Abstract
Tau is a highly abundant and multifunctional brain protein that accumulates in neurofibrillary tangles (NFTs), most commonly in Alzheimer’s disease (AD) and primary age-related tauopathy. Recently, microRNAs (miRNAs) have been linked to neurodegeneration; however, it is not clear whether miRNA dysregulation contributes to tau neurotoxicity. Here, we determined that the highly conserved brain miRNA miR-219 is downregulated in brain tissue taken at autopsy from patients with AD and from those with severe primary age-related tauopathy. In a Drosophila model that produces human tau, reduction of miR-219 exacerbated tau toxicity, while overexpression of miR-219 partially abrogated toxic effects. Moreover, we observed a bidirectional modulation of tau levels in the Drosophila model that was dependent on miR-219 expression or neutralization, demonstrating that miR-219 regulates tau in vivo. In mammalian cellular models, we found that miR-219 binds directly to the 3′-UTR of the tau mRNA and represses tau synthesis at the post-transcriptional level. Together, our data indicate that silencing of tau by miR-219 is an ancient regulatory mechanism that may become perturbed during neurofibrillary degeneration and suggest that this regulatory pathway may be useful for developing therapeutics for tauopathies.
Introduction
MicroRNAs (miRNAs) are powerful regulators of neuronal gene expression that contribute to both physiological and pathological processes (1). These small noncoding RNAs bind recognition motifs in multiple target mRNAs and silence expression through post-transcriptional mechanisms, such as translational repression or transcript destabilization, enabling them to serve as master regulators of transcriptional networks (2). miRNAs are implicated in diverse brain functions, including development, cognition, and synaptic plasticity (3). Expression-profiling studies indicate that alterations in miRNAs occur in the brains of Alzheimer’s disease (AD) patients, but the functional implications of these changes remain unclear (4). Here, we show that miR-219 is downregulated in AD and primary age-related tauopathy, modulates tau toxicity in vivo, and regulates tau expression at the post-transcriptional level through direct interaction with the tau mRNA.
Results and Discussion
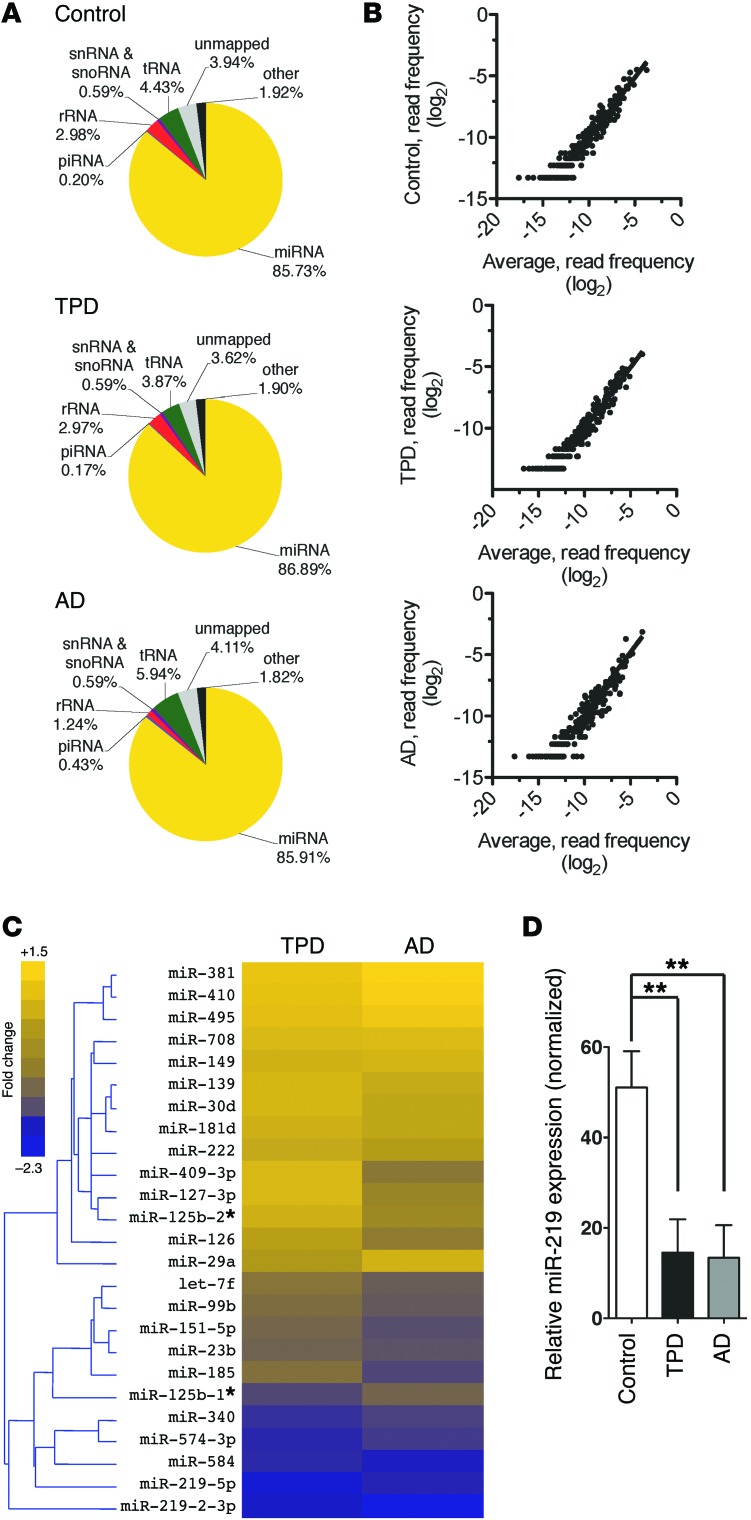
First, we investigated whether dysregulation of miRNA expression is associated with neurofibrillary degeneration. Abnormal accumulation of tau occurs in various pathological settings, with AD and primary age-related tauopathy being the most common (5). Using an established small RNA–profiling protocol (6), we compared miRNA expression in postmortem brain samples from control patients with postmortem brain samples of either AD or severe primary age-related tauopathy, often termed tangle-predominant dementia (TPD) (ref. 5 and Supplemental Table 1; supplemental material available online with this article; doi:10.1172/JCI78421DS1). Patients with TPD share many features with AD patients, including amnestic dementia, but develop neurofibrillary tangles (NFTs) independently of amyloid-β peptide (Aβ) (7). Using these cases, we sought to pinpoint changes that are associated with neurofibrillary degeneration. We obtained an average of 12.9 million sequence reads for each sample (range = 3.8–47.2 million) (Supplemental Table 2). All the small RNA classes showed a similar distribution of reads, and 86% of the reads mapped to known miRNAs (Supplemental Figure 1A and Figure 1A). Regression analysis revealed a strong correlation among normalized read counts (average r2 = 0.88, range = 0.76–0.96)(Figure 1B).
Figure 1. miR-219 is downregulated in autopsy brain tissue from AD and TPD patients.
(A) Pie charts showing the proportion of RNA-sequencing reads mapping to noncoding RNA subgroups (average in each comparison group). piRNA, piwi-interacting RNA; snoRNA, small nucleolar RNA; tRNA, transfer RNA. (B) Correlations of the normalized read frequencies of all detected miRNAs between representative subjects and the average from all profiles demonstrate a high degree of correlation. (C) Heatmap diagram with unsupervised hierarchical clustering showing changes in the levels of the most significant miRNAs identified in AD (n = 6) and TPD (n = 3) brain tissue samples relative to those of controls (n = 7). Statistical analysis was performed using 1-way ANOVA (P < 0.05, unadjusted). Data are shown as a pseudocolored heatmap (log2-transformed relative expression values of the normalized read frequency). (D) qPCR confirmed decreased levels of miR-219 in TPD (n = 19) and AD (n = 7) brain tissue samples compared with those of controls (n = 20). SNORD24 levels were used for normalization. **P ≤ 0.01 by 2-tailed Student’s t test.
To generate miRNA signatures, we compared the read frequencies between brain samples from AD, TPD, and control subjects (Supplemental Figure 1B). When ranked by significance, we found differences in 25 miRNAs among AD, TPD, and control brain samples (Figure 1C). Eleven miRNAs were different between TPD and control samples, 20 between AD and control samples, and 6 between TPD and AD samples. We focused on miR-219-5p (abbreviated hereafter as miR-219), as it showed the most dramatic difference between samples from patients and controls. Intriguingly, miR-219 is highly enriched in the brain (8). Further, miR-219 is a highly conserved “ancient” miRNA (9), showing 100% sequence identity among numerous species (Supplemental Figure 2). miR-219 can arise from 2 distinct precursors, miR-219-1 or miR-219-2, that produce identical mature 5p species but diverge in their 3p forms (Supplemental Figure 2). Since we observed negligible levels of miR-219-1-3p in our profiles, we inferred that differences in the miR-219-2 precursor might be responsible for the changes in miR-219 (Supplemental Table 3). Notably, we did not detect a difference in neuronal miR-124, suggesting that our observed differences might not be secondary to neuronal loss, but that compensatory changes may play a role. To validate our findings, we used quantitative PCR (qPCR). Once again, we observed a significant downregulation of miR-219 in both AD and TPD samples when compared with those from controls (Figure 1D). While there is currently no consensus as to which miRNAs are altered in AD, this finding is consistent with those of other studies (10–12).
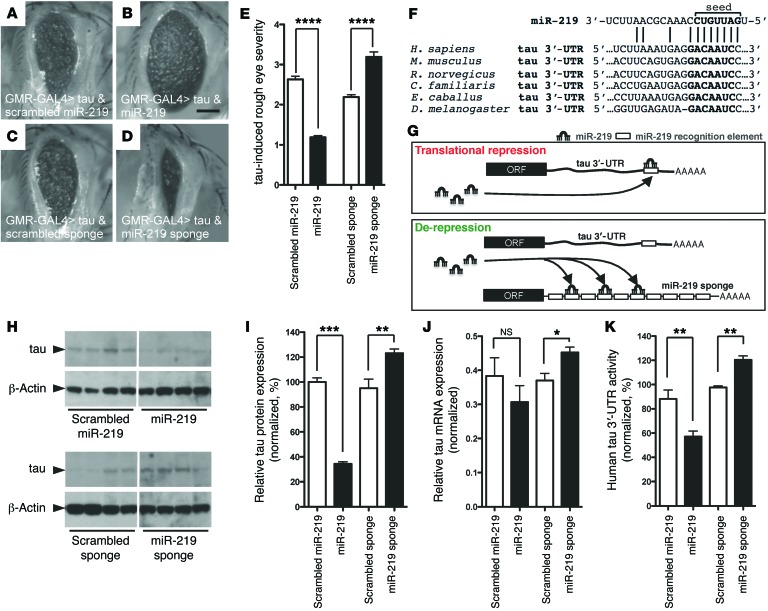
Next, we asked whether miR-219 influences tau toxicity in vivo. Transgenic Drosophila that express human tau are useful for modeling fundamental mechanisms of tau biology and tauopathy (13). Furthermore, many cellular mechanisms are conserved between Drosophila and humans, including miRNA regulation (14). Using a conditional expression system, we overexpressed either miR-219 or a miR-219–inhibiting sponge, a recombinant transcript containing multiple tandem miR-219 binding sites that significantly reduces miRNA levels (15). When human tau was expressed in the Drosophila eye alone or was coexpressed with a scrambled miR-219 control, a severe rough eye phenotype was produced (Supplemental Figure 3 and Figure 2A). When human tau and miR-219 were coexpressed, a partial reversal of the rough eye phenotype was observed, consistent with a protective role (Figure 2B). In contrast, coexpression of human tau and a miR-219–inhibiting sponge resulted in an exacerbation of the phenotype, demonstrating bidirectionality (Figure 2, C and D). Quantitative assessment of these phenotypes revealed that these findings are highly significant (Figure 2E and Supplemental Figure 4), indicating that miR-219 influences tau toxicity in this system.
Figure 2. In vivo regulation of tau toxicity and expression by miR-219.
(A and B) Coexpression of miR-219 partially suppressed the human tau–induced rough eye phenotype compared with that seen in the scrambled miR-219. (C and D) Coexpression of the miR-219 sponge with human tau exacerbated the phenotype compared with that observed in the scrambled sponge. Scale bar: 100 μm. (E) Semiquantitative assessments of the rough eye phenotype (n = 38/group). (F) miR-219 recognition element in the tau 3′-UTR. (G) Schematic illustrating regulation of tau by miR-219 and de-repression with the sponge. (H and I) Quantitative immunoblot of extracts from flies expressing miR-219 in the brain revealed decreased Drosophila tau protein levels compared with those detected in the scrambled control. Expression of the miR-219 sponge shows increased tau protein levels compared with those in the scrambled control (noncontiguous from the same gel). (J) qPCR shows that the tau mRNA levels were unchanged in the miR-219–expressing flies, but there was an increase in tau mRNA levels in flies expressing the miR-219 sponge compared with that observed in the scrambled control. (K) Flies that coexpress miR-219 and luciferase fused to the human tau 3′-UTR showed reduced activity compared with that seen in the scrambled control. Conversely, the miR-219 sponge significantly increased 3′-UTR activity compared with that in the scrambled control. Flies expressing a GAPDH 3′-UTR fused to luciferase were used for normalization. Data are representative of 3 experiments. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 by 2-tailed Student’s t test; ****P ≤ 0.0001 by Mann-Whitney U test.
This finding prompted us to investigate how miR-219 might regulate tau toxicity. In theory, this could occur either directly by altering tau levels, or indirectly through other factors. Using algorithms based on sequence homology, we found that among all the significantly downregulated miRNAs identified in our profiling studies, only miR-219 is predicted to target tau. The mature miR-219-2-3p is not predicted to target tau. Notably, the predicted miR-219 interaction with the tau mRNA is broadly conserved among species (Figure 2F). A conserved miR-219 recognition element is not contained in the 3′-UTR of other microtubule-associated proteins, including MAP1a, MAP1b, MAP2, and MAP4, suggesting a relative selectivity for MAPT-derived transcripts. Thus, miR-219 is the only highly conserved, brain-enriched miRNA predicted to target tau that we observed to be downregulated in AD and TPD.
We next tested the hypothesis that miR-219 ameliorates tau toxicity by direct silencing of tau expression through the highly conserved recognition element in the tau 3′-UTR (Figure 2G). The Drosophila tau gene encodes a protein with 66% amino acid similarity to human tau and shares many critical features, including microtubule-binding domains (16). Examination of the Drosophila genome revealed that the fly tau gene has a previously incompletely annotated 3′-UTR that extends for 1,439 bp and contains a single miR-219 recognition element (Supplemental Table 4). In fact, of all the miRNAs predicted to target human tau, the miR-219 recognition element is the only one present in Drosophila, suggesting a striking evolutionarily conserved relationship. This finding allowed us to ask whether tau is regulated by miR-219 in vivo in Drosophila. Our expression system enabled us to increase miR-219 levels 8.7-fold over baseline in the fly brain, whereas the sponge reduced miR-219 levels by 31.7% (Supplemental Figure 5, A and B). When miR-219 was overexpressed in the fly brain, we found a highly significant reduction in tau protein levels (63.8%; P < 0.001) compared with those in the scrambled miRNA control (Figure 2, H and I). Depletion of miR-219 levels with the sponge inhibitor resulted in a significant increase in tau protein levels (23.1%; P < 0.05) compared with those in controls (Figure 2, H and I). Expression of the miR-219 sponge resulted in a significant increase (22%; P < 0.05) in tau mRNA levels (Figure 2J). However, the tau mRNA levels were unchanged following expression of miR-219, suggesting effects on both mRNA stability and translation.
We extended our analysis to confirm that miR-219 regulates human tau 3′-UTR activity in vivo. We generated transgenic Drosophila that express luciferase fused to the human tau 3′-UTR. Overexpression of miR-219 significantly reduced the luciferase activity of the human tau 3′-UTR constructs compared with that observed in the scrambled control (P < 0.01; Figure 2K). In contrast, the miR-219 sponge inhibitor significantly increased luciferase activity (P < 0.01; Figure 2K), further supporting the presence of an evolutionarily conserved mechanism of tau regulation.
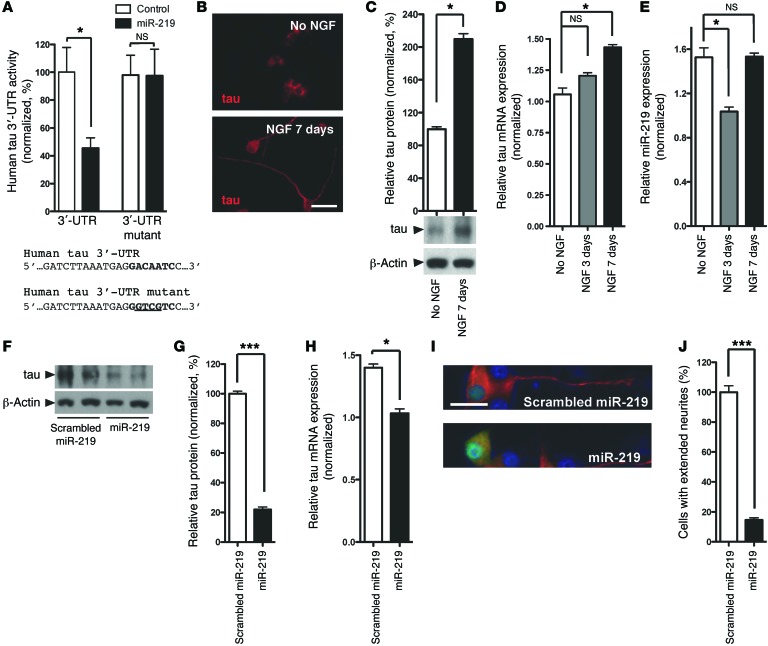
Next, we confirmed that miR-219 silences tau expression in human cells and asked whether this occurs through a direct interaction with the tau 3′-UTR. We inserted the full-length human tau 3′-UTR into a dual-luciferase reporter construct downstream of the Renilla luciferase and transiently cotransfected this construct along with a miR-219 miRNA mimic into human neuroblastoma cell cultures and compared them with control cells. We found that the miR-219 mimic significantly reduced luciferase activity when compared with that in the scrambled control (P < 0.05), while site-directed mutagenesis of the miR-219 recognition element abrogated silencing (Figure 3A). These findings demonstrate that silencing of tau expression by miR-219 occurs through a direct interaction with the predicted and highly conserved recognition element in the tau 3′-UTR.
Figure 3. miR-219 directly regulates tau expression and attenuates neurite outgrowth in mammalian cell cultures.
(A) Cotransfection of human neuroblastoma cells (SH-SY5Y) with a miR-219 mimic and a dual-luciferase human tau 3′-UTR reporter demonstrated reduced expression compared with that seen in the scrambled miRNA control. Mutagenesis of the miR-219 recognition element abrogated silencing. (B) Immunofluorescence on rat pheochromocytoma (PC12) cells using antisera targeting total tau (tauC) showed extension of processes following treatment with 100 nM NGF. Scale bar: 25 μm. (C) Immunoblot using tauC demonstrated increased tau protein levels following NGF treatment. (D) An increase in tau mRNA levels was observed following NGF treatment. (E) A transient decrease in miR-219 levels (normalized to SNORD24) occurred 3 days after NGF treatment and returned to baseline levels by day 7. (F and G) Quantitative immunoblot analysis using PC12 cells transduced with the lentiviral miR-219 vector revealed reduced tau protein levels compared with those detected in the scrambled miRNA control when differentiated for 7 days. (H) qPCR showed reduced tau mRNA levels in PC12 cells transduced with the lentiviral miR-219 vector. (I) Immunofluorescence microscopy using tauC (red) showed neurite outgrowth in untransduced PC12 cells, but cells transduced with lentiviral miR-219 (green) failed to extend neurites, as judged by the merged image (lower panel; yellow). (J) Quantification of neurite extension revealed a significant decrease in the numbers of tau-positive neurites in cells transduced with miR-219 compared with that observed in untransduced cells. Data are representative of 3 experiments. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 by 2-tailed Student’s t test.
Given that miR-219 can interact with the tau 3′-UTR, it was next necessary to validate these findings in a more physiological context. Functional trend analysis (17) suggests that miR-219 plays a role in neuron differentiation, axon development, and gene expression (Supplemental Table 5). Previous work indicates that tau plays a functional role during neurite outgrowth (18, 19). Using PC12 cells exposed to nerve growth factor (NGF), a well-established neurite outgrowth model (20), we confirmed that NGF induces differentiation into neuron-like cells with concomitant increases in tau mRNA and protein levels (Figure 3, B–D). Intriguingly, we also found that NGF induced a transient but significant downregulation of miR-219 that returned to baseline levels once the cells had fully differentiated (P < 0.05), demonstrating an inverse correlation between tau protein synthesis and miR-219 levels (Figure 3E). To determine whether changes in miR-219 influence tau expression, we expressed miR-219 in PC12 cells that were exposed to NGF and measured tau levels. Lentiviral expression of miR-219 resulted in a significant reduction in both tau protein and mRNA levels (Figure 3, F–H). While we observed a 26.2% decrease in tau mRNA levels following miR-219 treatment (P < 0.05), we detected a disproportionally large 76.7% reduction in tau protein levels (P < 0.001). In addition, cells transduced with miR-219 failed to extend tau-positive processes compared with those that were transduced with the scrambled control (Figure 3, I and J). Altogether, these results suggest that NGF-induced tau synthesis is regulated by miR-219.
In conclusion, these results suggest that tau toxicity is modulated by miR-219 through direct regulation of tau synthesis. This conclusion is based on the fact that miR-219 specifically binds the tau 3′-UTR in vitro and is bidirectionally regulated by miR-219 overexpression and inhibition in vivo. Our findings are consistent with miR-219 influencing tau expression at the post-transcriptional level, either through mRNA destabilization or translational repression, but this may differ depending on the experimental context. Further validation in other disease models and behavioral studies would be valuable. It is unlikely that miR-219 is alone among miRNAs in its ability to regulate human tau, but the role of miR-219 is of critical interest, given the extraordinary and unique conservation of its recognition element in the tau 3′-UTR. Further studies will provide a better understanding of the regulation of tau and advance our understanding of the pathogenesis of neurofibrillary degeneration and may guide us toward the development of novel therapeutic strategies.
Methods
Detailed Supplemental Methods are available online.
Small-RNA profiling.
RNA-sequencing data sets were deposited in the NCBI’s Gene Expression Omnibus database (GEO GSE63501).
Statistics.
Statistical significance was determined by 1-way ANOVA and Tukey’s test or by a 2-tailed Student’s t test using GraphPad Prism software (GraphPad Software). A P value of less than 0.05 was considered significant. For all figures in which error bars are shown, data represent the mean ± SEM. Statistical outliers and specimens with measurement errors were excluded.
Study approval.
Studies using autopsy tissue were approved by the IRB of Columbia University. Written informed consent was provided by the next of kin. Drosophila studies are not subject to IRB oversight.
Supplementary Material
Acknowledgments
This project was supported by NIH grants P30AG036453, K08NS072235, R01CA159227, R01MH080442, R01NS069695, P50AG08702, R01AG037212, P01AG07232, R01NS042859, P30AG028383, P50AG05131, U24NS072026, and P30AG19610. Additional support was provided by the Alzheimer’s Association (NIRG-11-204450); the Louis V. Gerstner Jr. Foundation; the Howard Hughes Medical Institute (HHMI); the BrightFocus Foundation; the Arizona Department of Health Services; the Arizona Biomedical Research Commission; and the Michael J. Fox Foundation. We express our deepest gratitude to the patients and staff of the contributing centers. Finally, we thank Jean-Paul Vonsattel, Etty Cortes, and Arlene Lawton for neuropathology support, Eric Lai for the transgenic Drosophila, and Nick Lowe for antisera.
Neil Renwick’s present address is: Laboratory of Translational RNA Biology, Department of Pathology and Molecular Medicine, Queen’s University, Kingston, Ontario, Canada.
Tudor A. Fulga’s present address is: Weatherall Institute, Oxford University, Oxford, United Kingdom.
John F. Crary’s present address is: Icahn School of Medicine at Mount Sinai, Department of Pathology, Mount Sinai School of Medicine, New York, New York, USA.
Conflict of interest: Thomas Tuschl is a cofounder of and advisor to Alnylam Pharmaceuticals and an advisor to Regulus Therapeutics. The Biogen Idec Foundation currently supports David Van Vactor.
Reference information:J Clin Invest. 2015;125(2):681–686. doi:10.1172/JCI78421.
References
- 1.Abe M, Bonini NM. MicroRNAs and neurodegeneration: role and impact. Trends Cell Biol. 2013;23(1):30–36. doi: 10.1016/j.tcb.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35(5):325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau P, Frigerio CS, De Strooper B. Variance in the identification of microRNAs deregulated in Alzheimer’s disease and possible role of lincRNAs in the pathology: The need of larger datasets. Ageing Res Rev. 2014;17:43–53. doi: 10.1016/j.arr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Crary JF, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafner M, et al. RNA-ligase-dependent biases in miRNA representation in deep-sequenced small RNA cDNA libraries. RNA. 2011;17(9):1697–1712. doi: 10.1261/rna.2799511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santa-Maria I, et al. The MAPT H1 haplotype is associated with tangle-predominant dementia. Acta Neuropathol. 2012;124(5):693–704. doi: 10.1007/s00401-012-1017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5(3):R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christodoulou F, et al. Ancient animal microRNAs and the evolution of tissue identity. Nature. 2010;463(7284):1084–1088. doi: 10.1038/nature08744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cogswell JP, et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimers Dis. 2008;14(1):27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- 11.Wang WX, Huang Q, Hu Y, Stromberg AJ, Nelson PT. Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: white matter versus gray matter. Acta Neuropathol. 2011;121(2):193–205. doi: 10.1007/s00401-010-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau P, et al. Alteration of the microRNA network during the progression of Alzheimer’s disease. EMBO Mol Med. 2013;5(10):1613–1634. doi: 10.1002/emmm.201201974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papanikolopoulou K, Skoulakis EM. The power and richness of modelling tauopathies in Drosophila. Mol Neurobiol. 2011;44(1):122–133. doi: 10.1007/s12035-011-8193-1. [DOI] [PubMed] [Google Scholar]
- 14.Dai Q, Smibert P, Lai EC. Exploiting Drosophila genetics to understand microRNA function and regulation. Curr Top Dev Biol. 2012;99:201–235. doi: 10.1016/B978-0-12-387038-4.00008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loya CM, Lu CS, Van Vactor D, Fulga TA. Transgenic microRNA inhibition with spatiotemporal specificity in intact organisms. Nat Methods. 2009;6(12):897–903. doi: 10.1038/nmeth.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidary G, Fortini ME. Identification and characterization of the Drosophila tau homolog. Mech Dev. 2001;108(1–2):171–178. doi: 10.1016/s0925-4773(01)00487-7. [DOI] [PubMed] [Google Scholar]
- 17.Berriz GF, Beaver JE, Cenik C, Tasan M, Roth FP. Next generation software for functional trend analysis. Bioinformatics. 2009;25(22):3043–3044. doi: 10.1093/bioinformatics/btp498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol. 1985;101(5 pt 1):1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drubin D, Kobayashi S, Kellogg D, Kirschner M. Regulation of microtubule protein levels during cellular morphogenesis in nerve growth factor-treated PC12 cells. J Cell Biol. 1988;106(5):1583–1591. doi: 10.1083/jcb.106.5.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.