06-0443. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Rudolf M, Funk M, Hofmann-Rummelt C, Franz-Haas NS, Aherrahrou Z, Schlotzer-Schrehardt U. Ultrastructural changes in a murine model of graded Bruch membrane lipoidal degeneration and corresponding VEGF164 detection. Invest. Ophthalmol. Vis. Sci. 2008;49:390–398. doi: 10.1167/IOVS.07-0227. [DOI] [PubMed] [Google Scholar]
- Schonfeld G. Familial hypobetalipoproteinemia: a review. J. Lipid Res. 2003;44:878–883. doi: 10.1194/jlr.R300002-JLR200. [DOI] [PubMed] [Google Scholar]
- Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. I. Focal increases in arterial LDL concentration precede development of fatty streak lesions. Arteriosclerosis. 1989a;9:895–907. doi: 10.1161/01.atv.9.6.895. [DOI] [PubMed] [Google Scholar]
- Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis. 1989b;9:908–918. doi: 10.1161/01.atv.9.6.908. [DOI] [PubMed] [Google Scholar]
- Scott BL, Bazan NG. Membrane docosohexanoate is supplied to the developing brain and retina by the liver. Proc. Natl. Acad. Sci. 1989;86:2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest JP, Jones MK, Dashti N. N-terminal domain of apolipoprotein B has structural homology to lipovitellin and microsomal triglyceride transfer protein: a “lipid pocket” model for self-assembly of apob-containing lipoprotein particles. J. Lipid Res. 1999;40:1401–1416. [PubMed] [Google Scholar]
- Seifert PS, Hugo F, Hansson GK, Bhakdi S. Prelesional complement activation in experimental atherosclerosis. Terminal C5b-9 complement deposition coincides with cholesterol accumulation in the aortic intima of hypercholesterolemic rabbits. Lab. Invest. 1989;60:747–754. [PubMed] [Google Scholar]
- Selkoe DJ, Ihara Y, Salazar FJ. Alzheimer’s disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982;215:1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Sellers JA, Shelness GS. Lipoprotein assembly capacity of the mammary tumor-derived cell line C127 is due to the expression of functional microsomal triglyceride transfer protein. J. Lipid Res. 2001;42:1897–1904. [PubMed] [Google Scholar]
- Seth A, Cui J, To E, Kwee M, Matsubara J. Complement-associated deposits in the human retina. Invest. Ophthalmol. Vis. Sci. 2008;49:743–750. doi: 10.1167/iovs.07-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PK. Molecular mechanisms of plaque instability. Curr. Opin. Lipidol. 2007;18:492–499. doi: 10.1097/MOL.0b013e3282efa326. [DOI] [PubMed] [Google Scholar]
- Sheraidah G, Steinmetz R, Maguire J, Pauleikhoff D, Marshall J, Bird AC. Correlation between lipids extracted from Bruch’s membrane and age. Ophthalmology. 1993;100:47–51. doi: 10.1016/s0161-6420(13)31712-6. [DOI] [PubMed] [Google Scholar]
- Shotton D, Severs N. An introduction to freeze fracture and deep etching. In: Shotton D, Severs N, editors. Rapid Freezing, Freeze Fracture, and Deep Etching. New York, NY: Wiley-Liss, Inc; 1995. pp. 1–30. [Google Scholar]
- Shoulders CC, Shelness GS. Current biology of MTP: implications for selective inhibition. Curr. Top. Med. Chem. 2005;5:283–300. doi: 10.2174/1568026053544560. [DOI] [PubMed] [Google Scholar]
- de Silva HV, Stuart WD, Duvic CR, Wetterau JR, Ray MJ, Ferguson DG, Albers HW, Smith WR, Harmony JA. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J. Biol. Chem. 1990;265:13240–13247. [PubMed] [Google Scholar]
- Sivaprasad S, Bailey TA, Chong VN. Bruch’s membrane and the vascular intima: is there a common basis for age-related changes and disease? Clin. Exp. Ophthalmol. 2005;33:518–523. doi: 10.1111/j.1442-9071.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- Skålen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Borén J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- Small DM. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988;8:103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- Small DM, Shipley GG. Physical-chemical basis of lipid deposition in atherosclerosis. Science. 1974;185:222–229. doi: 10.1126/science.185.4147.222. [DOI] [PubMed] [Google Scholar]
- Smith E. Relationship between plasma lipids and arterial tissue lipids. Nutr. Metab. 1973;15:17–26. doi: 10.1159/000175418. [DOI] [PubMed] [Google Scholar]
- Smith EB. The relationship between plasma and tissue lipids in human atherosclerosis. Adv. Lipid Res. 1974;12:1–49. doi: 10.1016/b978-0-12-024912-1.50008-9. [DOI] [PubMed] [Google Scholar]
- Smith JD. Apolipoproteins and aging: emerging mechanisms. Ageing Res. Rev. 2002;1:345–365. doi: 10.1016/s1568-1637(02)00005-3. [DOI] [PubMed] [Google Scholar]
- Smith EB, Evan PH, Downham MD. Lipid in the aortic intima. The correlation of morphological and chemical characteristics. J. Atheroscler. Res. 1967;7:171–186. doi: 10.1016/s0368-1319(67)80079-6. [DOI] [PubMed] [Google Scholar]
- Smith EB, Slater RS. The microdissection of large atherosclerotic plaques to give morphologically and topographically defined fractions for analysis. Atherosclerosis. 1972;15:37–56. doi: 10.1016/0021-9150(72)90036-6. [DOI] [PubMed] [Google Scholar]
- Souied EH, Benlian P, Amouyel P, Feingold J, Lagarde JP, Munnich A, Kaplan J, Coscas G, Soubrane G. The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am. J. Ophthalmol. 1998;125:353–359. doi: 10.1016/s0002-9394(99)80146-9. [DOI] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siR-NAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- Spaide R, Ho-Spaide W, Browne R, Armstrong D. Characterization of peroxidized lipids in Bruch’s membrane. Retina. 1999;19:141–147. doi: 10.1097/00006982-199902000-00010. [DOI] [PubMed] [Google Scholar]
- Sparks CE, Hnatiuk O, Marsh JB. Hepatic and intestinal contribution of two forms of apolipoprotein B to plasma lipoprotein fractions in the rat. Can. J. Biochem. 1981;59:693–699. doi: 10.1139/o81-096. [DOI] [PubMed] [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 1996;37:2724–2735. [PubMed] [Google Scholar]
- Spraul CW, Grossniklaus HE. Characteristics of drusen and Bruch’s membrane in postmortem eyes with age-related macular degeneration. Arch. Ophthalmol. 1997;115:267–273. doi: 10.1001/archopht.1997.01100150269022. [DOI] [PubMed] [Google Scholar]
- Starita C, Hussain AA, Pagliarini S, Marshall J. Hydrodynamics of ageing Bruch’s membrane: implications for macular disease. Exp. Eye Res. 1996;62:565–572. doi: 10.1006/exer.1996.0066. [DOI] [PubMed] [Google Scholar]
- Starita C, Hussain AA, Patmore A, Marshall J. Localization of the site of major resistance to fluid transport in Bruch’s membrane. Invest. Ophthalmol. Vis. Sci. 1997;38:762–767. [PubMed] [Google Scholar]
- Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull WI, Jr, Richardson M, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of the intima of human arteries and of its atherosclerosis-prone regions. Circulation. 1992;85:391–405. doi: 10.1161/01.cir.85.1.391. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Thematic review series: the Pathogenesis of Atherosclerosis. An interpretive history of the cholesterol controversy: part II: the early evidence linking hypercholesterolemia to coronary disease in humans. J. Lipid Res. 2005;46:179–190. doi: 10.1194/jlr.R400012-JLR200. [DOI] [PubMed] [Google Scholar]
- Strunnikova N, Baffi J, Gonzalez A, Silk W, Cousins SW, Csaky KG. Regulated heat shock protein 27 expression in human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2001;42:2130–2138. [PubMed] [Google Scholar]
- Sun K, Cai H, Tezel TH, Paik D, Gaillard ER, Del Priore LV. Bruch’s membrane aging decreases phagocytosis of outer segments by retinal pigment epithelium. Mol. Vis. 2007;13:2310–2319. [PubMed] [Google Scholar]
- Suzuki M, Kamei M, Itabe H, Yoneda K, Bando H, Kume N, Tano Y. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol. Vis. 2007;13:772–778. [PMC free article] [PubMed] [Google Scholar]
- Tabas I. Nonoxidative modifications of lipoproteins in atherogenesis. Annu. Rev. Nutr. 1999;19:123–139. doi: 10.1146/annurev.nutr.19.1.123. [DOI] [PubMed] [Google Scholar]
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- Tamai K, Spaide RF, Ellis EA, Iwabuchi S, Ogura Y, Armstrong D. Lipid hydroperoxide stimulates subretinal choroidal neovascularization in the rabbit. Exp. Eye Res. 2002;74:301–308. doi: 10.1006/exer.2001.1121. [DOI] [PubMed] [Google Scholar]
- Tamminen M, Mottino G, Qiao JH, Breslow JL, Frank JS. Ultrastructure of early lipid accumulation in ApoE-deficient mice. Arterioscler Thromb. Vasc. Biol. 1999;19:847–853. doi: 10.1161/01.atv.19.4.847. [DOI] [PubMed] [Google Scholar]
- Temel RE, Hou L, Rudel LL, Shelness GS. ACAT2 stimulates cholesteryl ester secretion in apoB-containing lipoproteins. J. Lipid Res. 2007;48:1618–1627. doi: 10.1194/jlr.M700109-JLR200. [DOI] [PubMed] [Google Scholar]
- Tezel TH, Del Priore LV, Kaplan HJ. Reengineering of aged Bruch’s membrane to enhance retinal pigment epithelium repopulation. Invest. Ophthalmol. Vis. Sci. 2004;45:3337–3348. doi: 10.1167/iovs.04-0193. [DOI] [PubMed] [Google Scholar]
- Thakkinstian A, Bowe S, McEvoy M, Smith W, Attia J. Association between apolipoprotein E polymorphisms and age-related macular degeneration: a HuGE review and meta-analysis. Am. J. Epidemiol. 2006;164:813–822. doi: 10.1093/aje/kwj279. [DOI] [PubMed] [Google Scholar]
- Tian J, Ishibashi K, Reiser K, Grebe R, Biswal S, Gehlbach P, Handa JT. Advanced glycation endproduct-induced aging of the retinal pigment epithelium and choroid: a comprehensive transcriptional response. Proc. Natl. Acad. Sci. U S A. 2005;102:11846–11851. doi: 10.1073/pnas.0504759102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torzewski M, Klouche M, Hock J, Messner M, Dorweiler B, Torzewski J, Gabbert HE, Bhakdi S. Immunohistochemical demonstration of enzymatically modified human LDL and its colocalization with the terminal complement complex in the early atherosclerotic lesion. Arterioscler Thromb. Vasc. Biol. 1998;18:369–378. doi: 10.1161/01.atv.18.3.369. [DOI] [PubMed] [Google Scholar]
- Triviño A, Ramírez AI, Salazar JJ, de Hoz R, Rojas B, Padilla E, Tejerina T, Ramírez JM. A cholesterol-enriched diet induces ultrastructural changes in retinal and macroglial rabbit cells. Exp. Eye Res. 2006;83:357–366. doi: 10.1016/j.exer.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol. Vis. 2006a;12:1319–1333. [PubMed] [Google Scholar]
- Tserentsoodol N, Sztein J, Campos M, Gordiyenko NV, Fariss RN, Lee JW, Fliesler SJ, Rodriguez IR. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol. Vis. 2006b;12:1306–1318. [PubMed] [Google Scholar]
- Umeda S, Suzuki MT, Okamoto H, Ono F, Mizota A, Terao K, Yoshikawa Y, Tanaka Y, Iwata T. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis) Faseb J. 2005;19:1683–1685. doi: 10.1096/fj.04-3525fje. [DOI] [PubMed] [Google Scholar]
- Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiin-flammatory properties of HDL. J. Clin. Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002;105:939–943. doi: 10.1161/hc0802.104327. [DOI] [PubMed] [Google Scholar]
- Veniant MM, Beigneux AP, Bensadoun A, Fong LG, Young SG. Lipoprotein size and susceptibility to atherosclerosis–insights from genetically modified mouse models. Curr. Drug Targets. 2008;9:174–189. doi: 10.2174/138945008783755629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeff FH, Sisson RJ. Basophilic staining of Bruch’s membrane. Arch. Ophthalmol. 1926;55:125–127. [Google Scholar]
- Verschuren WM, Jacobs DR, Bloemberg BP, Kromhout D, Menotti A, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, et al. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA. 1995;274:131–136. [PubMed] [Google Scholar]
- Walton KW, Dunkerley DJ. Studies on the pathogenesis of corneal arcus formation. II. Immunofluorescent studies on lipid deposition in the eye of the lipid-fed rabbit. J. Pathol. 1974;114:217–229. doi: 10.1002/path.1711140406. [DOI] [PubMed] [Google Scholar]
- Wang X, Greilberger J, Ratschek M, Jurgens G. Oxidative modifications of LDL increase its binding to extracellular matrix from human aortic intima: influence of lesion development, lipoprotein lipase and calcium. J. Pathol. 2001;195:244–250. doi: 10.1002/path.935. [DOI] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009a;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li C-M, Rudolf M, Belyaeva OV, Kedishvili NY, Chung BH, Curcio CA. Lipoprotein particles of intraocular origin in human Bruch membrane: an unusual lipid profile. Invest. Ophthalmol. Vis. Sci. 2009b;50:870–877. doi: 10.1167/iovs.08-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch. Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- Waugh DA, Small DM. Identification and detection of in situ cellular and regional differences of lipid composition and class in lipid-rich tissue using hot stage polarizing light microscopy. Lab. Invest. 1984;51:702–714. [PubMed] [Google Scholar]
- Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992;258:999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim. Biophys. Acta. 1997;1345:136–150. doi: 10.1016/s0005-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- White CR, Datta G, Mochon P, Zhang Z, Kelly O, Curcio C, Parks D, Palgunachari M, Handattu S, Gupta H, et al. Vasculoprotective effects of apolipoprotein mimetic peptides: an evolving paradigm in HDL therapy. Vasc. Dis. Prev. 2009;6:122–130. doi: 10.2174/1567270000906010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb. Vasc. Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of atherogenesis reinforced. Curr. Opin. Lipidol. 1998;9:471–474. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. Lipoprotein retention–and clues for atheroma regression. Arterioscler Thromb. Vasc. Biol. 2005;25:1536–1540. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]
- Wolter JR, Falls HF. Bilateral confluent drusen. Arch. Ophthalmol. 1962;68:219–226. doi: 10.1001/archopht.1962.00960030223013. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Tian J, Yang Y, Cutler RG, Wu T, Telljohann RS, Mattson MP, Handa JT. Oxidized low density lipoproteins induce a pathologic response by retinal pigmented epithelial cells. J. Neurochem. 2008;105:1187–1197. doi: 10.1111/j.1471-4159.2008.05211.x. [DOI] [PubMed] [Google Scholar]
- Yao Z, Tran K, McLeod RS. Intracellular degradation of newly synthesized apolipoprotein B. J. Lipid Res. 1997;38:1937–1953. [PubMed] [Google Scholar]
- Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, et al. Complement C3 variant and the risk of age-related macular degeneration. N. Engl. J. Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- Yokota T, Igarashi K, Uchihara T, Jishage K-i, Tomita H, Inaba A, Li Y, Arita M, Suzuki H, Mizusawa H, Arai H. Delayed-onset ataxia in mice lacking alpha -tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. PNAS. 2001;98:15185–15190. doi: 10.1073/pnas.261456098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Pathophysiology of age-related macular degeneration. Surv. Ophthalmol. 1987;31:291–306. doi: 10.1016/0039-6257(87)90115-9. [DOI] [PubMed] [Google Scholar]
- Yuan C, Petty C, O’Brien KD, Hatsukami TS, Eary JF, Brown BG. In vitro and in situ magnetic resonance imaging signal features of atherosclerotic plaque-associated lipids. Arterioscler Thromb. Vasc. Biol. 1997;17:1496–1503. doi: 10.1161/01.atv.17.8.1496. [DOI] [PubMed] [Google Scholar]
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N. Engl. J. Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- Zak Z, Lagrost L, Gautier T, Masson D, Deckert V, Duverneuil L, De Barros JP, Le Guern N, Dumont L, Schneider M, et al. Expression of simian CETP in normolipidemic Fisher rats has a profound effect on large sized apoE-containing HDL. J. Lipid Res. 2002;43:2164–2171. doi: 10.1194/jlr.m200253-jlr200. [DOI] [PubMed] [Google Scholar]
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch. Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- Zhang WY, Gaynor PM, Kruth HS. Apolipoprotein E produced by monocyte-derived macrophages mediates cholesterol efflux that occurs in the absence of added cholesterol acceptors. J. Biol. Chem. 1996;271:28641–28646. doi: 10.1074/jbc.271.45.28641. [DOI] [PubMed] [Google Scholar]